Summary
Type III interferons (IFNs) or IFN‐λs (IFN‐λ1/IL29, IFN‐λ2/interleukin (IL)−28A and IFN‐λ3/IL‐28B) consist of a recently identified group of IFNs, implicated initially in several human diseases, including cancer and autoimmunity. In this study, we sought to investigate the expression of type III IFNs and their common receptor IFN‐λR1/IL‐28Ra in Sjögren's syndrome (SS). Type III IFN expression was examined in minor salivary gland tissues (MSG), peripheral blood mononuclear cells (PBMCs), sera and resting or Toll‐like receptor (TLR)‐stimulated salivary gland epithelial cells (SGEC) from SS patients and sicca‐complaining controls. All type III IFN family members were detected in ductal and acinar epithelia of MSGs from both SS patients and sicca controls. IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B were also expressed in infiltrating mononuclear cells. In SS patients with intermediate MSG lesions, the epithelial expression of IFN‐λ2/IL‐28A was more intense compared to sicca controls (P < 0·05). The receptor IFN‐λR1/IL‐28Ra was detected in all types of cells except fibroblasts, and was exceptionally strong in plasmatocytoid dendritic cells, indicating that they are susceptible to type III IFN‐mediated regulation. In the periphery, only IFN‐λ1/IL‐29 was detected in the sera and was elevated significantly in SS patients with intermediate MSG inflammatory lesions compared to sicca controls (P = 0·0053). None of the type III IFNs was expressed constitutively in resting SGECs; they were all induced readily by TLR‐3 stimulation, suggesting that the in‐situ epithelial expression can be attributed to local microenvironment. Type III IFNs are expressed in MSGs in a similar pattern to type I IFNs and their expression is probably subjected to micro‐environmental regulation, suggesting that they are implicated in the inflammatory processes occurring in the affected exocrine glands.
Keywords: autoimmunity, cytokines, inflammation, TLRs
Introduction
Sjögren's syndrome (SS) is a chronic autoimmune disease with both organ‐specific and systemic features, characterized by dysfunction of the exocrine glands. Clinical symptoms extend from dry eyes and dry mouth to various extraglandular manifestations including arthritis, Raynaud's phenomenon, liver, lung and renal involvement, as well as vasculitis. Approximately 5% of patients may develop B cell lymphoma. Despite extensive studies, the aetiopathology of SS remains largely unknown. However, it is well established that epithelial cells, which are the targets of SS‐related inflammation, are also the key regulators of autoimmune responses by mediating the recruitment, activation and differentiation of inflammatory cells 1. Furthermore, epithelia of minor salivary glands (MSG) of SS patients display an activated phenotype, evident both in‐situ and in long‐term cultures, consisting of elevated expression of various immunomodulatory molecules, compared to control individuals 1. Although the offending factor(s) for epithelial cell activation in SS are not known, viral infection has long been suspected. Indeed, several types of viruses have been reported to harbour SS patients, whereas Toll‐like receptor (TLR)−3 signalling that participates in innate viral responses has been implicated in SS pathogenesis 1, 2, 3. In this context, types I and II interferons (IFNs), that are cytokines implicated in the regulation of innate and anti‐viral responses, appear to have a primary role in SS 2, 3, 4, 5.
Recently, a novel family of IFNs, namely the type III IFN family, has been described. In humans, it consists of three distinct molecules, IFN‐λ1/interleukin (IL)−29, IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B, which all bind to a common receptor, IFN‐λR1/IL‐10R (IFN‐λR1/IL‐28Ra). They are expressed mainly by mucosal epithelia at barrier tissues and by several types of immune cells. Anti‐viral activity protecting epithelial surfaces of the gut, lung, urogenital, gastrointestinal tract and liver has been attributed initially to type III IFNs 6, whereas recent data implicate them in the regulation of both innate and adaptive immune responses in a manner comparable to type I IFNs. Following the first observations, the interest has been focused on their role in various inflammatory disorders and recently in autoimmune diseases. Indeed, increased expression of type III IFNs has been described in the periphery and affected tissues of patients with cutaneous lupus erythematosus 7, systemic lupus erythematosus 8, psoriasis 9, rheumatoid arthritis 10 and systemic sclerosis 11.
The aim of the present study was to investigate the presence of type III IFNs in SS. The expression of the three family members and their common receptor are examined in the MSG tissues and the periphery, including both peripheral blood mononuclear cells (PBMCs) and serum, as well as long‐term cultured salivary gland epithelial cells (SGEC).
Materials and methods
Reagents
Rabbit polyclonal antibodies to human IFN‐λ1/IL‐29 (Santa Cruz, Dallas, Texas, USA), IFN‐λ2/IL‐28A (Antibodies‐online, Atlanta, GA, USA), IFN‐λ/IL‐28B (Bioss, Woburn, MA, USA), IFN‐λR1/IL‐28Ra (Novus Biologicals, Littleton, CO, USA) and CD3 (Dako, Glostrup, Denmark) were used. Mouse‐monoclonal antibodies to human CD20 (clone L26) and CD68 (clone PG‐M1) were from Dako and rat monoclonal to CD123 (clone 6H6) and CD303 (clone 201A) from Biolegend (San Diego, CA, USA). F(ab’)2‐goat anti‐rabbit immunoglobulin (Ig)G antibody with Alexa Fluor®488 and F(ab’)2‐goat anti‐mouse IgG with Alexa Fluor®555 were from Life Technologies (Carlsbad, CA, USA). Enzyme‐linked immunosorbent assays (ELISAs) for human IFN‐λ2/IL‐28A, IFN‐λ/IL‐28B and IFN‐λ1/IL‐29 were from Biolegend (Assay Biotech, Sunnyvale, CA, USA) and Affymetrix eBioscience (San Diego, CA, USA), respectively.
