Summary
Cytokines such as tumour necrosis factor (TNF)‐α, interleukin (IL)‐12, interferon (IFN)‐γ, IL‐23 and, more recently, IL‐9, have been implicated in the initiation/maintenance of inflammation in psoriasis and psoriatic arthritis (PsA). In the present study we aimed to characterize the role of γδ T cells in peripheral blood and synovial fluid of PsA patients and to investigate their response to in‐vitro stimulation with antigen or cytokines (IL‐9 and IL‐23). γδ T cells isolated from peripheral blood mononuclear cells and synovial fluid were analysed by flow cytometry to evaluate the phenotype and cytokine production. IL‐23R and IL‐9R gene expression were also evaluated by reverse transcription–polymerase chain reaction (RT–PCR). Peripheral blood mononuclear cells (PBMC), sorted γδ T cells and γδ cell lines were also stimulated in vitro with isopentenyl pyrophosphate (IPP), recombinant IL‐9 or recombinant IL‐23. Our results show an expansion of γδ T cells with a predominant effector memory phenotype in peripheral blood and synovium of untreated PsA patients, which reverses significantly after treatment with anti‐TNF‐α or anti‐IL‐12/IL‐23R monoclonal antibodies (mAbs). Moreover, in PsA patients γδ T cells activation is driven prevalently by IL‐9/IL‐9R interaction, and not only by IL‐23/IL‐23R. Together these findings indicate γδ T cells and IL‐9 as new players in the pathogenesis of PsA.
Keywords: γδ‐T cells, IL‐9, IL‐9R, psoriatic arthritis
Introduction
Psoriatic arthritis (PsA) is a heterogeneous chronic inflammatory disease that affects peripheral and axial joints, enthesis and other organs (ileum, nails, skin) of people with psoriasis (PsO) or with affected relatives. Not everyone who has psoriasis will develop psoriatic arthritis, even though the conditions are often related. The immune system is responsible for both, and inflammation leads to damage. Skin symptoms usually appear first, but arthritis symptoms sometimes appear months, or even years, before skin problems. Joint manifestations precede the onset of the cutaneous lesions in approximately 20% of patients with psoriatic arthritis (PsA). If there is a family history of psoriasis, these patients are diagnosed as having psoriatic arthritis sine psoriasis. The pathogenesis of PsA remains poorly understood, but both genetic and environmental factors appear to contribute to the onset and severity of the disease.
Cytokines such as tumour necrosis factor (TNF)‐α, interleukin (IL)‐12, interferon (IFN)‐γ and, in particular, IL‐23 are released from inflammatory and resident cells, and have been implicated in the initiation/maintenance of inflammation in psoriasis (Ps) and PsA 1. In particular, activation of the IL‐23 axis with the production of IL‐17 and IL‐22 downstream cytokines was demonstrated to elicit an inflammatory response resulting in inflammation of the skin, enthesis and synovium 1, 2. More recently IL‐9, another pleiotropic cytokine with proinflammatory activity, was found to be expressed highly in peripheral blood, synovium and gut of patients with PsA 3.
CD4 and also CD8 T cells were regarded originally to represent the major sources of these effector cytokines, but other T cell types may also contribute to their production.
γδ T cells, a population of unconventional T cells bridging innate and adaptive immunity and thus contributing to inflammation and autoimmunity, have been shown to be expanded in peripheral blood of PsA patients 4. In particular, the majority of γδ T cells in peripheral blood have the Vγ9Vδ2 T cell receptor. These cells recognize antigen in a major histocompatibility complex‐independent manner and develop strong cytolytic and T helper type 1 (Th1)‐like effector functions. Vγ9Vδ2 T cells respond to phosphoantigens such as (E)‐4‐hydroxy‐3‐methyl‐but‐2‐enyl pyrophosphate (HMBPP), which is synthesized in bacteria, and isopentenyl pyrophosphate (IPP), which is produced in eukaryotic cells through the mevalonate pathway. In physiological conditions, the generation of IPP is not sufficient for the activation of γδ T cells. Dysregulation of the mevalonate pathway in activated/transformed cells leads to accumulation of IPP and γδ T cell activation 5, 6.
