Summary
Congenital heart block (CHB) is a potentially lethal condition characterized by a third‐degree atrioventricular block (AVB). Despite anti‐Ro52 antibodies being detected in nearly 90% of mothers of affected children, CHB occurs in only 1–2% of anti‐Ro/Sjögren's‐syndrome‐related antigen A (SSA) autoantibody‐positive pregnancies. Maternal antibodies have been suggested to bind molecules crucial to fetal cardiac function; however, it remains unknown whether a single antibody profile associates with CHB or whether several specificities and cross‐reactive targets exist. Here, we aimed to define further the reactivity profile of CHB‐associated antibodies towards Ro52p200 (amino acid 200‐239). We first analysed reactivity of a monoclonal anti‐Ro52 antibody shown to induce AVB in rats (7.8C7) and of sera from anti‐Ro52p200 antibody‐positive mothers of children with CHB towards a panel of modified Ro52p200 peptides, and subsequently evaluated their potential to induce AVB in rats upon transfer during gestation. We observed that CHB maternal sera displayed a homogeneous reactivity profile targeting preferentially the C‐terminal part of Ro52p200, in contrast to 7.8C7 that specifically bound the p200 N‐terminal end. In particular, amino acid D233 appeared crucial to maternal antibody reactivity towards p200. Despite low to absent reactivity towards rat p200 and different binding profiles towards mutated rat peptides indicating recognition of different epitopes within Ro52p200, immunoglobulin (Ig)G purified from two mothers of children with CHB could induce AVB in rats. Our findings support the hypothesis that several fine antibody specificities and cross‐targets may exist and contribute to CHB development in anti‐Ro52 antibody‐positive pregnancies.
Keywords: anti‐Ro52 antibodies, AV block, congenital heart block, neonatal lupus erythematosus, SSA
Introduction
Congenital heart block (CHB) is an often‐lethal condition that may affect the children of women with anti‐Sjögren's‐syndrome‐related antigen A (SSA)/Ro and anti‐SSB/La autoantibodies 1, 2, 3, 4, 5. It is characterized by a block of signal conduction at the atrioventricular (AV) node (third‐degree AV block, AVB) in the otherwise anatomically normal fetal heart and usually develops during weeks 18‐24 of pregnancy 3, 6, 7. CHB is often preceded or accompanied by other cardiac pathologies, including lower‐degree AV block, prolonged isovolumetric contraction time, sinus bradycardia or more severe manifestations such as dilated myocardiopathy or endocardial fibroelastosis 8, 9, 10, 11.
Although anti‐SSA/Ro autoantibodies, and more particularly anti‐Ro52 antibodies, are found in approximately 90% of mothers of children with CHB, the risk for CHB is only 1–2% in single anti‐SSA/Ro antibody‐positive pregnancies 12, 13, 14. Defining a maternal antibody profile associated more closely with CHB remains a challenge; however it is important for both identification of high‐risk pregnancies and further understanding of the molecular mechanisms underlying antibody‐associated CHB. Maternal autoantibodies have been suggested to exert a pathogenic effect on the fetal heart by cross‐reacting to a cardiac‐specific molecule and interfering with calcium regulation and excitation–contraction coupling. In support of this hypothesis, anti‐Ro52 antibodies have been reported to induce bradycardia and complete AVB rapidly in Langendorff‐perfused hearts 15, and antibodies to amino acids 200–239 (p200) of Ro52 have been shown to disturb calcium homeostasis in cultured neonatal cardiomyocytes 16, 17. Several potential cross‐reactive targets have been suggested, the most likely candidates being L‐type calcium channel subunits 18, 19, 20. However, the question remains of whether there is indeed one single specific antibody profile underlying most cases of autoimmune‐associated CHB, or whether there may be several antibody specificities and cross‐targets involved.
In this study, we aimed to define further the epitope‐binding specificity of CHB‐associated anti‐Ro52 antibodies by analysing the reactivity of both a CHB‐inducing monoclonal antibody and sera from mothers of children with CHB towards a series of modified Ro52p200 peptides. We then evaluated the potential of anti‐Ro52 antibodies with different reactivity profiles to induce cardiac conduction disturbances in vivo in rat pups upon transfer during gestation.