Patients
Forty‐six consecutive patients with primary SS, as diagnosed by the American–European classification criteria 12, and 17 non‐SS sicca‐complaining individuals were included into the study. SS patients were categorized according to the grade of MSG infiltration in those with mild (SS‐I, n = 16; Tarpley score: 1+, one to two focal infiltrates per lobule), intermediate (SS‐II, n = 14; Tarpley score: 2+, ≥ 3 focal infiltrates per lobule) and severe inflammatory lesions (SS‐III, n = 16; Tarpley score: ≥ 3+, diffuse infiltrates associated with severe destruction of acinar glandular structures and loss of tissue architecture), as described previously 13. In all SS patients, the biopsy focus score (lymphocytic foci/4 mm2 of tissue) was ≥ 1. None of the SS patients had evidence of lymphoma, sarcoidosis or infection by hepatitis B, hepatitis C or human immunodeficiency virus. Furthermore, none of the patients received biological agents before biopsy. The characteristics of the SS patients studied are summarized in Table 1.
Table 1.
Characteristics of the individuals included in the study.
| Features | Non‐SS sicca controls (n=17) | Total SS (n = 46) |
SS‐I (n = 16) |
SS‐II (n = 14) |
SS‐III (n = 16) |
|
|---|---|---|---|---|---|---|
| General | Age (years), median (range) | 52 | 54 | 55 | 55.5 | 48 |
| (33–74) | (18–74) | (18–74) | (36–70) | (24–72) | ||
| No. of SS criteria fulfilled, median (range) | 2 | 4 | 4 | 4 | 4.5 | |
| (1–2) | (2–6) | (3–5) | (2–5) | (3–6) | ||
| Mean duration (years) of sicca symptoms, median (range) | 3 | 4 | 3 | 2·5 | 5 | |
| (0·33–5) | (0·33–17) | (1–5) | (1–10) | (0.33–17) | ||
| Histological (MSG biopsy) | Biopsy focus score (number of lymphocytic foci/4 mm2), median (range) | 0·00 | 2·75 | 1·23 | 2·99 | 5.56 |
| (0·00–0·35) | (1–12) | (1–1·44) | (1·6–3·64) | (2.75–12) | ||
| Tarpley biopsy score, median (range) | 0 | 2 | 1 | 2 | 3 | |
| (0–0) | (1–4) | (1–1) | (2–2) | (3–4) | ||
| Clinical | Arthralgias/arthritis (%) | 58·8 | 36·96 | 43·75 | 7·14 | 31.25 |
| SG enlargement (SGE) (%) | 0 | 28·26 | 6·25 | 21·42 | 56.25 | |
| Raynaud's phenomenon (%) | 11·76 | 32·61 | 31·25 | 28·57 | 37.5 | |
| Parenchymal organ involvement (%) | 0 | 8·7 | 6·25 | 7·14 | 12.5 | |
| Lung involvement (%) | 0 | 4·35 | 6·25 | 0 | 6.25 | |
| Renal involvement (%) | 0 | 4·35 | 0 | 7·14 | 6.25 | |
| Liver involvement (%) | 0 | 0 | 0 | 0 | 0 | |
| Indicative of vascular involvement (%) | 5·88 | 21·74 | 6·25 | 0 | 56.25 | |
| Palpable purpura (%) | 0 | 13·04 | 0 | 0 | 37.5 | |
| Peripheral neuropathy (%) | 5·88 | 2·17 | 0 | 0 | 6.25 | |
| Lymphoma (%) | 0 | 4·35 | 0 | 0 | 12.5 | |
| Laboratory | Anti‐Ro/SSA and/or La/SSB‐positive (%) | 5·8 | 58·7 | 25 | 71·42 | 75 |
| Anti‐Ro/SSA‐positive (%) | 5·8 | 54·35 | 25 | 64·28 | 75 | |
| Anti‐La/SSB‐positive (%) | 0 | 36·96 | 6 | 50 | 56.25 | |
| Rheumatoid factor positive (%) | 11·76 | 34·78 | 13 | 35·71 | 56.25 | |
| C3‐levels, median (range) | 87 | 98 | 90 | 108·5 | 94.5 | |
| (63–171) | (63–181) | (66·8–181) | (79–144) | (63–156) | ||
| C4‐levels, median (range) | 28 | 22 | 36·2 | 22 | 17 | |
| (12–52) | (3·9–47) | (12–45·6) | (10–47) | (3.9–41) | ||
| Cryoglobulinaemia (%) | 0 | 6·52 | 0 | 0 | 18.75 | |
| Hypergammaglobulinaemia (%) | 0 | 28·26 | 0 | 28·57 | 56.25 | |
| Leucopenia (%) | 0 | 8·7 | 6·25 | 7·14 | 12.5 | |
| ESSDAI score, median (range) | NA | 2 | 2 | 2 | 5 | |
| (0–9) | (0–5) | (0–6) | (0–9) | |||
ESSDAI = European League Against Rheumatism Sjögren's Syndrome Disease Activity Index; SS = Sjögren's syndrome; MSG = minor salivary gland.
