Summary
Neurotensin (NT) is a gut hormone functioning proinflammatory through nuclear factor kappa B (NF‐κB) and interleukin (IL)−8 secretion or anti‐inflammatory through epidermal growth factor receptors. NT mRNA is down‐regulated in duodenal biopsies of children with untreated coeliac disease. The aim of this study was to investigate if plasma pro‐NT levels correlated with the degree of intestinal mucosal damage and tissue transglutaminase autoantibody (tTGA) levels in children with coeliac disease. Fasting plasma samples from 96 children with coeliac disease and 89 non‐coeliac disease controls were analysed for NT precursor fragment pro‐NT 1–117 by a chemiluminometric immunoassay. Pro‐NT levels were compared with NT mRNA from duodenal biopsies, assessed previously with quantitative polymerase chain reaction (PCR). Illumina core exome arrays were used for human leucocyte antigen (HLA) typing and the Marsh criteria applied to score mucosal damage. Tissue TGA was measured by radio binding assay. A general linear model compared pro‐NT levels with diagnosis of coeliac disease, Marsh score and HLA DQ haplotype. Spearman's rank test was used to compare pro‐NT levels with tTGA, age and duodenal NT mRNA levels, respectively. Plasma pro‐NT levels were elevated in children with coeliac disease (median 23 pmol/l higher, P = 0·003) and in those with severe intestinal mucosal damage (median 24 pmol/l higher for ≥ Marsh 3b versus not, P = 0·0004). Pro‐NT levels correlated further with tTGA (r 2 = 0·22, P = 0·002), but not with duodenal NTS mRNA levels (r 2 = −0·12, P = 0·14). Pro‐NT was not associated with any of the HLA risk‐haplotypes. Elevated peripheral pro‐NT levels reflect more severe forms of active coeliac disease, indicating a potential role of NT in intestinal inflammation.
Keywords: coeliac disease, children, inflammation, neurotensin
Introduction
Neurotensin (NT) is a gut hormone secreted by specific endocrine cells scattered throughout the epithelial layer of the small intestine 1, 2, located predominantly in jejunum and ileum 3. NT is processed from a 170 amino acid‐long NT/neuromedin (NMN) precursor protein, which is tissue cleaved specifically to generate mature NT and NMN as well as specific N‐terminal extended forms of the two peptides in the gut 4, 5, 6. Secreted NT can be difficult to detect in blood plasma due to its rapid degradation 7, 8. However, an immunoassay was developed that detect the pro fragment of NT (NT/NMN 1–117), shown to be completely stable in human plasma 9. Fat is the most potent inducer of NT secretion, resulting in decreased gastrointestinal motility and gastric acid secretion 10, 11. NT is also suggested to play a role in intestinal inflammation as measured in colonic intestinal cell lines, either as a proinflammatory peptide through nuclear factor kappa B (NF‐κB) activation and increased interleukin (IL)−8 secretion or as an anti‐inflammatory peptide through epidermal growth factor receptor activation 12.
Coeliac disease is a gluten‐dependent intestinal small bowel disorder characterized by intestinal epithelial inflammation and villous atrophy 13. Autoantibodies against the endogenous protein tissue transglutaminase (tTG) is a highly specific and sensitive marker of coeliac disease 14, although in most cases a histological assessment is necessary to confirm the diagnosis. The severity of the histological lesion is graded with a six‐category Marsh score 15 and the degree of intestinal damage is reflected moreover by the plasma levels of tTG autoantibodies (tTGA) 16. Certain human leucocyte antigen (HLA) DQ alleles are necessary for coeliac disease and more than 90% of all affected patients carry DQ2.5 (A1*05‐B1*02) and the remainder mainly DQ8 (A1*0301‐B1*0302) 17. Children homozygous for the DQ2.5 haplotype have been shown to be at higher risk for coeliac disease 18. Even so, some individuals can be tTGA‐positive or carry HLA risk alleles without developing coeliac disease.