Studies carried out on psoriasis (Ps) and PsA‐like disease mice models have demonstrated that Th17 cells were not the primary source of IL‐17 and IL‐22, but these cytokines were also produced by a skin‐invading population of γδ T cells 7. Neutralization of IL‐17, produced mainly by γδ T cells, blocked disease symptoms completely in these mice 7, 8, 9.
More recently, activated Vγ6+CD27– γδ T cells, another subset of γδ T cells expressing the invariant Vγ6/Vδ1 T cell receptor (TCR) and developing only in the late‐embryonic thymus, were demonstrated to be abundant in non‐inflamed entheseal tissue and to constitute the large majority of RORγt + IL‐23R+ enthesis‐resident lymphocytes able to increase in numbers under inflammatory conditions and to produce IL‐17 efficiently 10.
In the present study we aimed to: (1) characterize more clearly the frequency and the phenotype of γδ T cells in peripheral blood and synovial fluid of PsA patients, (2) investigate their cytokine production profile, (3) study their response to in‐vitro stimulation with isopentenyl pyrophosphate (IPP) or cytokines (IL‐9 and IL‐23) and (4) to study changes in their function and cytokine production after treatment with cytokine‐blocking agents.
Here we demonstrate an expansion of γδ T cells with a predominant effector memory phenotype in peripheral blood and synovium of untreated PsA patients, which reverses significantly after treatment with anti‐TNF‐α or anti‐IL‐12/IL‐23R monoclonal antibodies (mAbs). At the same time we demonstrate that γδ T cells activation is driven prevalently by IL‐9/IL‐9R interaction, and not only by IL‐23/IL‐23R in PsA. Together, these findings may indicate γδ T cells and IL‐9 as new players in the pathogenesis of PsA.
Material and methods
Patients
Forty patients with PsA classified according to the CASPAR criteria 11, 12 (12 patients with predominant axial involvement), 10 patients with osteoarthritis (OA), five patients with rheumatoid arthritis (RA), five patients with Ps and 20 healthy donors (HD) were enrolled into this study. Table 1 shows the baseline characteristics of patients and controls. Blood samples were collected at baseline and after 12 weeks of therapy with adalimumab (n = 26) or ustekinumab (n = 14). Synovial fluid samples (from knees) were obtained from 12 untreated PsA patients and 10 OA patients. PsA disease activity was measured by the Disease Activity Index for Psoriatic Arthritis (DAPSA) 13. Active disease for patients with axial involvement was assessed by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 14. Collection of samples and all experimental protocols were approved by the ethical committee and the institutional review board of the University of Palermo, and methods were carried out in accordance with the approved guidelines. Informed consent was obtained from each patient and controls.
Table 1.
Baseline characteristics of patients and controls
| PsA axial (n = 12) | PsA peripheral (n = 28) | Osteoarthritis (n = 10) | Controls (n = 20) | |
|---|---|---|---|---|
| Age, mean (range) years | 32 (24–61) | 27 (20–57) | 46 (32–69) | 34 (28–69) |
| Sex, no. (%) female | 6 (50) | 12 (43) | 7 (70) | 14 (70) |
| HLA‐B27 (%) | 3 | 5 | 0 | – |
| Disease duration, mean (range) months | 52 (10–65) | 40 (10–64) | 33 (11–140) | – |
| CRP (mg/l), mean (range) | 3.7 (1.6–5) | 4.5 (3–12) | 2 (1.5–3) | – |
| DAPSA score, mean (range) | – | 5.7 (3.6–10) | – | – |
| BASDAI score, mean (range) | 5.2 (4–7) | – | – | – |
| Salazopyrin (%) | – | 7 (25) | – | – |
| Methotrexate (%) | – | 7 (25) | – | – |
| Adalimumab (%) | 8 (66) | 18 (64) | – | – |
| Ustekinumab (%) | 4 (34) | 10 (36) | – | – |
PsA = psoriatic arthritis; HLA = human leucocyte antigen; CRP = C‐reactive protein; DAPSA = Disease Activity Index for Psoriatic Arthritis; BASDAI = Bath Ankylosing Spondylitis Disease Activity index.