Materials and methods
Patient sera and monoclonal antibody
Sera were obtained from 18 anti‐Ro52p200 antibody‐positive mothers of children diagnosed with second‐ or third‐degree AVB (CHB mother sera). The samples were collected prospectively from 2001 to 2012 at the Rheumatology Unit of the University‐Hospital of Padua, Italy and stored at −80°C. The study was carried out in accordance with the principles outlined in the Declaration of Helsinki and all participants gave informed consent. The generation and characterization of the monoclonal antibody 7.8C7 has been described previously 21.
Purification of human IgG antibodies
Immunoglobulin fractions containing immunoglobulin (Ig)G were purified from the serum of two mothers whose fetuses were diagnosed with third‐degree AVB (CHB mothers 1 and 2) and from the serum of a healthy donor (control) by protein A‐Sepharose gel separation (HiTrap Protein A HP columns; GE Healthcare, Uppsala, Sweden; chromatography system; ÄKTA, GE Healthcare).
Peptides
N‐term, mid, C‐term and rat‐to‐human (r2h) mutated peptides were synthesized at the Department of Medical Biophysics, Linköping University, Linköping, Sweden. All other peptides were purchased from Thermo BioSciences, Ulm, Germany. Peptide purity was confirmed by high‐performance liquid chromatography and mass spectrometry.
Enzyme‐linked immunosorbent assay (ELISA)
Peptide ELISA was performed as described previously 22, 23. Sera were tested at a dilution of 1 : 500 and the monoclonal antibody 7.8C7 at a concentration of 1 µg/ml. The specificity and affinity of purified IgG to different p200 peptides were assessed in competition experiments performed by preincubating serum (dilution 1 : 2000) with peptides in different concentrations ranging from 0·1 to 1 mg/ml for 1 h at 20°C prior to analysis in ELISA.
Circular dichroism spectroscopy
Circular dichroism spectra of peptides were recorded with a ChiraScan CD spectrometer (Applied Photophysics, Leatherhead, UK). All spectra were analysed at 25°C over the wavelength range 195 to 280 nm with a step size of 0·5 nm, a bandwidth of 1·5 nm, an average collection time of 2 s per point and an equilibration time of 1 min in a 0·1‐cm cuvette. The CD spectra were averaged from four wavelengths scans and blanked against the vehicle solution [0·1% trifluoroacetic acid (TFA) buffer]. In the absence of W, F or Y residues in the peptides, the concentration was estimated based on dry peptide weight, thus limiting the evaluation of secondary structure to a qualitative assessment.
Experimental animals and antibody transfer
Dark Agouti rats (Charles Rivers, Sulzfeld, Germany) were kept and bred in the animal facility at the Center for Molecular Medicine at the Karolinska Institute, Stockholm, Sweden. All experimental protocols were approved by the Stockholm North Ethics Committee. Fifteen‐week‐old female rats were injected intraperitoneally with 4 mg of purified IgG on day 7 after mating.
Electrocardiogram (ECG) recording
Three‐lead ECGs were recorded within 24 h of birth on conscious pups, as described previously 16. PR intervals were corrected for heart rate variation by expressing them as PR/√RR.
Statistical analysis
Statistical analysis was performed using the Mann–Whitney U‐test. A P‐value < 0·05 was considered significant.
