Summary
New data suggest the involvement of rotavirus (RV) in triggering autoimmunity in coeliac disease (CD) by molecular mimicry between the human‐transglutaminase protein and the dodecapeptide (260‐271 aa) of the RV protein VP7 (pVP7). To assess the role of RV in the onset of CD, we measured anti‐pVP7 antibodies in the sera of children with CD and of control groups. We analysed serum samples of 118 biopsy‐proven CD patients and 46 patients with potential CD; 32 children with other gastrointestinal diseases; 107 no‐CD children and 107 blood donors. Using enzyme‐linked immunosorbent assay (ELISA) assay, we measured immunoglobulin (Ig)A–IgG antibodies against the synthetic peptides pVP7, the human transglutaminase‐derived peptide (476–487 aa) which shows a homology with VP7 protein and a control peptide. The triple‐layered RV particles (TLPs) containing the VP7 protein and the double‐layered RV‐particles (DLPs) lacking the VP7 protein were also used as antigens in ELISA assay. Antibody reactivity to the RV‐TLPs was positive in 22 of 118 (18%) CD patients and in both paediatric (17 of 107, 16%) and adult (29 of 107, 27%) control groups, without showing a statistically significant difference among them (P = 0·6, P = 0·1). Biopsy‐proven CD patients as well as the adult control group demonstrated a high positive antibody reactivity against both pVP7 (34 of 118, 29% CD patients; 66 of 107, 62% adult controls) and control synthetic peptides (35 of 118, 30% CD patients; 56 of 107, 52% adult controls), suggesting a non‐specific response against RV pVP7. We show that children with CD do not have higher immune reactivity to RV, thus questioning the molecular mimicry mechanism as a triggering factor of CD.
Keywords: coeliac disease, molecular mimicry, rotavirus, VP7
Introduction
Infectious agents have been thought to play a role in the aetiopathogenesis of coeliac disease (CD) 1, a gluten‐dependent autoimmune disorder in genetically predisposed individuals carrying the human leucocyte antigen (HLA)‐DQ2 or ‐DQ8 haplotypes. Induction of autoimmunity is often supposed to occur by molecular mimicry, a mechanism that can be defined as recognition by the immune system of defined structures present in microorganisms (bacteria, parasites or viruses) 2 capable of inducing responses that are cross‐reactive with self‐molecules of the host. Immune‐mediated diseases in which molecular mimicry has been proposed include acute rheumatic fever 3, Guillain–Barré syndrome with anti‐myelin antibodies induced by Campylobacter jejuni lipo‐oligosaccharides 4 and narcolepsy caused by antibodies against the influenza virus nucleoprotein induced by an influenza A H1N1 vaccine that cross‐reacts with the human hypocretin receptor 2 5, 6.
In the case of CD, a region of sequence homology has been reported between A‐gliadin and the E1b protein of human intestinal adenovirus 12, suggesting a pathogenic role of past adenovirus infections with the development of cross‐reactive antibodies elicited by these infections 7. However, subsequent studies failed to show antibodies to adenovirus E1b protein 8, as well as the presence of adenovirus 12 in the intestine of both untreated adult and paediatric CD patients 9 in comparison to controls.
Recent data seem to support the involvement of rotavirus (RV) in facilitating the onset of CD 10. A random peptide library was screened to isolate a dodecamer peptide (VVKVGGSSSLGW), which was recognized by the serum immunoglobulins of some untreated CD patients. This dodecamer peptide (pCP) showed weak homology with the amino acid sequence 260–271 of RV serotype I major neutralizing protein VP7 and several other human proteins [heat shock protein (HSP)60, myotubularin related protein 2 (MTMR2), Toll‐like receptor (TLR)‐4] and in particular with the amino acid sequence 476–487 of human tissue type 2 transglutaminase (tTG) protein, the main autoantigen involved in the pathogenesis of CD. The affinity‐purified antibodies of untreated coeliac patients’ serum samples against pCP were shown to bind recombinant human transglutaminase as well as the other human proteins, and they seemed to exert some biological activities in vitro increasing epithelial cell permeability and monocyte activation 10. It was also claimed that the anti‐RV‐VP7 antibodies might have much greater diagnostic predictive value for CD than the well‐established CD diagnostic markers (anti‐transglutaminase and anti‐endomysium antibodies) 11. However, antibody reactivity against the RV‐VP7 peptide was measured in only a few children before and after the CD diagnosis, and the antibody reactivity was very low after onset of CD.