Paraffin‐embedded MSG tissues, serum, PBMCs and SGECs are kept routinely and used after informed consent from each patient undergoing a procedure for SS diagnosis. The samples used in the experimental sets of this study were obtained from the aforementioned pool of patients and controls. The study was approved by the Ethics Committee of the School of Medicine, National University of Athens, Greece (Protocol no. 5107).
Immunohistochemical analysis of salivary gland biopsies
Paraffin‐embedded MSG tissues from 46 SS patients (16 with mild, 14 with intermediate and 16 with severe MSG autoimmune lesions) and 17 non‐SS sicca‐complaining controls were analysed. Each MSG biopsy tissue consisted of at least four glands. Non‐malignant parotid gland tissues were obtained from five individuals subjected to parotidectomy due to mixed tumours and served as a second control group of non‐autoimmune salivary gland tissue.
The in‐situ expression of type III IFNs (IFN‐λ1/IL‐29, IFN‐λ2/IL‐28A and IFN‐λ/IL‐28B) and their common receptor (IFN‐λR1/IL‐28Ra) was analysed immunohistochemically by a standard technique using the EnVision system (Dako) 2. Antigen retrieval was performed by microwaving in 10 mM‐Tris/1 mM‐ethylenediamine tetraacetic acid (EDTA) (pH 9·0). Non‐immune fetal bovine serum (10%) and 0·5% H2O2 in methanol were used to block non‐specific antibody binding and endogenous peroxidase activity, respectively. Permeabilization by 0·1% Triton‐X‐100 in blocking buffer was used for IFN‐λ1/IL‐29, IFN‐λ2IL‐28A and IFN‐λ/IL‐28B staining. Negative control staining was performed by replacing primary with irrelevant isotype‐matched antibodies. Staining with primary antibodies was performed overnight in a humidified chamber at 4°C. In certain experiments, the type(s) of the cells expressing IFN‐λR1/IL‐28Ra was also investigated by standard double immunofluorescence staining using primary antibodies against cell markers specific for T cells, B cells, macrophages and plasmacytoid dendritic cells (pDCs) (CD3, CD20, CD68, CD123 and CD303, respectively) and Alexa Fluor®‐conjugated secondary antibodies. Briefly, immunostaining with IFN‐λR1/IL‐28Ra and cell type‐specific markers was performed overnight, as described previously in immunohistochemistry, followed by a 30‐min incubation with the appropriate Alexa Fluor®‐conjugated secondary antibodies at room temperature and mounting with ProLong® Gold anti‐fade reagent with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Life Technologies). Images were acquired by an Olympus FV1000 confocal laser scanning microscope.
The positive staining and signal intensity in the whole tissue surface and in the secretory ductal epithelium of MSGs was analysed using Image J software in serial images covering the entire area of MSG biopsies (at least four glands per section) that were acquired by a computerized image‐acquisition system connected to a Carl‐Zeiss Axioskop‐40 microscope at ×20‐objective magnification analysis system. Values were expressed as a percentage of the total tissue area and average intensity of positive pixels.
PBMCs and sera
PBMCs obtained from 13 SS patients (four with mild, five with intermediate and four with severe MSG lesions) and 10 non‐SS sicca‐complaining controls were analysed. PBMCs were isolated by standard density gradient centrifugation. RNA isolation and cDNA preparation was performed as described previously 2. The levels of type III IFNs mRNA expression was estimated by quantitative real‐time polymerase chain reaction (PCR), as described below.
Sera were obtained from 21 healthy donors, nine non‐SS sicca‐complaining controls and 33 SS patients (nine with mild, 12 with intermediate and 12 with severe MSG infiltrates) at the time of diagnostic MSG biopsy and kept at −30°C until assessment with commercially available ELISAs specific for IFN‐λ1/IL‐29 (sensitivity: 8‐pg/mL), IFN‐λ2/IL‐28A (sensitivity: 15·6 pg/ml) and IFN‐λ3/IL‐28B (sensitivity: 31 pg/ml; for sera 1·55 ng/ml), according to the manufacturer's instructions.
SGEC lines and TLR stimulation
Non‐neoplastic, long‐term cultured SGEC lines were established from a lobule of MSG taken during the diagnostic biopsy, as described previously 2. The expression of type III IFNs was studied in 14 SGEC lines from SS patients with mild, intermediate and severe MSG infiltrates (five, four and five, respectively) and eight from non‐SS sicca‐complaining controls. As TLR‐3 stimulation has been shown to induce type I IFN expression in SGECs, its effect on the expression of type III IFNs has also been studied. Therefore, SGECs were left untreated or stimulated with the TLR‐3 ligand polyI:C (5 μg/ml; Sigma, St Louis, MO, USA) at various time‐points (6, 12, 24, 48 and 72 h), as described previously 2. Treatment with the TLR‐4 ligand lipopolysaccharide (LPS) (1 μg/ml; Sigma) served as control TLR treatment. At the end of treatments, the cells, as well as the cell‐free culture supernatants, were harvested and kept at −80°C till use. Cell pellets were used for RNA isolation and cDNA preparation as before. The effect of TLR signalling on the expression of type III IFNs was assessed at the mRNA level by real‐time quantitative PCR (qPCR) in reverse‐transcribed RNA from untreated or TLR‐treated SGECs and at protein level by measuring cytokine secretion in culture supernatants by specific commercial ELISAs, according to the manufacturer's instructions.