Isolation of peripheral blood and synovial fluid mononuclear cells, preparation of γδ T cell lines and flow cytometry
Peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) were isolated from heparinized blood and synovial fluid samples, respectively, by Ficoll‐Hypaque (Sigma, St Louis, MO, USA) density‐gradient centrifugation. RPMI‐1640 medium supplemented with 2 nM L‐glutamine, 5 mM Hepes, 100 U/μg/ml penicillin/streptomycin and 10% fetal calf serum (FCS) (all obtained from BioWhittaker Italia, Rome, Italy) was used throughout experiments. Cell viability (trypan blue dye exclusion) was always > 95%.
Polyclonal Vδ2 T cell lines were generated by culture of PBMC with zoledronic acid 0·5 μM (Novartis, Basel, Switzerland) and IL‐2 50 UI/ml (Proleukin; Novartis) for 2 weeks, as described previously 15. The heterogeneous population of γδ T cells produced from ex‐vivo culture reproduce perfectly in large scale the small pool of γδ T cells present in vivo, leading us to study their phenotype, their functions and the secreted proinflammatory cytokines.
PBMC, sorted γδ T cells (by enriching PBMCs using a γδ T cell isolation kit from Miltenyi Biotec, Bergisch Gladbach, Germany) and γδ cell lines (obtained as described above) were stimulated with IPP (100 μM; Sigma), recombinant IL‐9 (2 ng/ml; BD Biosciences, San Josè, CA, USA) or recombinant IL‐23 (10 ng/ml; BD Biosciences) at 37°C in 5% CO2. After 2 h of incubation, brefeldin A (10 μg/ml; Sigma) was added, and after 16 h of incubation cells were collected and stained with the following monoclonal antibodies: anti‐CD45 (BD Biosciences), anti‐TCRVδ2 (BD Biosciences), anti‐CD45RA (BD Biosciences), anti‐CD27 (BD Biosciences), anti‐IFN‐γ (BD Biosciences), anti‐IL‐17 (R&D Systems, Minneapolis, MN, USA), anti‐IL‐22 (R&D Systems), anti‐IL‐23R (R&D Systems) and anti‐IL‐9R (R&D Systems). Isotype‐matched antibodies of irrelevant specificities were used as a negative control. Cells were incubated with mAbs for 30 min on ice and washed twice in phosphate‐buffered saline (PBS) containing 0·1% (w/v) NaN3 (Sigma). After surface staining, the cells were fixed with 2% (w/v) paraformaldehyde (Sigma) for 30 min at 4°C and then treated with a permeabilization solution (BD Biosciences) for 10 min at room temperature and stained with antibodies for intracellular antigens for 30 min at 4°C. Cells were acquired by a fluorescence activated cell sorter (FACS) Canto II flow cytometer (BD Biosciences) and analysis was performed using FlowJo analysis software (TreeStar Inc., Ashland, OR, USA). The gating strategy involved progressively measuring total cells, viable cells only, lymphomonocytes and specific cell types. For every sample, 100 000 nucleated cells were acquired and values are expressed as percentage of viable lymphomonocytes, as gated by forward‐ and side‐scatter. Negative control (background) values were not subtracted, as the median backgrounds for isotype‐matched mAbs was 0·0028% (range = 0·000–0·0063%). Samples were considered positive if the number of cells was equal to or greater than 0·01% and at least 10 clustered events were apparent. This empirical cut‐off value was predicted to be > 90% different from background at an α of 0·05.