The outcomes of these studies have remained controversial 12, 13, 14, and the role of RV infection in the CD process is still unclear. This study was carried out with the objective of verifying the findings from previous studies 10, 11 by measuring immunoglobulin (Ig)G and IgA antibodies against purified RV particles, one of them containing the native form of VP7. It is expected that sera from patients who react with the VP7‐derived peptide, if they are the consequence of RV infection, should also be positive for the whole VP7 protein as well as for the dominant RV antigen VP6. Thus, in a large number of serum samples of both untreated young children with CD and age‐matched controls, we measured IgG and IgA antibodies against purified RV particles and compared these antibody values with those obtained by determining reactivity against the dodecamer peptide (pCP), the RV‐VP7‐derived peptide, the tTG 476‐487‐derived peptide and the human tTG enzyme to check differences in antibody recognition between RV synthetic and native antigens. We also verified the CD diagnostic sensitivity of anti‐VP7 peptide antibodies in comparison to the anti‐transglutaminase antibodies among patients with potential CD, which are characterized by the presence of pathological concentrations of serum anti‐transglutaminase antibodies but with normal intestinal mucosa.
Materials and methods
Study design
The study was conducted at the Institute of Child Health Burlo Garofolo from May 2014 to May 2015 and was composed of the following study groups:
Biopsy‐proven CD children diagnosed according to the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) criteria 15 with intestinal lesion ranging from 3a to 3c based on Oberhuber's classification and tested positive for serum anti‐tTG antibodies, concentration greater than five times the cut‐off values (normal value < 7 U/ml) and IgA anti‐endomysium antibodies (AEA).
Symptomatic children with potential CD, defined by the presence of both positive serum anti‐tTG antibodies values and IgA anti‐endomysium antibodies (AEA) and of CD‐related human leucocyte antigen (HLA) but with normal intestinal mucosa.Children with non‐gluten‐related gastrointestinal diseases and with no personal or family history of CD.
Children who had made a spontaneous recovery from food allergy 16 and with no personal or family history of CD as a control group
Healthy young blood donors and with no family history of CD as another control group.
The serum samples were collected during clinical visit for the diagnosis of CD and other medical conditions (i.e. food‐dependent allergy, inflammatory bowel diseases) and stored in three aliquots in our biobank at −30°C. All the serum samples were evaluated for the IgA and IgG antibodies against the purified RV particles and the synthetic peptides using an enzyme‐linked immunosorbent assay (ELISA) assay. The investigation was approved by the hospital's Independent Ethics Committee (CE/V‐5/2014).
Antigens
Viral particles
We produced and purified two types of RV particles: triple‐layered particles (TLPs, whose outer layer is formed by the VP7 and VP4 antigens) and double‐layered particles (DLPs, which lack the outer layer and only expose the middle layer formed by the VP6 antigen), as described previously 17. The purified particles were analysed by Western blot and both DLPs and TLPs contain the two structural viral proteins VP1 and VP2, while only TLPs contain VP7 (Supporting information, Fig. S1). The integrity of viral particles was assessed by non‐denaturing agarose gel electrophoresis (Supporting information, Fig. S2).
Peptides
The four synthetic peptides 10: (1) the dodecameric celiac peptide pCP (VVKVGGSSSLGW); (2) the RV VP7‐derived peptide pVP7 (VIQVGGSNVLDI) comprising the amino acid sequence 260–271; (3) the human tissue type 2 transglutaminase‐derived peptide ptTG (RIRVGQSMNMGS) comprising amino acids 476–487; and (4) an irrelevant control peptide pCTRL (VTLPKDSDVELP) were synthesized using the solid‐phase peptide synthesis method and molecular weights were confirmed by matrix‐assisted laser desorption ionization time‐of‐flight (MALDI‐TOF) mass spectrometry by Primm (Milan, Italy).