Studies on NT in coeliac disease pathogenesis are scarce, and existing results are diverse. An early study showed that untreated coeliac disease patients displayed increased postprandial plasma NT release compared to healthy controls and patients on a gluten‐free diet 19. Another conflicting study observed impaired postprandial plasma NT release in untreated coeliac disease patients, despite increased preprandial plasma NT levels 20. In the search for candidate genes in coeliac disease, we recently observed markedly decreased fasting NT mRNA levels in distal duodenum of children with untreated coeliac disease compared to children without signs of intestinal injury 21. The aim of the present study was to investigate if proneurotensin (pro‐NT) plasma levels reflect the level of inflammation in paediatric coeliac disease and also to reveal if the change of NT at transcriptional level in duodenum is reflected by change in blood plasma levels in a paediatric coeliac disease cohort.
Materials and methods
Subjects and diagnostic classification
Small intestinal biopsies and peripheral blood samples were collected consecutively from children who underwent upper endoscopy at the paediatric gastroenterology unit in Malmö, Sweden between 2010 and 2012 21, 22. All children were fasting prior to endoscopy procedure performed in general sedation with propofol. Children positive for tTGA and showing characteristic villous atrophy of the distal part of duodenum (Marsh ≥ 2) were defined as having coeliac disease, whereas tTGA‐positive children having a Marsh < 2 were defined as having potential coeliac disease. Children tTGA‐negative with a normal biopsy were included as disease controls. Included in this study were 215 children: 96 with untreated coeliac disease, 30 with potential coeliac disease and 89 disease controls (Table 1). All participants were informed about the aim of the study and a parental written consent was obtained for each child. The local ethical committee approved the study.
Table 1.
Clinical characteristics according to diagnostic status: coeliac disease, potential coeliac disease and disease controls
|
Coeliac disease n = 96 |
Potential coeliac disease n = 30 |
Disease control n = 89 |
P‐value | |
|---|---|---|---|---|
| Age, years | 6·9 (3·9) | 8·7 (5·0) | 12·2 (4·3) | < 0·001 |
| Sex, n (%) | 0·785 | |||
| Female | 57 (64) | 20 (67) | 58 (60) | |
| Male | 32 (36) | 10 (33) | 38 (40) | |
| HLA* genotype, n (%) | < 0·001 | |||
| DQ2.5/2.5 | 26 (87) | 3 (10) | 1 (3) | |
| DQ2.5/X | 20 (44) | 13 (28) | 13 (28) | |
| DQ2.5/8 | 19 (63) | 5 (19) | 3 (11) | |
| DQ8/X | 17 (42) | 3 (7) | 21 (51) | |
| OTHER | 7 (10) | 6 (9) | 57 (81) | |
| tTGA* (U/ml) | 138·4 (209·7) | 14·7 (35·7) | 1·1 (1·4) | < 0·001 |
Continuous variables are presented as mean (standard deviation). The P‐values were calculated using analysis of variance and χ2 test. *HLA = human leucocyte antigen; tTGA = tissue transglutaminase autoantibodies.
Proneurotensin analysis
NT mRNA levels in duodenal biopsies from study participants was investigated previously by quantitative polymerase chain reaction (PCR), as described previously 21. Plasma was isolated after 10 min centrifugation at 1000 g and then frozen until pro‐NT analysis was conducted. Pro‐NT levels were measured in plasma by a chemiluminometric sandwich immunoassay to detect a pro‐NT precursor fragment (pro‐NT 1–117) based on an assay described elsewhere 9.
Tissue transglutaminase analysis
The radio ligand binding assay was used to determine plasma levels of immunoglobulin (Ig)A against tTGA according to previously described methodology 23. The cut‐off level of normal tTGA was set to 4 U/ml, as estimated from receiver operating characteristic (ROC) curves of healthy blood donors. Samples were analysed in duplicate and antibody levels determined from a standard curve of pooled positive tTGA samples using the GraphPad Prism version 6.0 software. Mean tTGA levels in coeliac disease patients and controls are expressed in Table 1.