Real‐time quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Total RNA was extracted with the ABI PRISM 6100 Nucleic Acid PrepStation (Applied Biosystems through Life Technologies Italia, Monza, Italy), according to the manufacturer's instructions. Random hexamers and a Moloney murine leukaemia virus (MMLV) reverse transcriptase kit (Stratagene, La Jolla, CA, USA) were used for cDNA synthesis. Transcripts were quantified by real‐time qRT–PCR on an ABI PRISM 7700 Sequence Detector (Applied Biosystems) with Applied Biosystems’ predesigned TaqMan gene expression assays and reagents according to the manufacturer's instructions. The following probes were used (identified by Applied Biosystems assay identification number): IL9R, HS_00602538_M1 and IL23R, Hs00332759_m1. For each sample, mRNA abundance was normalized to the amount of 18S rRNA.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, CA, USA). Multiple groups were analysed using one‐way analysis of variance (anova) and pairwise comparisons using the Mann–Whitney U‐test, with Bonferroni's correction to adjust significance levels for multiple comparisons where appropriate. Correlation analyses were performed using the Spearman's rank correlation coefficient. P‐values less than 0·05 were considered significant.
Results
Patients
Patients at baseline showed active disease defined as a DAPSA score > 14 [mean 26 (range = 18–32)]. All the patients were treated previously with non‐steroidal anti‐inflammatory drugs (NSAIDs) and a patient 14 with an anti‐TNF‐α with insufficient control of the disease. At the moment of the first bleeding (baseline) they were in drug washout by at least 4 weeks to avoid any action of other drugs on γδ T cells. Fourteen patients had taken methotrexate or salazopyrin after baseline in association with adalimumab or ustekinumab. After 12 weeks of therapy all the patients showed a significant improvement of signs and symptoms, achieving a significant reduction of DAPSA score [5 (range = 2–7)] and of BASDAI [mean 5·2 (range 4–7)] indicating a good clinical response to therapies.
Expansion of Vγ9Vδ2 T cells in peripheral blood and synovial fluid of PsA patients
In line with earlier reports, the mean frequency of peripheral blood Vγ9Vδ2 T cells in patients with active PsA was significantly higher than in HD (8 versus 5%) and decreased significantly to mean values 1·85% after therapy with either adalimumab (2%) or ustekinumab (1·7%). No difference was observed among patients treated or not with methotrexate (n = 7) or salazopiryn (n = 7) (data not shown), indicating that either adalimumab or ustekinumab reversed the expansion of Vγ9Vδ2 T cells consistently in the peripheral blood of PsA patients (Fig. 1a). According to data from the literature the percentage of Vγ9Vδ2 T cells from RA (2·1%) and Ps patients (11%) were lower and higher, respectively, than in HD and PsA (Fig. 1a). No differences were observed among peripheral blood Vγ9Vδ2 T cells of HD or OA subjects (data not shown).
Figure 1.
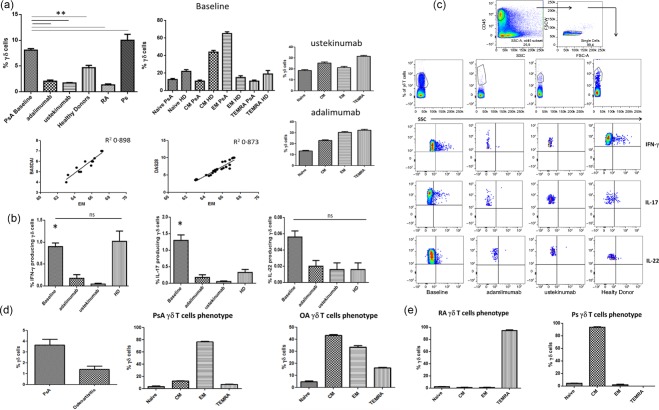