Quantitative real‐time polymerase chain reaction (qPCR)
The levels of type III IFNs mRNA expression were estimated by qPCR using SYBR Green‐I (Molecular Probes, Eugene, OR, USA). Human glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) served as the reference gene. All samples were run in duplicate. The relative quantification of PCR products was performed by the 2–ΔΔCT method 2. Primer sequences for IFN‐λ1/IL‐29, IFN‐λ2/IL‐28A‐IFN‐λ3/IL‐28B and GAPDH mRNAs (Cybergreen® Gene Expression Assays; Applied Biosystems) were as follows: human IL (hIL)−29 forward: 5′‐GGACGCCTTGGAAGAGTCACT‐3′, hIL‐29‐reverse: 5′‐AGAAGCCTCAGGTCCCAATTC‐3′; hIL‐28AB forward: 5′‐CTGCCACATAGCCCAGTTCA‐3′, hIL‐28AB reverse: 5′‐AGAAGCGACTCTTCTAAGGCATCTT‐3′; and hGAPDH forward: 5′‐AGGTGGTCTCCTCTGACTTC‐3′, hGAPDH‐reverse 5′‐CTGTTGCTGTAGCCAAATTCG‐3′.
Statistics
Statistical analyses were performed by the unpaired two‐tailed non‐parametric t‐test and one‐way analysis of variance (anova) using GraphPad Prism version 4.0 software (GraphPad Software, San Diego, CA, USA). Only statistically significant differences (P ≤ 0·05) are reported.
Results
Expression of type III IFNs and their common receptor IFN‐λR1/IL‐28Ra in MSG biopsies
The microscopic evaluation of immunohistochemical staining revealed that all members of the type III IFN family (IFN‐λ1/IL‐29, IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B) and their common receptor IFN‐λR1/IL‐28Ra were expressed in the MSGs of both SS patients and non‐SS sicca‐complaining controls. IFN‐λ1/IL‐29 staining was observed in ductal and acinar epithelium and endothelia. IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B were expressed in ductal and acinar epithelium, as well as infiltrating MNCs (Fig. 1). A similar expression pattern of IFN‐λ1/IL‐29 and IFN‐λ3/IL‐28B was observed between SS and non‐SS sicca controls, whereas IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B staining seemed more intense in SS patients, and particularly in those with intermediate lesions (Fig. 1). To validate the initial microscopic observations, morphometric analysis of the percentage of tissue surface and the intensity of positive signal in total tissue area or ductal epithelium was employed. This approach revealed that both the percentage of tissue surface and the intensity of positive signals of IFN‐λ2/IL‐28A in the total tissue area, as well as ductal epithelium, were statistically significantly higher in MSGs of SS with intermediate infiltrates (SS‐II) compared to sicca controls (P < 0·05), as well as SS patients with mild (SS‐I) (P < 0·05) (Fig. 2, Table 2). None of the other type III IFNs or the IFN‐λR1/IL‐28Ra was found to be expressed statistically significantly differentially between SS patients and non‐SS sicca controls or among the three SS subgroups (Table 2).
Figure 1.
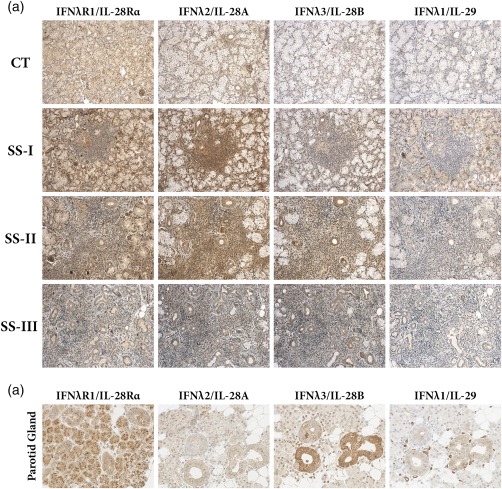
Expression of type III interferons (IFNs) (IFN‐λ1/interleukin (IL)−29, IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B) and their signalling receptor (IFN‐λR1/IL‐28Rα) (a) in minor salivary gland (MSG) tissues that belong to the three Sjögren's syndrome (SS) subgroups, as these classified by the grade of the inflammatory lesion (SS‐I: mild, n = 16; SS‐II: intermediate, n = 14; and SS‐III: severe, n = 16 MSG lesions. Sicca controls CT, n = 17), (b) in non‐malignant parotid glands of patients undergoing parotidectomy due to mixed tumours. Original magnification ×200.
Figure 2.