Classification of intestinal biopsies according to Marsh criteria
The same pathologist scored all biopsies, blinded to the results of tTGA levels and clinical data, according to Marsh criteria with some slight modifications 15, 24. A Marsh score 0 (M0) was defined as a mucosa with normal villous architecture and normal crypt height; M1 as M0 but the epithelium showing an intraepithelial lymphocyte (IEL) count of ≥ 25 IEL per 100 enterocytes; M2 as M1 but with crypt hyperplasia; M3a as M2 but with partial villous atrophy; M3b as M3a but with subtotal villous atrophy, and finally M3c as M3b but with total villous atrophy. Due to only three biopsies being determined as M2, this category was merged with category M3a. The Marsh distribution among the investigated children was 86 (40%) as M0, 40 (19%) as M1, 20 (9%) as M2/M3a, 27 (13%) as M3b and 42 (20%) as M3c.
HLA genetic markers
HLA was typed with Illumina Core Exome single nucleotide polymorphism (SNP) arrays using tagged SNPs that predict the HLA‐DQ alleles with high accuracy, SNP rs2187668 for DQ2.5 (A1*0501‐B1*0201) and rs7454108 for DQ8 (A1*0301‐B1*0302) 25. HLA‐DQ haplotypes were divided into five genotype groups: (1) DQ2.5/2.5 (homozygous DQ2.5), (2) DQ2.5/x (heterozygous DQ2.5/x, where x is any other haplotype than DQ8 or DQ2.5), (3) DQ2/8 (heterozygous patients carrying DQ2.5/DQ8), (4) DQ8/x (homozygous DQ8/8 or heterozygous DQ8/x, where x is any other haplotype than DQ8 or DQ2.5) and (5) other (patients carrying neither DQ2.5 nor DQ8). The distribution of HLA genotypes between coeliac disease patients and controls is presented in Table 1.
Statistical analyses
The comparison of age, sex and tTGA levels between the diagnostic groups was assessed using analysis of variance (anova) or χ2 test. Spearman's rank test was used to correlate pro‐NT with tTGA, duodenal NT mRNA levels and age. The general linear model was used to compare pro‐NT levels between diagnosis, Marsh score and HLA genotype. Disease controls, M0 and non‐HLA DQ2 or DQ8 children constituted as reference in each general linear model analysis. Pro‐NT and tTGA variables were log‐transformed due to their skewed distribution. For a clearer visualization of the result, children were divided into three equally sized groups (tertiles) based on their plasma pro‐NT levels: low, medium and high levels. A P‐value of < 0·05 was considered significant for all the tests performed.
Results
Pro‐NT levels were higher in children with coeliac disease (median 93·7 pmol/l; interquartile range (IR) = 83·1) compared to the disease controls (median = 70·5 pmol/l; IR = 75·6) (P = 0·003), but not when children with potential coeliac disease (median = 83·7 pmol/l; IR = 82·8) were compared to disease controls (P = 0·077). A positive correlation was observed between tTGA and pro‐NT levels in plasma (r 2 = 0·22, P = 0·002) (Fig· 1) and, as expected, tTGA levels and Marsh score correlated strongly with one another (r 2 = 0·74, P < 0·001).
Figure 1.
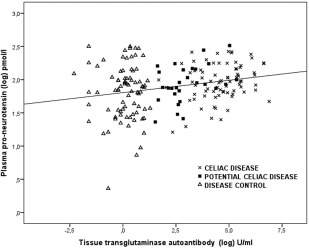
Correlation between proneurotensin (log) and tissue transglutaminase autoantibody (log) levels in plasma, r 2 = 0·22, P = 0·002 (Spearman's rank test). Different symbols define disease diagnosis; crosses for coeliac disease patients, filled squares for potential coeliac disease patients and triangles for disease controls.