Phenotypical analysis and cytokine production of Vγ9Vδ2T cells from peripheral blood and synovial fluid of psoriatic arthritis (PsA) patients. (a) Percentages of total Vγ9Vδ2 T cells, their naive (Tnaive) (CD45RA+CD27+), T central memory (TCM) (CD45R−CD27+), T effector memory (TEM) (CD45RA−CD27−), effector memory terminally differentiated (TEMRA) (CD45RA+CD27−) subsets and correlation between percentage of Vγ9Vδ2 TEM subset and disease activity index (BASDAI and DAS28). (b) Mean percentage of interferon (IFN)‐γ‐ and interleukin (IL)‐17‐producing Vγ9Vδ2 T cells. (c) Dot‐plot analysis and gating strategy of one representative PsA patient and one control [healthy donor (HD)]. (d) Percentages of total synovial fluid (SF) Vγ9Vδ2 T cells and mean percentage of Vγ9Vδ2 T cells subsets from PsA patients and controls [osteoarthritis (OA)]. Data are expressed as mean [standard error of the mean (s.e.m.)]. *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
Vγ9Vδ2 T cell subsets are characterized by distinct migratory routes and effector functions, based on their expression of CD45RA and CD27. A remarkable change in the distribution of functional subsets in PsA was observed. T central memory (TCM) cells were the major Vγ9Vδ2 T cell subset in the peripheral blood of HD, and T effector memory (TEM) cells were the dominant population in untreated PsA patients with active disease, while the other Vγ9Vδ2 T cell subsets, naive (Tnaive) and effector memory terminally differentiated (TEMRA), were represented poorly in both controls and patients (Fig. 1a). Most notably, the number of Vγ9Vδ2 cells with a TEM phenotype was correlated directly with BASDAI and DAS28 activity scores and reduced significantly by anti‐cytokine therapy (Fig. 1a). Conversely, the proportion of Vγ9Vδ2 cells with Tnaive, TCM and TEMRA phenotypes were distributed equally in treated and untreated PsA patients (Fig. 1a). A completely different phenotype was found in RA and Ps patients, with a dominant population of TEMRA in RA and CM in Ps (Fig. 1e).
The phenotypical modifications of Vγ9Vδ2 T cells from the peripheral blood of PsA patients were accompanied by modifications in functional responses in vitro. As shown in Fig. 1b, the production of IL‐17 upon in‐vitro stimulation with IPP was found to be increased significantly in untreated patients compared to HD and decreased consistently after anti‐cytokine therapy with both mAb anti‐ TNF‐α and anti‐IL‐12/IL‐23. The production of IFN‐γ was comparable in HD and PsA patients after IPP and was reduced consistently in patients after therapy. No IL‐22 production was observed in patients and controls. Fig. 1c shows the FACS analysis of cytokine production by Vγ9Vδ2 T cells of one individual from any tested group.
We examined further the frequency and functional activity of Vγ9Vδ2 T cells in the synovial fluid (SF) of patients with active PsA. Due to inability to obtain SF from normal subjects, SFs from patients with OA were used as controls. The percentage of total Vγ9Vδ2 T cells and their TEM subset was increased significantly in SF of PsA patients compared to patients with OA (Fig. 1d). In addition to an increase in the proportions of Vγ9Vδ2 TEM cells, within the Vγ9Vδ2 T cell compartment we found a significantly increased frequency of IFN‐γ+ and IL‐17+ cells in the PsA SF compared to OA (Fig. 2a). Cumulative data from PsA and OA patients and FACS analysis of cytokine expression by Vγ9Vδ2 T cells of one individual from any tested group are shown in Fig. 2a,b. The frequency of IFN‐γ+‐ and IL‐17+‐producing Vγ9Vδ2 + T cells was observed to be higher in SF than in the peripheral blood compartment of patients (n = 12) when both samples were compared (Fig. 2c).
Figure 2.