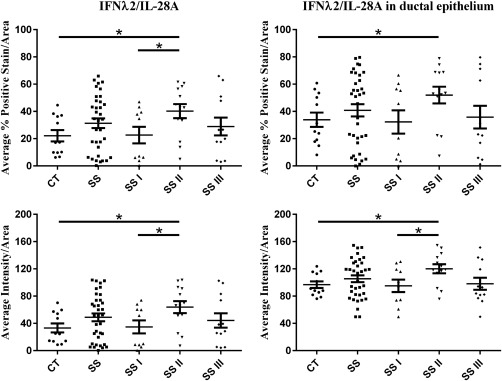
Morphometric analysis of in‐situ interferon (IFN)‐λ2/interferon (IL)−28A‐positive signal per tissue area, per ductal area and respective stain intensity of positive signal in minor salivary glands (MSGs) of Sjögren's syndrome (SS) patients with mild (SS‐I), intermediate (SS‐II) and severe (SS‐III) MSG lesions and sicca controls (CT). Statistically significant differences are indicated.
Table 2.
Morphometric analysis of type III interferon (IFN) staining in minor salivary gland (MSG) tissues.
| Type III IFNs | CT | SS | SS‐I | SS‐II | SS‐III | Significance | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean value ± s.e. | CT versus SS‐II* | SS‐I versus SS‐II* | |||||||
| Total tissue | IFN‐λ1/IL‐29 | %Average positive stain/area | 5·47 ± 2·18 | 4·33 ± 0·87 | 5·92 ± 1·72 | 4·10 ± 1·65 | 3·01 ± 0·97 | n.s. | n.s. |
| Average intensity/area | 8·10 ± 3·28 | 6·30 ± 1·31 | 8·73 ± ±2·64 | 5·94 ± 2·42 | 4·32 ± 1·44 | n.s. | n.s. | ||
| IFN‐λ2/IL‐28A | %Average positive stain/area | 22·12 ± 4·14 | 31·32 ± 3·55 | 22·63 ± 6·06 | 40·23 ± 5·16 | 28·91 ± 6·54 | P = 0·0125 | P = 0·0376 | |
| Average intensity/area | 33·32 ± 6·57 | 48·84 ± 5·79 | 34·82 ± 9·59 | 63·87 ± 8·77 | 44·26 ± 10·48 | P = 0·0114 | P = 0·0373 | ||
| IFN‐λ3/IL‐28B | %Average positive stain/area | 0·16 ± 0·03 | 0·18 ± 0·02 | 0·19 ± 0·04 | 0·25 ± 0·04 | 0·13 ± 0·03 | n.s. | n.s. | |
| Average intensity/area | 24·29 ± 5·03 | 27·18 ± 3·33 | 27·96 ± 5·82 | 37·90 ± 7·05 | 19·17 ± 4·20 | n.s. | n.s. | ||
| Ductal epithelium | IFN‐λ1/IL‐29 | %Average positive stain/area | 3·38 ± 2·52 | 2·25 ± 0·63 | 2·86 ± 1·16 | 1·93 ± 0·83 | 2·04 ± 1·41 | n.s. | n.s. |
| Average intensity/area | 56·05 ± 7·43 | 67·19 ± 3·13 | 70·80 ± 4·50 | 64·28 ± 6·40 | 67·14 ± 4·95 | n.s. | n.s. | ||
| IFN‐λ2/IL‐28A | %Average positive stain/area | 33·88 ± 5·28 | 40·78 ± 4·52 | 32·26 ± 8·54 | 51·97 ± 6·13 | 35·77 ± 8·35 | P = 0·0367 | NS | |
| Average intensity/area | 96·71 ± 4·81 | 105·40 ± 4·96 | 95·15 ± 9·16 | 120·00 ± 6·67 | 98·04 ± 8·81 | P = 0·0103 | P = 0·0352 | ||
| IFN‐λ3/IL‐28B | %Average positive stain/area | 43·86 ± 7·86 | 47·35 ± 4·33 | 48·82 ± 8·47 | 56·97 ± ±7·68 | 37·01 ± 5·86 | n.s. | n.s. | |
| Average intensity/area | 118·00 ± 6·85 | 121·30 ± 4·06 | 120·60 ± 7·63 | 129·40 ± 7·87 | 114·40 ± 5·45 | n.s. | n.s. | ||
Sicca controls (CT) = sicca‐complaining non‐SS controls; Sjögren's syndrome (SS)‐I = patients with mild MSG infiltrates; SS‐II with intermediate and SS‐III with severe; n.s. = not significant; IL = interleukin; s.e. = standard error. *Statistical analysis revealed significant differences only between SS‐II (intermediate MSG inflammatory lesions) and CT, as well as SS‐I (mild lesions) and SS‐II.
The common IFN‐λR1/IL‐28Ra was expressed in almost all cell types comprising the MSG, including infiltrating MNCs in SS lesions, except fibroblasts (Fig. 1). Among infiltrating MNCs, a type with characteristic pDC‐like morphology exhibited exceptionally strong staining for IFN‐λR1/IL‐28Ra. Thus, double immunofluorescent analysis for IFN‐λR1/IL‐28Ra and markers specific for T cells, B cells, macrophages and pDCs was employed to identify the type of the strongly expressing cells. Indeed, it was found that strong IFN‐λR1/IL‐28Ra staining was mainly on pDCs (Fig. 3).
Figure 3.