Antibody determination by ELISA
Plates (Nunc, Sigma‐Aldrich, Milan, Italy) were coated with purified particles or synthetic peptides at a concentration of 5 µg/ml in phosphate‐buffered saline (PBS) overnight (O/N) at 4°C. After blocking with PBS 3% Tween (PBST) for 1 h at room temperature (RT), 100 µl of serum samples diluted 1 : 400 in PBST were incubated for 2 h at RT. Plates were then washed with PBST and anti‐human alkaline phosphatase‐conjugated IgA or IgG were incubated for 2 h at RT. The immunocomplexes were revealed with the p‐nitrophenilphosphate substrate and the plates were read at 405 nm. The results were expressed as optical density (OD) (serum sample OD–anti‐human antibody OD). OD values higher than the mean OD values + 2 standard deviations for each antigen of 50 healthy childrens’ serum samples (24F, 26M, mean aged 2–9 years) were considered positive. The IgA normal values were: 0·07 for pCP, 0·081 for ptTG, 0·07 for pVP and 0·082 for pCTRL; and the normal values for IgG were: 0·496 for pCP, 0·531 for ptTG, 0·652 for pVP7 and 0·713 for pCTRL.
Serum IgA and IgG anti‐tTG antibodies were measured by ELISA following the manufacturer's instructions (Eurospital, Trieste, Italy). All the assays were performed by operators (F. Z., V. S., S. Q., L. D. L., S. V.) blind to the patients’ clinical and laboratory data.
Statistical analysis
Statistical comparison between positive serological data of the different groups was performed using the two‐tailed Fisher's exact test. The Kruskal–Wallis test was used to compare serological data against TLPs by age between all CD patients and control children. A value of P < 0·05 was considered significant. In order to evaluate the interassay precision of the ELISA method for the antibody measurement against both the synthetic peptides and the RV particles the coefficient of variation (CV) was calculated using three replicates from four serum samples on 2 consecutive days.
Results
Subjects
Informed consent was obtained from a total of 410 subjects (303 children and 107 young adults) who were divided into the following groups (Table 1)
Table 1.
Clinical and laboratory data of the study groups
| Study groups | Number | Sex | Mean age, years (range) | Positivity for HLA‐DQ2/‐DQ8 (%) | Anti‐transglutaminase antibodies (mean ± standard deviation) | |
|---|---|---|---|---|---|---|
| IgA | IgG | |||||
|
Coeliac disease (CD) |
118 | 77F–41M | 7 (1–23) | 118 (100%) | 148 ± 35 | 98 ± 67 |
|
Potential CD (POT) |
46 | 25F–21M | 7·5 (1–16) | 46 (100%) | 85 ± 20 | 38 ± 28 |
|
Gastrointestinal diseases (GD) |
32 | 14F–18M | 9 (1–14) | 15 (47%) | – | – |
|
Healthy children (HC) |
107 | 37F–70M | 7.6 (2–17) | 41 (38%) | – | – |
|
Healthy adults (HA) |
107 | 42F–65M | 28 (18–40) | 30 (28%) | – | – |
HLA = human leucocyte antigen.
One hundred and eighteen biopsy‐proven CD patients (77F, 41M; median age 7 years, range 1–23 years), suffering from diarrhoea (80 subjects), abdomen distention (65), anaemia (25), type 1 diabetes (10), tested positive for both CD‐related HLA and serum IgA/IgG anti‐tTG antibodies (mean value 148 ± 35, range 65–250 U/ml for IgA; mean value 98 ± 67, range 3–120 U/ml for IgG) and AEA (group defined as CD).