Results
An AVB‐inducing monoclonal antibody and sera from mothers of children with CHB exhibit differential reactivity profiles to Ro52p200
Antibody reactivity to the 40 amino acid long Ro52p200 peptide has been associated with CHB both in humans 21, 24 and in animal models 16, 17. To define further the Ro52p200 epitope(s) recognized preferentially by CHB‐associated antibodies, we first assessed the reactivity of a monoclonal anti‐human Ro52p200 antibody shown previously to induce AVB in a rat model (7.8C7) 16 and of sera from anti‐Ro52p200 antibody‐positive mothers of children with CHB (CHB mothers) towards two largely overlapping Ro52 peptides encompassing the p200 region, p197 and p200 (Fig. 1a). Strikingly, while the anti‐Ro52p200 monoclonal antibody bound to p200 and p197 to a similar extent (Fig. 1b), most sera from CHB mothers showed little to no reactivity to p197, despite binding to p200 (Fig. 1c). p197 differs from p200 by only a few amino acids; however, it has been shown to exhibit a lesser degree of α‐helical structure and overall stability than p200 25. Lack of binding of maternal CHB sera to p197 could therefore be due to either the loss of a dominant epitope in the C‐terminal end of p200, or the loss of a structure‐dependent epitope, or both.
Figure 1.
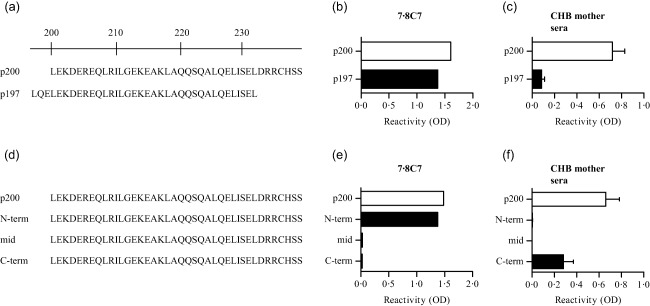
Different reactivity profiles of congenital heart block (CHB)‐associated anti‐Ro52 antibodies to p200. (a) Amino acid sequence of the human Ro52 p200 and p197 peptides. (b,c) Reactivity to p200 and p197 of the anti‐Ro52 monoclonal antibody 7.8C7 (b) and of CHB mother sera [n = 18, mean ± standard error of the mean (s.e.m.)] (c). (d) Truncated p200 peptides; the amino acids retained in the truncated peptides compared to full‐length p200 are indicated in black letters. (e,f) Reactivity to truncated p200 peptides of 7.8C7 (e) and CHB mother sera (n = 18, mean ± s.e.m.) (f).
Subsequent analysis of reactivity towards overlapping truncated p200 peptides (Fig. 1d) revealed that the anti‐p200 monoclonal antibody 7.8C7 targets an epitope located within the N‐terminal part of the p200 peptide (Fig. 1e). By contrast, sera from CHB mothers exhibited a surprisingly homogeneous profile that differed radically from that of 7.8C7, as they bound only to the truncated C‐term p200 peptide (Fig. 1f). Reactivity to this peptide was nevertheless lower than that observed towards the full‐length p200, indicating that CHB mothers' sera not only target the C‐terminal part of the p200 peptide, but also probably recognize additional epitopes within p200, the presence of which is dependent upon an intact structure lost in the truncated peptides.
Sera from CHB mothers bind preferentially to a dominant epitope in the C‐terminal end involving amino acid D233
To define further the epitope specificity of CHB‐associated human sera, we took advantage of the fact that, despite a high homology between human and rat Ro52p200 peptides (Fig. 2a), most sera from CHB mothers show little to no reactivity towards rat p200 compared to human p200 (Fig. 2b). We therefore generated three different peptides identical in sequence to rat p200 except for a few selected amino acids that were substituted for their respective human counterparts (rat‐to‐human, r2h, peptides) (Fig. 2a). The selection of amino acids to mutate was based on their chemical properties (charge, polarity or side chain) and their predicted position based on a helical wheel sketching assuming an α‐helical fold of the peptide 22. As the lack of reactivity of CHB maternal sera observed towards the p197 and truncated p200 peptides could be due to a loss of peptide structure, we first verified by circular dichroism that both the r2h peptides and the rat p200 peptide had a secondary structure and α‐helical contribution similar to that observed previously for p200 (Fig. 2c–f) 22.
Figure 2.