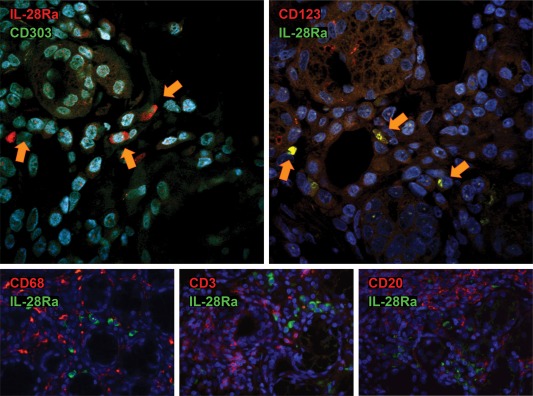
Representative double immunofluorescent staining for the common type III interferon (IFN) signalling receptor [IFN‐λR1/interleukin (IL)−28Rα] and markers of inflammatory cells [plasmatocytoid dendritic cells (pDCs): CD303 and CD123, macrophages: CD68, T lymphocytes: CD3 and B lymphocytes: CD20] in a minor salivary gland (MSG) tissue from a Sjögren's syndrome (SS) patient. Co‐localization is indicated with yellow arrows. Original magnification ×1000.
To investigate the expression of type III IFNs and their receptor in non‐autoimmune salivary tissues, non‐malignant parotid glands from individuals subjected to parotidectomy due to mixed tumours were tested. The expression pattern of type III IFNs and their receptor was similar with that observed in MSGs (Fig. 1).
Expression of type III IFN family in the periphery
At the mRNA level, none of the type III IFNs (IFN‐λ1/IL‐29, IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B) was detected in PBMCs obtained from SS patients or sicca‐complaining controls (data not shown). Furthermore, we were unable to detect IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B in the serum of either SS patients or non‐SS sicca controls. On the contrary, IFN‐λ1/IL‐29 was detected in sera of SS patients, non‐SS sicca controls and healthy individuals (Fig. 4). Its levels were found significantly higher in sera of SS patients with intermediate MSG infiltrates, compared to the other SS subgroups and non‐SS sicca controls [mean concentration (pg/ml) ± standard error (s.e.): 82·33 ± 37·19, 32·63 ± 20·78, 293·3 ± 118·6, 18·33 ± 7·63, 762·0 ± 284·6 and 30·82 ± 8·86 in healthy, SS‐sicca controls, total SS patients, SS‐I, SS‐II and SS‐III subgroup, respectively; P = 0·0053] (Fig. 4). The in‐depth analysis of these findings indicate that this elevated expression is due most probably to three of the SS‐II patients studied that expressed exceptionally high sera levels (> 2200 pg/ml) of IFN‐λ1/IL‐29. The analysis of their medical records did not reveal association with clinical or laboratory features or subjacent infection, although the latter cannot be excluded. Furthermore, serum levels were not found to associate with in‐situ epithelial type III expression (data not shown).
Figure 4.
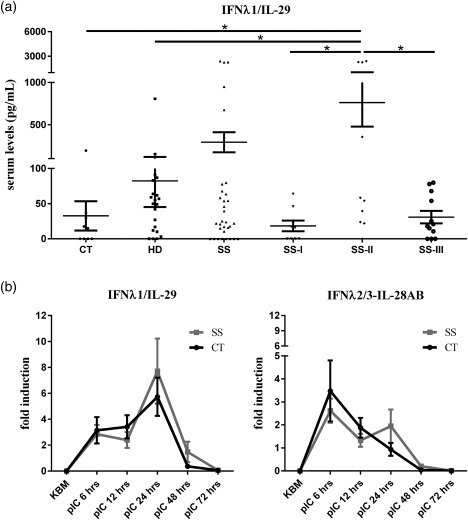
(a) Detection of interferon (IFN)‐λ1/interleukin (IL)−29 in sera of healthy donors (HD), sicca controls (CT), total number of Sjögren's syndrome (SS) patients and SS patients classified by the grade of the inflammatory lesion [SS‐I: mild; SS‐II: intermediate and SS‐III: severe minor salivary gland (MSG) lesions]. Statistically significant differences are indicated. (b) Expression of IFN‐λ1/IL‐29 and IFN‐λ2/3‐IL‐28A/B mRNA in long‐term cultured salivary gland epithelial cells (SGECs) established from SS patients and non‐SS CTs following Toll‐like receptor (TLR)−3 ligation. Resting SGECs or treated with pI:C for various time‐points are shown. mRNA expression was assessed by quantitative real‐time polymerase chain reaction.
The expression of type III IFNs in long‐term cultured SGECs is induced upon TLR‐3 activation
Long‐term cultured, non‐neoplastic SGECs were not found to express constitutively any of the type III IFN family members at the mRNA or protein level (Fig. 4). However, polyI:C‐mediated stimulation of TLR‐3 was found to readily induce the expression of the mRNA of all type III IFNs from 6 h in a biphasic way (Fig. 4). The first peak of type III IFNs mRNA was observed at 6 h followed by a second increment starting from 12 h with a peak at 24 h and subsequent reduction to minimal levels at 72 h (Fig. 4). The second peak of IFN‐λ2/IL‐28A‐IFN‐λ3/IL‐28B was similar to that observed at 6 h, and evident only in SGECs from SS patients (Fig. 4). On the protein level, the secretion of IFN‐λ1/IL‐29 was induced upon 6‐h polyI:C treatment and peaked at 48 h, and was not found to differ significantly between SGECs obtained from patients and sicca‐complaining controls (data not shown). On the contrary, treatment of SGECs with the TLR‐4 ligand, LPS, had no effect in the expression of type III IFN‐λs in the mRNA or protein level (data not shown).