Forty‐six children with potential CD (25F, 21M; median age 7·5 years, range 1–16 years) suffering from recurrent abdominal pain (30), abdomen distension (10), anaemia (eight) with CD‐related HLA, tested positive for serum IgA/IgG anti‐tTG antibodies (mean value 85 ± 20, range 15–120 U/ml for IgA; mean value 38 ± 28, range 2–97 U/ml for IgG) and AEA but with normal small‐bowel biopsy (group defined as POT).
Thirty‐two children (14F, 18M; median age 9 years, range 1–14 years), suffering from other gastrointestinal diseases: Crohn (10), ulcerative colitis (nine) eosinophilic oesophagitis (13), 15 of which carrying the CD‐related HLA‐DQ2 or ‐DQ8 and all of them were tested negative for CD‐related autoantibodies (group defined as GD).
One hundred and seven children (37F, 70M; median age 7·6 years, range 2–17 years), who had made a spontaneous recovery from food allergy and who were tested negative for CD‐related autoantibodies, 41 of them positive for HLA‐DQ2 or ‐DQ8 (group defined as HC).
One hundred and seven blood donors (42F, 65M; median age 28 years, range 18–40 years), tested negative for CD‐related autoantibodies and 30 of 107 were positive for HLA‐DQ2 or ‐DQ8 (group defined as HA).
Serum antibodies
The interassay test of the ELISA method against the two RV particles produced CV mean values of 7·5 ± 0·9% for IgA and 8 ± 1·2% for IgG. The results (Table 2a) showed that 22 of 118 (18·5%) CD patients, 17 of 107 (16%) HC and 29 of 107 (27%) HA were tested positive for TLP‐particles containing VP7 antigen without showing a statistically significant difference among them (CD versus HC: P = 0·6, CD versus HA: P = 0·1). No potential CD patients (POT) were tested positive for anti‐TLP antibodies. A significantly higher positivity for DLP particles not containing VP7 antigen was observed in both HA and GD study groups in comparison to the other groups (HA versus CD: P < 0·001, HA versus HC: P < 0·001, GD versus CD: P < 0·001, GD versus HC: P < 0·001, HA versus GD: P = 0·3).
Table 2.
Subjects tested positive for immunoglobulin (Ig)A and/or IgG against the rotavirus particles (a) and the four synthetic peptides (b)
| (a) | Antibody positivity to TLPs | Antibody positivity to DLPs | ||||||
|---|---|---|---|---|---|---|---|---|
| Study groups (no.) | IgA | IgG | IgA+IgG | Total positive (%) | IgA | IgG | IgA+IgG | Total positive (%) |
| CD (118) | 12 | 4 | 6 | 22 (18·5%) | 5 | 13 | 9 | 27 (23%) |
| POT (46) | – | – | – | – | 2 | – | 1 | 3 (6·5%) |
| GD (32) | – | – | 1 | 1 (3%) | 5 | 1 | 12 | 18 (56%) |
| HC (107) | 3 | 9 | 5 | 17 (16%) | – | 3 | 9 | 12 (11%) |
| HA (107) | 14 | 14 | 1 | 29 (27%) | 6 | 36 | 6 | 48 (45%) |
| (b) | Antibody positivity to pCP | Antibody positivity to ptTG | Antibody positivity to pVP7 | Antibody positivity to pCTRL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Groups (N°) | IgA | IgG | IgA+IgG | Total positive (%) | IgA | IgG | IgA+IgG | Total positive (%) | IgA | IgG | IgA+IgG | Total positive (%) | IgA | IgG | IgA+IgG | Total positive (%) |
| CD (118) | 22 | – | 6 | 28 (24%) | 14 | – | 2 | 16 (13.5%) | 34 | – | – | 34 (29%) | 34 | – | 1 | 35 (30%) |
| POT (46) | 4 | 2 | – | 6 (13%) | 1 | – | – | 1 (2%) | 4 | – | – | 4 (9%) | 2 | – | – | 2 (4%) |
| GD (32) | 1 | – | – | 1 (3%) | – | – | – | – | 1 | – | – | 1 (3%) | 1 | – | – | 1 (3%) |
| HC (107) | 3 | 1 | – | 4 (4%) | 1 | 1 | 1 | 3 (3%) | 2 | 1 | 1 | 4 (4%) | 1 | – | 1 | 2 (2%) |
| HA (107) | 14 | 30 | 4 | 48 (45%) | 4 | 32 | 10 | 46 (43%) | 2 | 48 | 16 | 66 (62%) | 4 | 52 | – | 56 (52%) |
pCP = dodecamer peptide; ptTG = transglutaminase‐derived peptide; pVP7 = VP7‐derived peptide; pCTRL = irrelevant control peptide; TLP = triple‐layered rotavirus (RV) particles; DLP = double‐layered RV particles; CD = coeliac disease; POT = potential CD; GD = gastrointestinal disease; HC = healthy controls; HA = healthy adults.