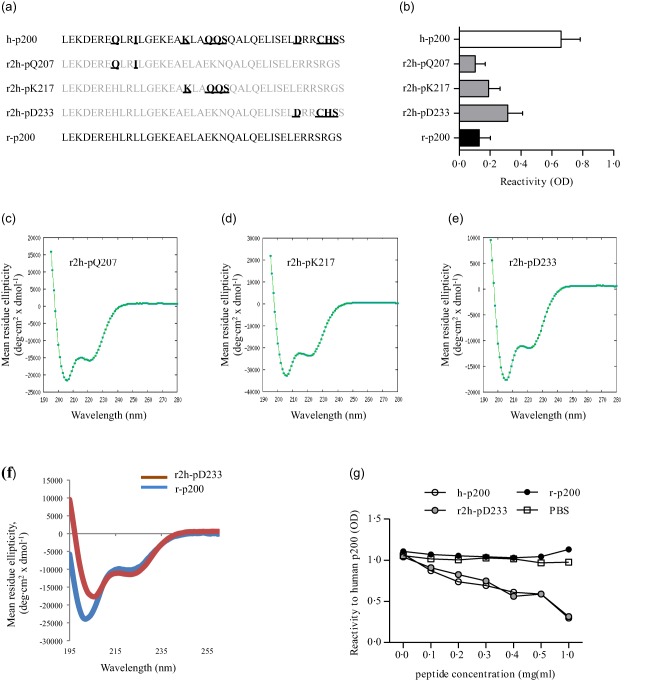
Fine specificity of anti‐Ro52p200 antibodies from congenital heart block (CHB) mothers. (a) Amino acid sequence of human p200 (h‐p200), rat p200 (r‐p200), and rat‐to‐human mutated p200 peptides (r2h‐pQ207, r2h‐pK217 and r2h‐pD233). Mutated amino acids are highlighted for each r2h peptide; unchanged amino acids from the initial rat p200 peptide are indicated in grey. (b) Reactivity of CHB mother sera [n = 18, mean ± standard error of the mean (s.e.m.)] to p200 and r2h peptides. (c–f) Circular dichroism spectroscopy analysis of r2h and r‐p200 peptide secondary structure showing α‐helical contribution. (g) Competition assay showing the binding specificity of a CHB mother serum (CHB mother 1). [Colour figure can be viewed at wileyonlinelibrary.com]
Analysis of maternal sera binding to the mutated rat p200 peptides revealed that part of the reactivity observed towards human p200 was recapitulated by introducing rat‐to‐human amino acid mutations in the C‐terminal part of the rat p200 peptide (r2h‐pD233, Fig. 2b). By contrast, there was no gain in mean reactivity when amino acid substitutions were introduced in the N‐terminal or central part (r2h‐pQ207 and r2h‐pK217, Fig. 2b). Together, these data confirm the presence of a dominant epitope in the p200 C‐terminal part, while also supporting the existence of additional epitopes located in the middle stretch of p200. Of note, competition experiments with serum from a CHB mother (CHB mother 1) revealed that reactivity to Ro52‐p200 was decreased by preincubation with soluble r2h‐pD233 in a concentration‐dependent manner to a degree similar to preincubation with h‐p200 (Fig. 2g), suggesting that most of the reactivity to Ro52p200 in given CHB mother sera might be due to reactivity towards a dominant epitope in the C‐terminal part of p200 (involving amino acids 233 and 236–238).
To pinpoint the crucial amino acid(s) underlying the reactivity of maternal CHB sera to the C‐terminal part of the p200 peptide, we performed an alanine scan of that region (Fig. 3a). Peptides used in the alanine scan have already been described and were shown to have a similar secondary structure and α‐helical contribution as p200 22. Strikingly, the reactivity profile among the CHB maternal sera showed a consistently characteristic decrease in reactivity towards p200 when the aspartic acid at position 233 was substituted for an alanine (Fig. 3b). Single amino acid substitutions at other positions (234–239) did not affect antibody binding, suggesting that aspartic acid 233 is crucial to a dominant epitope recognized by sera from mothers of children with CHB.
Figure 3.

Amino acid D233 is crucial to a dominant epitope recognized by sera from mothers of children with congenital heart block (CHB). (a) Schematic view of p200 peptides containing point mutations (alanine scan). The amino acid substituted by an alanine is indicated in black. (b) Reactivity of CHB mother sera [n = 18, mean ± standard error of the mean (s.e.m.)] to mutated p200 peptides described in (a).