Pro‐NT levels in plasma did not correlate with NT mRNA levels in the distal duodenal mucosa (r 2= −0·12, P = 0·14), although plasma pro‐NT, correlated positively with biopsy Marsh score (r 2= 0·14, P = 0·037). Plasma pro‐NT levels in children with M3b (median = 119 pmol/l) and M3c (median = 95 pmol/l) categorized biopsies were higher than in children with M0 biopsies (median = 75 pmol/l) (P = 0·008 and P = 0·038, respectively) (Table 2a). Figure 2 illustrates the increased proportion of patients with high levels of pro‐NT in plasma (tertile 3) combined with a high duodenal Marsh score (M3b–M3c). The pro‐NT ranges for the tertiles were as follows; 2–64 pmol/l for tertile 1, 64–108 pmol/l for tertile 2 and 108–326 pmol/l for tertile 3.
Table 2 (a).
Median (interquartile range) proneurotensin (pro‐NT) plasma levels (pmol/l) according to Marsh score
| Marsh score | pro‐NT | P‐value |
|---|---|---|
| Marsh 3c | 95·0 (88·5) | 0·038 |
| Marsh 3b | 118·8 (99·7) | 0·008 |
| Marsh 2/3a | 64·3 (67·7) | 0·48 |
| Marsh 1 | 82·9 (65·5) | 0·63 |
| Marsh 0 | 75·2 (100·8) | Ref. |
Each Marsh category was compared to the M0 category using a general linear model.
Figure 2.
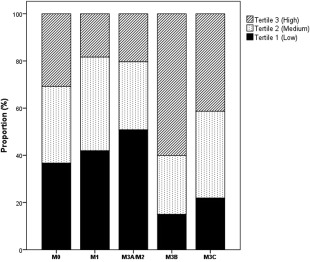
Proportion of study participants with low (tertile 1), medium (tertile 2) or high (Tertile 3) levels of plasma proneurotensin according to Marsh category. The tertiles were created by dividing proneurotensin levels from all study participants into three equally sized groups. M0 = normal mucosa (n = 74); M1 = lymphocyte infiltration (n = 36); M2/M3a = lymphocyte infiltration, crypt hyperplasia and partial villous atrophy (n = 20); M3b = lymphocyte infiltration, crypt hyperplasia and subtotal villous atrophy (n = 21); M3c = lymphocyte infiltration, crypt hyperplasia and total villous atrophy (n = 35).
All the children homozygous for HLA DQ2.5 presented slightly elevated plasma levels of pro‐NT (96 pmol/l) compared to children not carrying the DQ2.5 or DQ8 haplotypes (81 pmol/l), and this difference was borderline significant (P = 0·055) (Table 2b, Fig. 3a). Carriers heterozygous for DQ2.5 and DQ8 did not differ in pro‐NT levels compared to the DQ2.5 and DQ8‐negative individuals. Children with M0 and M1 biopsies and carrying the DQ8 haplotype presented low plasma pro‐NT levels (Fig. 3b), which was not observed when all Marsh categories were compared (Fig. 3a).
Table 2 (b).
Median (interquartile range) proneurotensin (pro‐NT) plasma levels (pmol/l) according to human leucocyte antigen (HLA) DQ genotype
| HLA group | pro‐NT | P‐value |
|---|---|---|
| DQ2.5/2.5 | 95·9 (74·8) | 0·055 |
| DQ2.5/8 | 76·4 (102·0) | 0·59 |
| DQ2.5/X | 82·9 (83·7) | 0·32 |
| DQ8/X | 77·9 (91·0) | 0·70 |
| Negative DQ2.5 and DQ8 | 81·2 (100·1) | Ref. |
Each HLA group was compared to the negative DQ2.5 and DQ8 carriers using a general linear model.
Figure 3.