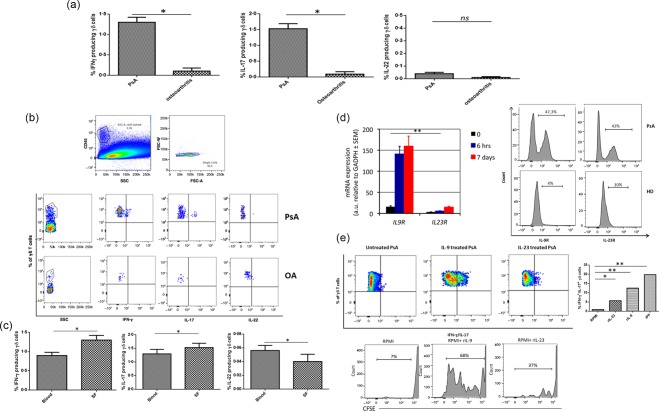
Cytokine production by Vγ9Vδ2 T cells from synovial fluid (SF) of psoriatic arthritis (PsA) patients and comparison of paired peripheral blood and synovial fluid (SF) samples from patients with PsA. Interleukin (IL)‐9R and IL‐23R expression and in‐vitro response to recombinant IL‐23 and IL‐9. (a) Mean percentages of interferon (IFN)‐γ, IL‐17‐producing Vγ9Vδ2 T cells in PsA and osteoarthritis (OA) patients. (b) Dot‐plot analysis of one representative PsA and OA patient. (c) Increased frequencies of IFN‐γ+ Vγ9Vδ2+ and IL‐17+ Vγ9Vδ2+ T in the PsA SF compared to peripheral blood. (d) Reverse transcription–polymerase chain reaction (RT–PCR) of IL‐9R and IL‐23R gene expression on Vγ9Vδ2 T cells, either unstimulated or stimulated with isopentenyl pyrophosphate (IPP) for 6 h or 7 days. (e) Fluorescence activated cell sorter (FACS) analysis of IL‐9R and IL‐23R expression by Vγ9Vδ2 T cells of PsA patients and healthy donors (HD). Dot‐plot analysis of IFN‐γ/IL‐17 producing Vγ9Vδ2 T cells and Vγ9Vδ2 T cell expansion from one representative PsA patient after in‐vitro stimulation with rIL‐9 or rIL‐23. Mean percentage of IFN‐γ/IL‐17‐producing Vγ9Vδ2 T cells after in‐vitro stimulation with recombinant IL (rIL)‐9 or rIL‐23. Data represent the mean values ± standard error of the mean (s.e.m.). *P < 0·05; **P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
Vγ9Vδ2 T cells of PsA patients express IL‐9R and IL‐23R and respond in vitro to IL‐23 and IL‐9
We therefore investigated whether IL‐23 and IL‐9 contributed to the expansion of IL‐17+ and IFN‐γ+ Vγ9Vδ2 T cells in PsA patients. Initially, we assessed the kinetics of expression of mRNA encoding for IL‐9 and IL‐23 receptors (R). In HD, resting unstimulated Vγ9Vδ2 T cells express low levels of IL9R mRNA and of IL23R mRNA (Fig. 2d). Vγ9Vδ2 TCR stimulation by antigen induced up‐regulation of IL9R mRNA and IL23R mRNA as early as 6 h, which was sustained up to 7 days after stimulation (Fig. 2d). Accordingly, FACS analysis of Vγ9Vδ2 T cells from the peripheral blood of HD show very low surface IL‐9R and IL‐23R expression but, interestingly, Vγ9Vδ2 T cells from the peripheral blood of PsA patients expressed both receptors at high levels (Fig. 2d).
To confirm that IL‐9 and/or IL‐23 signalling can drive a potentially pathogenic response in human Vγ9Vδ2 T cells, and to characterize the nature of this response, we stimulated purified Vγ9Vδ2 T cells from PsA patients with recombinant IL‐9 or IL‐23 and evaluated the IFN‐γ and IL‐17 production. Vγ9Vδ2 T cells from PsA patients exhibited a six‐ and 12‐fold increase in IL‐17 and IFN‐γ production in response to IL‐23 or IL‐9 exposure, respectively (Fig. 2e) that was comparable to in‐vitro stimulation with IPP (data not shown). In accordance with the reduced expression of IL‐23R and IL‐9R in HD, stimulation with recombinant IL‐9 or IL‐23 did not lead to a significant increase of IL‐17 and IFN‐γ production (data not shown).