Sera from CHB mothers with different binding specificities within p200 induce AVB in rat pups
The monoclonal antibody 7.8C7 binds equally well to the human and rat Ro52p200 peptides (data no shown), and has been shown to induce AVB in rats when transferred during gestation 16. By contrast, most sera from CHB mothers do not bind to the rat p200 peptide (Fig. 2b). We therefore asked whether IgG purified from such sera would also induce AVB in rats. In addition, we asked if different binding profiles towards the mutated rat‐to‐human peptides, which indicate recognition of different epitopes, would affect the potential pathogenic effect of the maternal sera on the rat fetal heart. We therefore selected sera from two CHB mothers that had different binding specificities to the mutated rat‐to‐human peptides for transfer experiments: serum from CHB mother 1 showed a somewhat restricted binding specificity, with evidence of reactivity towards the rat p200 peptide only when rat‐to‐human mutations were introduced in the C‐terminal part (r2h‐pD233) of p200, and not at other positions (Fig. 4a); serum from CHB mother 2 had a broader binding profile, with evidence of reactivity towards all rat‐to‐human mutated peptides and even towards the rat p200 peptide (Fig. 4b).
Figure 4.
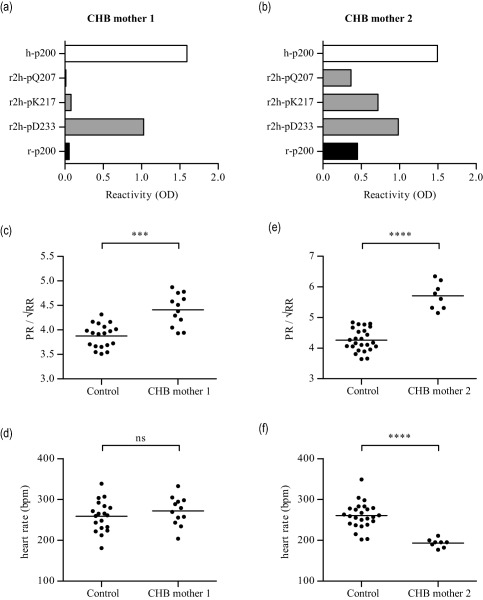
Anti‐Ro52p200 antibodies with different fine specificities induce atrioventricular block (AVB) in rats. (a, b) Reactivity profiles of the sera from two mothers of children with congenital heart block (CHB) (CHB mothers 1 and 2) to different p200 peptides (described in Fig. 2a). (c,d) PR interval (c) and heart rate (d) of rat pups exposed to CHB mother 1 immunoglobulin (Ig)G (n = 12) or control IgG (n = 19) during gestation. (e,f) PR interval (e) and heart rate (f) of rat pups exposed to CHB mother 2 IgG (n = 8) or control IgG (n = 25) during gestation. ***P < 0·001; ****P < 0·0001.
ECG recordings on neonatal rats revealed that pups exposed in utero to IgG purified from CHB mother 1 showed significantly longer PR intervals than pups born to mothers injected with control IgG (Fig. 4c), although no significant difference in heart rates was observed (Fig. 4d). Similar to IgG from CHB mother 1, IgG purified from CHB mother 2 also led to a significant prolongation of the PR interval in pups who had been exposed in utero compared to pups born to mothers who had received control IgG (Fig. 4e). However, while no effect on heart rate had been observed upon exposure to CHB mother 1 IgG, pups born to mothers who had received CHB mother 2 IgG showed significantly lower heart rates at birth than control pups (Fig. 4f). These data therefore suggest that anti‐Ro52 antibodies that recognize different epitopes within the p200 peptide may nevertheless similarly affect signal conduction at the AV node in rat pups upon transfer during gestation.