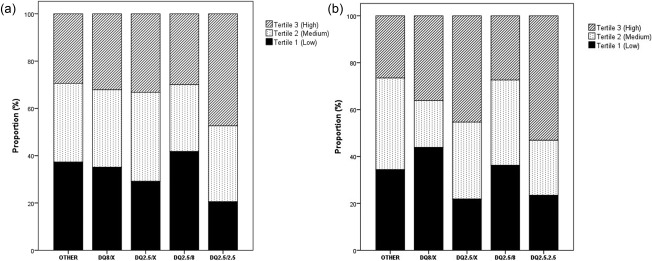
(a) Proportion of all study participants with low (tertile 1), medium (tertile 2) or high (tertile 3) levels of plasma proneurotensin according to human leucocyte antigen (HLA) DQ genotype. (b) Proportion of study participants (disease controls and potential coeliac disease patients) without crypt hyperplasia or villous atrophy (having M0 and M1 biopsies) with low (tertile 1), medium (tertile 2) or high (tertile 3) levels of plasma proneurotensin according to HLA DQ genotype.
Age had an impact on pro‐NT levels among the disease controls with a tendency to lower pro‐NT levels among older children (r 2= −0.17, P = 0·096) (Fig. 4). No correlation was observed between age and pro‐NT among children with coeliac disease or potential coeliac disease. The pro‐NT distribution was larger for the disease controls and also increased with age.
Figure 4.
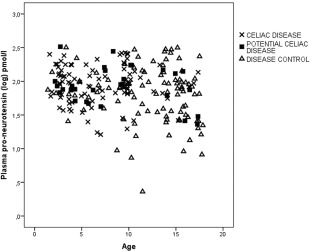
Correlation between proneurotensin (log) and age (years) for coeliac disease patients r 2 = −0·054; P = 0·61 (cross), potential coeliac disease patients r 2 = −0·069; P = 0·72 (filled square) and disease controls r 2 = −0·17; P = 0·096 (triangle), calculated using Spearman's rank test.
Discussion
In the present study, we found that untreated coeliac disease children had increased fasting plasma pro‐NT levels compared to disease controls. Interestingly, children with more severe intestinal mucosal changes also presented the highest pro‐NT levels. In agreement with our results, increased fasting NT levels were detected in two previous studies in adult coeliac disease patients 20, 26. In contrast to these former studies, others have shown that fasting NT levels are reduced significantly in children with untreated coeliac disease 27. The conflicting results between these studies may have several explanations.
First, pattern of hormone release could reflect the location of the mucosal damage. Ileal and jejunal perfusion studies showed that nutrient absorption was impaired in jejunum but enhanced in ileum from active coeliac disease patients 28. Furthermore, a postprandial rise in NT plasma levels has been observed previously in active coeliac disease patients compared to controls 26, 27. It is plausible that endocrine cells in the unaffected ileal mucosa of coeliac disease patients increase NT secretion to compensate for the deleterious mucosal degradation and loss of absorptive capacity in the proximal small intestine. This may explain the insignificant correlation we observed between plasma pro‐NT and NT mRNA levels in distal duodenum from children in our cohort.
Secondly, NT interaction with lymphocytes and expression of NT receptors on various immune cells is well known 12 but has, to our knowledge, not been investigated in coeliac disease. The increased NT plasma levels observed in children with Marsh 3b could be caused by the increased number of IELs in the mucosal epithelium. Marsh 3c patients have more severe intestinal atrophy, but the IEL level is often decreased among these patients; hence, this group presented lower median pro‐NT levels than M3b patients. Another finding that strengthens the interaction of NT with lymphocytes in this study is the observed correlation between plasma pro‐NT and tTGA levels. However, this correlation was weak and corresponds to the finding that potential coeliac disease patients have just slightly increased pro‐NT levels compared to disease controls.
Thirdly, differences between previous studies and our study could be the distribution of HLA‐risk haplotypes among the study participants. The HLA DQ2 but also DQ8 haplotypes are necessary for coeliac disease to develop, and there is a dose effect, with HLA DQ2.5 homozygotes being at highest risk for coeliac disease 17, 18. This study could not detect any large differences in pro‐NT levels between children with DQ2.5 or DQ8 and children negative for these haplotypes. Nevertheless, DQ2.5 homozygotes presented slightly elevated levels of pro‐NT compared to negative carriers. However, this increase could be a cause of the previously known association of DQ2.5 homozygosity with coeliac disease, although a higher proportion of DQ2.5 carriers without villous atrophy still presented high pro‐NT levels (Fig. 3b).