Discussion
This is the first demonstration of the expression of type III IFNs and their common receptor in biological samples of pSS. All members of type III IFN family (IFN‐λ1/IL‐29, IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B), as well as their common receptor IFN‐λR1/IL‐28Ra, were detected in the MSG epithelia and infiltrates of SS patients and non‐SS sicca‐complaining controls. As anticipated, the IFN‐λR1/IL‐28Ra receptor was found to be expressed by almost all types of cells comprising the MSGs, including epithelial and infiltrating MNCs, with stronger expression observed in infiltrating plasmatocytoid DCs. Although initial microscopic observation suggested that IFN‐λ2/IL‐28A and IFN‐λ3/IL‐28B expression may be stronger in SS patients, detailed morphometric analysis revealed that only IFNλ2/IL‐28A was expressed more intensively in SS patients with intermediate MSG infiltrates compared to sicca‐complaining controls. This pattern could be explained by stronger ductal epithelial staining. None of the cytokines was detected in PBMCs, whereas only IFN‐λ1/IL‐29 was detected in serum, due possibly to low concentration and/or restricted sensitivity of commercially available ELISAs. The levels of IFN‐λ1/IL‐29 were higher in sera of SS patients with intermediate lesions at MSGs, following the pattern of IFN‐λ2/IL‐28A in MSGs. Finally, long‐term cultured SGECs were not found to express constitutively any of the type III IFNs, but these were induced readily upon TLR‐3 stimulation, suggesting that the expression of type III IFNs is dependent upon on‐and‐off signals of innate immunity, in a pattern similar to that described for type I IFNs 2.
Although molecularly distinct, type III IFNs exert similar anti‐viral and immunoregulatory properties to type I IFNs. They are expressed and act upon various types of cells, including epithelial cells and various types of immunocytes, such as plasmacytoid DCs, conventional DCs and macrophages. They most probably possess a major role in mucosal barriers, including the gastrointestinal and respiratory tract, where they target primarily epithelial cells, protecting them from the frequent viral attacks that are typical for barrier tissues. Type I IFNs appear to induce a more systemic effect, often associated with deleterious consequences, including massive induction of proinflammatory cytokines and apoptosis‐inducing molecules on immune cells and blocking of adaptive immune responses by T or B cells 6, 14, 15. Thus, type III IFNs appear to be the weapons of choice in the restriction of local viral infections, whereas type I IFNs have a more prominent role in severe systemic responses. Apart from their significant anti‐viral and anti‐tumour function, recent reports have shown that type III IFNs possess a significant immune‐regulatory function at mucosal sites by activating diverse types of cells, including plasmatocytoid DCs, macrophages and NK cells, suppressing the T helper type 1 (Th1) or Th17 responses or neutrophils recruitment 16. The pattern of type III IFNs expression in MSGs of both SS patients and controls is similar to that of IFN‐β 2. The significance of higher type III IFN expression in SS patients with intermediate lesions is not clear. Considering the distinct composition of the inflammatory lesions in patients with distinct grade of infiltration 13, 17, 18, it possibly reflects the effect of distinct underlying pathogenetic processes. The findings of the present study implicate type III IFNs in the regulation of local autoimmune responses operated in SS. Although type III IFNs are expressed similarly between SS and control patients, the strong expression of IFN‐λR1/IL‐28Ra receptor in the plasmatocytoid DCs infiltrating the MSGs of SS patients is of particular interest, suggesting that epithelial type III expression may modulate the plasmatocytoid DC activation in SS autoimmune lesions. Plasmatocytoid DCs have been implicated in SS and are considered to have a major role in SS‐inflammatory responses by IFN‐α production 4, 19. From this perspective, it would be tempting to hypothesize that (a) type III IFNs modulate type I IFN responses or (b) type III IFNs modulate plasmatocytoid DCs directly. Types I (IFN‐α and IFN‐β) and II IFNs (IFN‐γ) have been implicated for a long time in the perpetuation of the inflammatory lesions of SS 4, 5, 19, 20, 21, 22, 23. Type I IFN responses are expressed mainly in ductal epithelia, whereas type II in immune cells invade the salivary glands 5. The pattern of IFN expression in MSGs has been reported to differ among SS patients and can be distinguished to type I‐predominant, type II‐predominant and types I/II‐mixed IFN, with type I to be associated with more intense infiltration, whereas the ratio of type II to type I IFN expression has been linked to lymphoma development 19, 22.
The in‐situ epithelial expression of type III IFNs deserves special attention. Long‐term cultured non‐neoplastic SGEC do not express type III IFNs constitutively; however, their expression is induced readily upon TLR‐3 ligation, in a pattern similar to that observed with TLR‐3‐induced IFN‐β by SGECs 2. This suggests that the in‐situ epithelial expression in MSGs is probably induced by tissue microenvironmental factors, including viral infections, free DNA released locally, stress‐induced activation, etc. 1. Although the expression of type III IFNs has not been found to differ significantly between SS patients and controls, our in‐situ and in‐vitro findings implicate them in the earlier courses of disease pathogenesis. A tempting hypothesis is that upon chronic stimulation by ‘external’ microenvironmental factor(s), glandular epithelia express type III IFNs which, in turn, interact with activated plasmatocytoid dendritic cells and regulate their function and, thus, the perpetuation of SS autoimmune inflammatory responses.