Discussion
Considering the relatively low penetrance of CHB in fetuses of anti‐Ro/SSA antibody‐positive women, efforts have been made to find an antibody profile that could predict CHB occurrence more accurately. Specificity towards amino acids 200–239 (p200) of Ro52 was suggested to associate with CHB in two studies 21, 24; however, no statistically significant differences in the prevalence of anti‐p200 antibodies were found between mothers of children with or without CHB in two other studies 26, 27. The Ro52p200 peptide is 40 amino acid long, and it is possible that specificity towards a particular epitope within p200, rather than reactivity to p200 in general, is associated with CHB. In the present study, we therefore evaluated the binding of CHB maternal sera to a series of truncated or mutated p200 peptides. Strikingly, we found that sera from 18 CHB mothers all bound preferentially to the C‐terminal part of p200, and that amino acid D233 in particular was crucial to maternal CHB sera binding to p200. By contrast, a monoclonal antibody (7.8C7) shown previously to induce AVB in rodents 16 targeted specifically an epitope within the N‐terminal end of p200. In addition, whereas 7.8C7 binds to the rat p200 peptide, maternal CHB sera showed little to no reactivity to rat p200. Introducing rat‐to‐human amino acid mutations within the C‐terminal part of the rat p200 peptide increased maternal CHB sera binding to some extent, further supporting the presence of a dominant epitope within the C‐terminal region and contrasting with the binding specificity of 7.8C7. Nevertheless, subsequent transfer of IgG purified from two CHB mothers with different reactivity profiles to the mutated rat p200 peptides into rats during gestation showed that, despite a reactivity profile different from that of 7.8C7 and different from each other, these CHB mother IgGs could also induce AVB in rat pups. These findings indicate that different fine specificities of anti‐Ro52 antibodies can similarly disrupt fetal cardiac conduction.
Maternal antibodies have been suggested to affect the fetal heart by cross‐reacting to a cardiac‐specific molecule and disturbing calcium homeostasis. Although we cannot exclude that there is an as‐yet‐unidentified common epitope targeted by both the maternal sera and monoclonal antibody tested here, our data suggest that these AVB‐inducing antibodies recognize different p200 epitopes and yet affect the fetal heart in a similar manner. This therefore supports the hypothesis of the existence of several cross‐reactive targets for CHB maternal sera in the fetal heart. Several well‐substantiated candidates have indeed been proposed, in particular different subunits of cardiac calcium channels 18, 19, 20. Altogether, it is possible that there is not one single CHB‐inducing antibody specificity, but rather several different specificities that may act in an additive fashion to induce substantial damage in the fetal heart and lead to complete third‐degree AVB.
In this study, we show that signs of AVB can be induced in rats upon transfer of human anti‐Ro52 antibody‐positive purified IgG. Importantly, although these findings indicate that cardiac manifestations similar to human CHB may be recapitulated in animal models, the phenotype observed in rodents remains mild, with the notable absence of complete third‐degree AVB 2, 28. One explanation may be that levels of IgG crossing the placenta in rodents are insufficient to lead to a full‐blown inflammation and fibrosis of the fetal AV node. Alternatively, it is likely that critical fetal susceptibility factors are absent in the mouse and rat strains studied so far. Indeed, we have shown previously that fetal MHC modulates the penetrance of first‐degree AVB in a rat model of CHB 29, and genetic variants modulating fetal cardiac function and/or inflammatory responses in the presence of maternal antibodies may contribute further to disease susceptibility and phenotype severity. This, in fact, mirrors the situation in humans, where the recurrence rate reaches only 12–20% despite the persistence of maternal antibodies 30, 31, 32, and where up to one‐third of fetuses of anti‐Ro52 antibody‐positive mothers have been described to develop a first‐degree AVB that, in the majority of cases, reverts spontaneously to normal heart rhythm before or at birth 11.
In all, we show here that anti‐Ro52 antibodies with different fine specificities can induce AV block in vivo upon transfer during gestation, supporting the idea that there may be not one, but several antibody profiles associated with CHB, as well as several cross‐targets recognized by maternal antibodies in the fetal heart. We also identify the C‐terminal part of the Ro52p200 peptide as a dominant epitope recognized by serum from mothers of children with CHB, with amino acid D233 being especially important for reactivity towards this epitope.