Some limitations with this study should be mentioned. Age was a strong confounder for coeliac disease with increased frequency of coeliac disease among young children (Table 1). However, the impact of age on pro‐NT levels seemed somewhat less pronounced compared with the impact of age on coeliac disease diagnosis. Furthermore, the increased pro‐NT distribution among the disease controls compared to the other diagnostic groups may reflect the heterogeneity of the control group. Hence, the disease controls were not healthy children, but constituted children investigated for various reasons of which some had inflammatory conditions in the gut. Access to reference levels for fasting pro‐NT in a healthy paediatric cohort comprised of both young and older children is warranted, but challenging from an ethical aspect. Furthermore, recent findings revealed that NT can pass the blood–brain barrier in both directions 29, which means that plasma levels of the protein are no longer represented only by the levels secreted from the small intestine. We had no access to peptide data for NT in duodenum, although we are in the process of trying to detect NT in duodenum by immunofluorescence.
In conclusion, this study found that children with untreated coeliac disease have elevated peripheral pro‐NT levels compared with disease controls. Pro‐NT levels also seem to reflect more severe forms of active coeliac disease, indicating a potential role of NT in small intestinal inflammation. Plasma pro‐NT may have a potential application as diagnostic marker in coeliac disease, and could complement present markers in the diagnosis of dubious cases or even replace the invasive endoscopic procedure.
Disclosure
The authors declare no disclosures.
Acknowledgements
C. M. planned the study, performed statistical analysis and wrote the manuscript. D. A. collected samples from study participants. A. T. N. and D. A. contributed to data interpretation and to critical revision of the manuscript. We would like to acknowledge Sphingotec GmbH in Germany and Olle Melander for their generosity in running the pro‐NT analysis without claims of reimbursement. Thanks also to all children and their families for contributing to this study.
References
- 1. Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 1973; 248:6854–61. [PubMed] [Google Scholar]
- 2. Hammer RA, Leeman SE, Carraway R, Williams RH. Isolation of human intestinal neurotensin. J Biol Chem 1980; 255:2476–80. [PubMed] [Google Scholar]
- 3. Egerod KL, Engelstoft MS, Grunddal KV et al A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP‐1, PYY, and neurotensin but not somatostatin. Endocrinology 2012; 153:5782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dobner PR, Barber DL, Villa‐Komaroff L, McKiernan C. Cloning and sequence analysis of cDNA for the canine neurotensin/neuromedin N precursor. Proc Natl Acad Sci USA 1987; 84:3516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carraway RE, Mitra SP. Differential processing of neurotensin/neuromedin N precursor(s) in canine brain and intestine. J Biol Chem 1990; 265:8627–31. [PubMed] [Google Scholar]
- 6. Carraway RE, Mitra SP, Paradise C. Characterization of large neuromedin‐N using antisera towards regions of the neurotensin neuromedin‐N precursor. Peptides 1991; 12:601–7. [DOI] [PubMed] [Google Scholar]
- 7. Barelli H, Foxthrelkeld JET, Dive V, Daniel EE, Vincent JP, Checler F. Role of endopeptidase‐3.4.24.16 in the catabolism of neurotensin, in‐vivo, in the vascularly perfused dog ileum. Br J Pharmacol 1994; 112:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friry C, Feliciangeli S, Richard F, Kitabgi P, Rovere C. Production of recombinant large proneurotensin neuromedin N‐derived peptides and characterization of their binding and biological activity. Biochem Biophys Res Commun 2002; 290:1161–8. [DOI] [PubMed] [Google Scholar]