Discussion
In this study, we confirm previous data 1, 4 regarding the role of Vγ9Vδ2 T cells in the pathogenesis of PsA and report for the first time, to our knowledge, that IL‐17‐ and IFN‐γ Vγ9Vδ2 T‐producing cells are expanded in the peripheral blood and accumulate in the synovial fluid of PsA patients with active disease, show a TEM phenotype and express both IL‐23R and IL‐9R. Stimulation with either the TCR agonist IPP or with recombinant IL‐9, rather than IL‐23, induces activation, expansion and cytokine production by Vγ9Vδ2 T cells, suggesting a major role of IL‐9 in PsA pathogenesis. Most notably, the proportion of Vγ9Vδ2 TEM cells producing IL‐17 and IFN‐γ correlates with disease activity and decreases upon treatment with either anti‐TNF‐α or anti IL‐12/IL‐23 mAbs. IL‐17 and IFN‐γ Vγ9Vδ2 T cells with a predominant TEM phenotype are also enriched in the SF of PsA patients, supporting further the hypothesis that they participate actively in the inflammatory response in PsA patients.
In‐vitro stimulation of Vγ9Vδ2 T cells induces the production of IL‐17 and IFN‐γ. This finding is consistent with the idea that Vγ9Vδ2 cells are polarized T cells, and that the cytokine milieu can further drive their differentiation.
A major role for IL‐23 has been demonstrated in psoriasis, and studies of human psoriatic lesions support a role for both IL‐12 and IL‐23 in the pathogenesis 16. The increased expression of the Th17 specifying transcription factor (RORC) and the Th17‐inducing cytokines (IL‐23, IL‐6, IL‐1β) in lesional psoriatic skin versus non‐lesional skin and skin of healthy volunteers suggests activation of the IL‐23/IL‐17 pathway in psoriasis 17.
Involvement of the IL‐23 pathway was also found in ankylosing spondylitis, and genetic association studies also indicate a key role for IL‐23R 18 in several chronic inflammatory conditions. However, such a unique role for the IL‐23 pathway cannot be translated to PsA, in which other cytokines appear to be involved. In this regard, we have demonstrated recently an over‐expression of IL‐9, produced mainly by Th9 cells in peripheral blood and synovial fluid of PsA patients 3.
IL‐9R levels have been found increased in lesional skin of psoriatic patients 19. IL‐9 also promotes skin inflammation in a mouse model of psoriasis 7. In this mouse model, treatment with anti‐IL‐17 mAb inhibited the psoriatic‐like skin phenotype and down‐regulated IL‐9 expression, indicating a role of IL‐9 and suggesting a link between IL‐9 and the Th17 pathway.
Previous studies have demonstrated that murine γδ T cells are an innate source of IL‐17 without the need for TCR engagement by antigen 20. A striking consequence of these findings is that the role of the TCR in IL‐17‐producing γδ T cells could be redundant, in line with their predetermined phenotype in the thymus without positive or negative selection.
Accordingly, murine γδ T cells acquire IL‐17‐producing potential in the neonatal thymus independently upon encountering the specific antigen 20. In contrast to mouse studies, TCR engagement is required for the differentiation of human IL‐17‐producing Vγ9Vδ2 T cells from naive precursors, which poses the question of how Vγ9Vδ2 T cells from PsA patients then respond to cytokines in an innate fashion. In peripheral blood and/or SF of PsA patients Vγ9Vδ2 T cells become activated by some as‐yet unknown antigens they recognize specifically and thus induce Vγ9Vδ2 T cells to express IL‐9R and IL‐23R. The studies reported here failed to show significant IL‐9R and IL‐23R expression by unstimulated Vγ9Vδ2 T cells from healthy individuals, which were up‐regulated following antigen stimulation in vitro. This implies that TCR activation is able to induce not only cytokine production but also to increase IL‐9R and IL‐23R expression levels.
In conclusion, our data provide for the first time evidence that IL‐17‐ and IFN‐γ Vγ9Vδ2‐producing T cells with a TEM phenotype are expanded in the peripheral blood and in the synovial fluid of PsA patients with active disease, express IL‐23R and IL‐9R and respond prevalently to IL‐9 in an innate fashion.
Disclosure
The authors declare no disclosures.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Prof. Triolo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. GG, FC, FD, GS, RG, GT.
Acquisition of data. GG, DDL, MLP, PR, PC, JJF.
Analysis and interpretation of data. GG, FC, DDL, MLP, PR, PC, GS, FD, JJF, RG, GT.