Disclosure
The authors declare they have no disclosures.
Author contributions
M. W. H. designed the study with input from A. H., S. E. S. and M. S. A. R. identified and recruited patients. A. H., V. O., M. H., J. B., L. O., M. A. and S. E. S. performed the experiments with guidance from M. S., S. E. S. and M. W. H.; A H., A. A., V. O. and M. A. analysed the data; A. H., A. A. and M. W. H. wrote the first draft of the manuscript, and all authors took part in revision until its final form.
Acknowledgements
We thank Linda Helmfors for excellent support in peptide synthesis. This study was supported by grants from the Swedish Research Council, the Swedish Heart‐Lung Foundation, the Stockholm County Council, the Karolinska Institute, the Swedish Rheumatism Association, King Gustaf the Vth 80‐year Foundation, the Freemason Children Foundation Stockholm, and the Torsten and Ragnar Söderberg Foundation.
References
- 1. Ambrosi A, Sonesson SE, Wahren‐Herlenius M. Molecular mechanisms of congenital heart block. Exp Cell Res 2014; 325:2–9. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosi A, Wahren‐Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res Ther 2012; 14:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman DM, Rupel A, Buyon JP. Epidemiology, etiology, detection, and treatment of autoantibody‐associated congenital heart block in neonatal lupus. Curr Rheumatol Rep 2007; 9:101–8. [DOI] [PubMed] [Google Scholar]
- 4. Michaelsson M, Engle MA. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin 1972; 4:85–101. [PubMed] [Google Scholar]
- 5. Wahren‐Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013; 382:819–31. [DOI] [PubMed] [Google Scholar]
- 6. Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum 2004; 50:173–82. [DOI] [PubMed] [Google Scholar]
- 7. Litsey SE, Noonan JA, O'Connor WN, Cottrill CM, Mitchell B. Maternal connective tissue disease and congenital heart block. Demonstration of immunoglobulin in cardiac tissue. N Engl J Med 1985; 312:98–100. [DOI] [PubMed] [Google Scholar]
- 8. Bergman G, Eliasson H, Bremme K, Wahren‐Herlenius M, Sonesson SE. Anti‐Ro52/SSA antibody‐exposed fetuses with prolonged atrioventricular time intervals show signs of decreased cardiac performance. Ultrasound Obstet Gynecol 2009; 34:543–9. [DOI] [PubMed] [Google Scholar]
- 9. Costedoat‐Chalumeau N, Amoura Z, Villain E, Cohen L, Piette JC. Anti‐SSA/Ro antibodies and the heart: more than complete congenital heart block? A review of electrocardiographic and myocardial abnormalities and of treatment options. Arthritis Res Ther 2005; 7:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution's experience of 30 years. J Am Coll Cardiol 2002; 39:130–7. [DOI] [PubMed] [Google Scholar]
- 11. Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren‐Herlenius M. Signs of first‐degree heart block occur in one‐third of fetuses of pregnant women with anti‐SSA/Ro 52‐kd antibodies. Arthritis Rheum 2004; 50:1253–61. [DOI] [PubMed] [Google Scholar]
- 12. Brito‐Zeron P, Izmirly PM, Ramos‐Casals M, Buyon JP, Khamashta MA. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol 2015; 11:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brucato A, Frassi M, Franceschini F et al Risk of congenital complete heart block in newborns of mothers with anti‐Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum 2001; 44:1832–5. [DOI] [PubMed] [Google Scholar]
- 14. Salomonsson S, Dzikaite V, Zeffer E et al A population‐based investigation of the autoantibody profile in mothers of children with atrioventricular block. Scand J Immunol 2011; 74:511–7. [DOI] [PubMed] [Google Scholar]
- 15. Boutjdir M, Chen L, Zhang ZH et al Arrhythmogenicity of IgG and anti‐52‐kD SSA/Ro affinity‐purified antibodies from mothers of children with congenital heart block. Circ Res 1997; 80:354–62. [DOI] [PubMed] [Google Scholar]