In summary, this study demonstrates the expression of type III IFNs in MSG tissues and SGECs, suggesting that they might participate in local anti‐viral and immune responses. Although their role(s) in disease pathogenesis has not been fully understood, the elevated in‐situ epithelial expression of IFN‐λ2/IL‐28A and the increased levels of IFN‐λ1/IL‐29 in sera of SS patients with certain histological phenotypes and the strong expression of the common receptor IFN‐λR1/IL‐28Ra by plasmatocytoid DCs suggest that they can be implicated in the pathogenesis of SS.
Disclosure
The authors have no financial or other potential conflicts of interest to declare.
Acknowledgements
We would like to thank Dr G. Baltatzis and the Professor of Pathology E. Patsouris for their substantial contribution to the confocal microscopy experiments. The research was financed by the ‘IKY Fellowships of Excellence for Postgraduate Studies in Greece–Siemens Program’ scholarship to E. A.
References
- 1. Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjogren's syndrome: what we know and what we should learn. J Autoimmun 2012; 39:4–8. [DOI] [PubMed] [Google Scholar]
- 2. Kyriakidis NC, Kapsogeorgou EK, Gourzi VC, Konsta OD, Baltatzis GE, Tzioufas AG. Toll‐like receptor 3 stimulation promotes Ro52/TRIM21 synthesis and nuclear redistribution in salivary gland epithelial cells, partially via type I interferon pathway. Clin Exp Immunol 2014; 178:548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spachidou M, Kapsogeorgou E, Moutsopoulos H, Manoussakis M. Toll‐like receptor‐3 triggering of salivary gland epithelial cells (SGEC) causes detachment‐induced cell death (anoikia) that is more pronounced in cells derived from Sjögren's syndrome patients. Ann of Rheum Dis 2007; 66: A36. [Google Scholar]
- 4. Gottenberg JE, Cagnard N, Lucchesi C et al Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci USA 2006; 103:2770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JC, Casciola‐Rosen L, Berger AE et al Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci USA 2012; 109:17609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wack A, Terczynska‐Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol 2015; 16:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zahn S, Rehkamper C, Kummerer BM et al Evidence for a pathophysiological role of keratinocyte‐derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol 2011; 131:133–40. [DOI] [PubMed] [Google Scholar]
- 8. Wu Q, Yang Q, Lourenco E, Sun H, Zhang Y. Interferon‐lambda1 induces peripheral blood mononuclear cell‐derived chemokines secretion in patients with systemic lupus erythematosus: its correlation with disease activity. Arthritis Res Ther 2011; 13:R88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolk K, Witte K, Witte E et al IL‐29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis. Sci Transl Med 2013; 5:204ra129. [DOI] [PubMed] [Google Scholar]
- 10. Wang F, Xu L, Feng X, Guo D, Tan W, Zhang M. Interleukin‐29 modulates proinflammatory cytokine production in synovial inflammation of rheumatoid arthritis. Arthritis Res Ther 2012; 14:R228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dantas AT, Goncalves SM, Pereira MC et al Interferons and systemic sclerosis: correlation between interferon gamma and interferon‐lambda 1 (IL‐29). Autoimmunity 2015; 48:429–33. [DOI] [PubMed] [Google Scholar]
- 12. Vitali C, Bombardieri S, Jonsson R et al Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European consensus group. Ann Rheum Dis 2002; 61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren's syndrome. J Autoimmun 2010; 34:400–7. [DOI] [PubMed] [Google Scholar]
- 14. Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol Rev 2013; 255:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lazear HM, Nice TJ, Diamond MS, Interferon l. Immune functions at barrier surfaces and beyond. Immunity 2015; 43:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galani IE, Koltsida O, Andreakos E. Type III interferons (IFNs): emerging master regulators of immunity. Adv Exp Med Biol 2015; 850:1–15. [DOI] [PubMed] [Google Scholar]
- 17. Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T‐regulatory cells in Sjogren's syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol 2008; 173:1389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kapsogeorgou EK, Christodoulou MI, Panagiotakos DB et al Minor salivary gland inflammatory lesions in Sjogren syndrome: do they evolve? J Rheumatol 2013; 40:1566–71. [DOI] [PubMed] [Google Scholar]
- 19. Nezos A, Gravani F, Tassidou A et al Type I and II interferon signatures in Sjogren's syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjogren's related lymphomagenesis. J Autoimmun 2015; 63:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brkic Z, Maria NI, van Helden‐Meeuwsen CG et al Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren's syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 2013; 72:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 1979; 301:5–8. [DOI] [PubMed] [Google Scholar]
- 22. Hall JC, Baer AN, Shah AA et al Molecular subsetting of interferon pathways in Sjogren's syndrome. Arthritis Rheumatol 2015; 67:2437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol 1994; 152:5532–9. [PubMed] [Google Scholar]