Acknowledgement
This study was supported in part by a grant of Ministero dell'Istruzione, dell'Università e della Ricerca Scientifica from Italy.
References
- 1. Frleta M, Siebert S, McInnes IB. The interleukin‐17 pathway in psoriasis and psoriatic arthritis: disease pathogenesis and possibilities of treatment. Curr Rheumatol Rep 2014; 16:414. [DOI] [PubMed] [Google Scholar]
- 2. Lories RJ, McInnes IB. Primed for inflammation: enthesis‐resident T cells. Nat Med 2012; 18:1018–9. [DOI] [PubMed] [Google Scholar]
- 3. Ciccia F, Guggino G, Ferrante A et al IL‐9 over‐expression and Th9 polarization characterize the inflamed gut, the synovial tissue and the peripheral blood of patients with psoriatic arthritis. Arthritis Rheum 2016; 68:1922–31. [DOI] [PubMed] [Google Scholar]
- 4. Kenna TJ, Davidson SI, Duan R et al Enrichment of circulating interleukin‐17‐secreting interleukin‐23 receptor‐positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis Rheum 2012; 64:1420–9. [DOI] [PubMed] [Google Scholar]
- 5. Burk MR, Mori L, De Libero G, Human V, gamma 9. V delta 2 cells are stimulated in a cross‐reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol 1995; 25:2052–8. [DOI] [PubMed] [Google Scholar]
- 6. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non‐peptide antigens recognized by human gamma delta T cells. Nature 1995; 375:155–8. [DOI] [PubMed] [Google Scholar]
- 7. Pantelyushin S, Haak S, Ingold B et al Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Investig 2012; 122:2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khmaladze I, Kelkka T, Guerard S et al Mannan induces ROS‐regulated, IL‐17A‐dependent psoriasis arthritis‐like disease in mice. Proc Natl Acad Sci USA 2014; 111:E3669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becher B, Pantelyushin S. Hiding under the skin: interleukin‐17‐producing gammadelta T cells go under the skin? Nat Med 2012; 18:1748–50. [DOI] [PubMed] [Google Scholar]
- 10. Reinhardt A, Yevsa T, Worbs T et al IL‐23‐dependent gammadelta T cells produce IL‐17 and accumulate in enthesis, aortic valve, and ciliary body. Arthritis Rheum 2016; doi: 10.1002/art.39732. [DOI] [PubMed]
- 11. Zlatkovic‐Svenda MI, Kerimovic‐Morina D, Stojanovic RM. Psoriatic arthritis criteria evaluation: CASPAR and modified CASPAR. Clin Exp Rheumatol 2011; 29:899–900. [PubMed] [Google Scholar]
- 12. Taylor W, Gladman D, Helliwell P et al Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54:2665–73. [DOI] [PubMed] [Google Scholar]
- 13. Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016; 75:811–8. [DOI] [PubMed] [Google Scholar]
- 14. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21:2286–91. [PubMed] [Google Scholar]
- 15. Todaro M, D'Asaro M, Caccamo N et al Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol 2009; 182:7287–96. [DOI] [PubMed] [Google Scholar]
- 16. Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL‐17A‐producing cells and are found in lesional skin. J Invest Dermatol 2011; 131:1853–60. [DOI] [PubMed] [Google Scholar]
- 17. Raychaudhuri SK, Saxena A, Raychaudhuri SP. Role of IL‐17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin Rheumatol 2015; 34:1019–23. [DOI] [PubMed] [Google Scholar]
- 18. Australo‐Anglo‐American Spondyloarthritis C, Reveille JD, Sims AM et al Genome‐wide association study of ankylosing spondylitis identifies non‐MHC susceptibility loci. Nat Genet 2010; 42:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh TP, Schon MP, Wallbrecht K, Gruber‐Wackernagel A, Wang XJ, Wolf P. Involvement of IL‐9 in Th17‐associated inflammation and angiogenesis of psoriasis. PLOS ONE 2013; 8:e51752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Brien RL, Roark CL, Born WK. IL‐17‐producing gammadelta T cells. Eur J Immunol 2009; 39:662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