- 16. Ambrosi A, Dzikaite V, Park J et al Anti‐Ro52 monoclonal antibodies specific for amino acid 200‐239, but not other Ro52 epitopes, induce congenital heart block in a rat model. Ann Rheum Dis 2012; 71:448–54. [DOI] [PubMed] [Google Scholar]
- 17. Salomonsson S, Sonesson SE, Ottosson L et al Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med 2005; 201:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qu Y, Baroudi G, Yue Y, Boutjdir M. Novel molecular mechanism involving alpha1D (Cav1.3) L‐type calcium channel in autoimmune‐associated sinus bradycardia. Circulation 2005; 111:3034–41. [DOI] [PubMed] [Google Scholar]
- 19. Qu Y, Xiao GQ, Chen L, Boutjdir M. Autoantibodies from mothers of children with congenital heart block downregulate cardiac L‐type Ca channels. J Mol Cell Cardiol 2001; 33:1153–63. [DOI] [PubMed] [Google Scholar]
- 20. Strandberg LS, Cui X, Rath A et al Congenital heart block maternal sera autoantibodies target an extracellular epitope on the alpha1G T‐type calcium channel in human fetal hearts. PLoS One 2013; 8:e72668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strandberg L, Ambrosi A, Espinosa A et al Interferon‐alpha induces up‐regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52‐specific monoclonal antibodies. J Clin Immunol 2008; 28:220–31. [DOI] [PubMed] [Google Scholar]
- 22. Ottosson L, Salomonsson S, Hennig J et al Structurally derived mutations define congenital heart block‐related epitopes within the 200‐239 amino acid stretch of the Ro52 protein. Scand J Immunol 2005; 61:109–18. [DOI] [PubMed] [Google Scholar]
- 23. Tonello M, Ruffatti A, Marson P et al Plasma exchange effectively removes 52‐ and 60‐kDa anti‐Ro/SSA and anti‐La/SSB antibodies in pregnant women with congenital heart block. Transfusion 2015; 55:1782–6. [DOI] [PubMed] [Google Scholar]
- 24. Salomonsson S, Dorner T, Theander E, Bremme K, Larsson P, Wahren‐Herlenius M. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum 2002; 46:1233–41. [DOI] [PubMed] [Google Scholar]
- 25. Salomonsson S, Ottosson L, Safsten P et al Cloning and characterization of two human Ro52‐specific monoclonal autoantibodies directed towards a domain associated with congenital heart block. J Autoimmun 2004; 22:167–77. [DOI] [PubMed] [Google Scholar]
- 26. Clancy RM, Buyon JP, Ikeda K et al Maternal antibody responses to the 52‐kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum 2005; 52:3079–86. [DOI] [PubMed] [Google Scholar]
- 27. Reed JH, Clancy RM, Lee KH, Saxena A, Izmirly PM, Buyon JP. Umbilical cord blood levels of maternal antibodies reactive with p200 and full‐length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken) 2012; 64:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki H, Silverman ED, Wu X et al Effect of maternal autoantibodies on fetal cardiac conduction: an experimental murine model. Pediatr Res 2005; 57:557–62. [DOI] [PubMed] [Google Scholar]
- 29. Strandberg LS, Ambrosi A, Jagodic M et al Maternal MHC regulates generation of pathogenic antibodies and fetal MHC‐encoded genes determine susceptibility in congenital heart block. J Immunol 2010; 185:3574–82. [DOI] [PubMed] [Google Scholar]
- 30. Ambrosi A, Salomonsson S, Eliasson H et al Development of heart block in children of SSA/SSB‐autoantibody‐positive women is associated with maternal age and displays a season‐of‐birth pattern. Ann Rheum Dis 2012; 71:334–40. [DOI] [PubMed] [Google Scholar]
- 31. Friedman DM, Kim MY, Copel JA et al Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008; 117:485–93. [DOI] [PubMed] [Google Scholar]
- 32. Julkunen H, Eronen M. The rate of recurrence of isolated congenital heart block: a population‐based study. Arthritis Rheum 2001; 44:487–8. [DOI] [PubMed] [Google Scholar]