- 9. Ernst A, Hellmich S, Bergmann A. Proneurotensin 1‐117, a stable neurotensin precursor fragment identified in human circulation. Peptides 2006; 27:1787–93. [DOI] [PubMed] [Google Scholar]
- 10. Spiller RC, Trotman IF, Higgins BE et al The ileal brake – inhibition of jejunal motility after ileal fat perfusion in man. Gut 1984; 25:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olsen PS, Pedersen JH, Kirkegaard P, Stadil F, Fahrenkrug J, Christiansen J. Neurotensin inhibits meal‐stimulated gastric‐acid secretion in man. Scand J Gastroenterol 1983; 18:1073–6. [DOI] [PubMed] [Google Scholar]
- 12. Zhao DZ, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides 2006; 27:2434–44. [DOI] [PubMed] [Google Scholar]
- 13. Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357:1731–43. [DOI] [PubMed] [Google Scholar]
- 14. Rostom A, Dube C, Cranney A et al The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology 2005; 128(4 Suppl.1):S38–46. [DOI] [PubMed] [Google Scholar]
- 15. Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother 2000; 54:368–72. [DOI] [PubMed] [Google Scholar]
- 16. Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol 2003; 36:219–21. [DOI] [PubMed] [Google Scholar]
- 17. Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2:647–55. [DOI] [PubMed] [Google Scholar]
- 18. Liu E, Lee HS, Agardh D. Risk of celiac disease according to HLA haplotype and country. N Engl J Med 2014; 371:1074. [DOI] [PubMed] [Google Scholar]
- 19. Bloom SR, Besterman HS, Sarson DI et al Hormone profile of celiac‐disease. Gastroenterology 1978; 74:1011. [Google Scholar]
- 20. Bardella MT, Fraquelli M, Peracchi M, Cesana BM, Bianchi PA, Conte D. Gastric emptying and plasma neurotensin levels in untreated celiac patients. Scand J Gastroenterol 2000; 35:269–73. [DOI] [PubMed] [Google Scholar]
- 21. Monten C, Gudjonsdottir AH, Browaldh L, Arnell H, Nilsson S, Agardh D et al Genes involved in muscle contractility and nutrient signaling pathways within celiac disease risk loci show differential mRNA expression. BMC Med Genet 2015; 16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monten C, Bjelkenkrantz K, Gudjonsdottir AH et al Validity of histology for the diagnosis of paediatric coeliac disease: a Swedish multicentre study. Scand J Gastroenterol 2016; 51:427–33. [DOI] [PubMed] [Google Scholar]
- 23. Kjelleras J, Vaziri‐Sani F, Agardh D. Improved efficacy by using the pTnT‐rhtTG plasmid for the detection of celiac disease specific tissue transglutaminase autoantibodies in radioligand binding assays. Scand J Clin Lab Invest 2011; 71:701–4. [DOI] [PubMed] [Google Scholar]
- 24. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992; 102:330–54. [PubMed] [Google Scholar]
- 25. Monsuur AJ, de Bakker PIW, Zhernakova A et al Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLOS ONE 2009; 4 (5): 10.1371/annotation/53480f56-4ef7-4877-ace7-e5892d392cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besterman HS, Bloom SR, Sarson DL et al Gut‐hormone profile in coeliac disease. Lancet 1978; 1:785–8. [DOI] [PubMed] [Google Scholar]
- 27. Hernanz A, Polanco I, Codoceo R, Lama R, Vazquez C. Gastrointestinal peptide profile in children with celiac disease. J Pediatr Gastroenterol Nutr 1987; 6:341–5. [DOI] [PubMed] [Google Scholar]
- 28. Silk DB, Kumar PJ, Webb JP, Lane AE, Clark ML, Dawson AM. Ileal function in patients with untreated adult coeliac disease. Gut 1975; 16:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gevaert B, Wynendaele E, Stalmans S et al Blood–brain barrier transport kinetics of the neuromedin peptides NMU, NMN, NMB and NT. Neuropharmacology 2016; 107:460–70. [DOI] [PubMed] [Google Scholar]