When we divided all patients with CD (CD + POT) and children control group (HC) by age, the analysis of IgA reactivity to the TLP particles containing the VP7 did not show any statistically significant difference. Regarding the IgG reactivity to TLPs, the control group in the 1–5‐year age range showed a significant IgG immunological response in respect to the CD patients (P = 0·0004) (Fig. 1).
Figure 1.
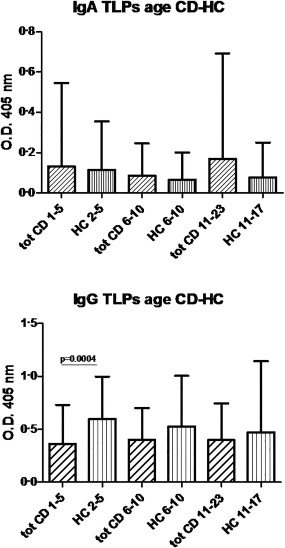
Immunoglobulin (Ig)A and IgG antibody concentrations against rotavirus‐triple‐layered particles (RV‐TLP) containing the native VP7 protein in patients with coeliac disease and the children control group divided by age. The antibody concentrations are expressed as optical density (OD), in sera of all coeliac patients (tot CD), and healthy children (HC) divided by age.
The interassay test for the four synthetic peptides produced CV mean values of 5·8 ± 1·7% for IgA and 6 ± 1·9% for IgG. A significantly higher positivity for the all synthetic peptides (including the control peptide) was observed in the HA study group in comparison to the other groups (P < 0·001) (Table 2b). Sera from the CD group demonstrated statistically high reactivity against ptTG, pVP7 and pCTRL synthetic peptides limited to IgA isotype in comparison to the other groups (P < 0·01). Among potential CD patients (POT), four of 46 (9%) and two of 46 (4%) were tested positive for both pVP7 and control peptide, respectively (Table 2b).
Discussion
In the present study, we found no difference in specific humoral immune response to the rotavirus particles containing the antigen VP7 between the untreated celiac patients and controls. All CD patients showed no significant reactivity against the dodecamer peptide (pCP), which has a weak homology with both the RV‐VP7 protein and the human type2 transglutaminase protein. Furthermore, the same immune reactivity to the synthetic sequence of the VP7 peptide was observed in both CD and HA study groups and was not specific, as it was present to the same degree against the irrelevant synthetic control peptide. Unless well validated 18, peptides could have unpredicted behaviours, as was observed with sera of the CD and control groups which showed relatively high values against all synthetic peptides, including the control group.
In general, no differences were found in the immune recognition of RV particles containing VP7 between patients with CD and controls when divided by age groups, thus indicating that RV infection was not associated significantly with the CD group. This is confirmed by the IgG reactivity of the CD and control groups against DLPs, which represents antibodies directed towards the highly immunogenic VP6 antigen protein. It should be noted that by determining reactivity against all the RV‐TLPs, we detected all anti‐VP7 antibodies, not only those against the peptide pVP7. Interestingly, among the youngest subjects (aged 1–5 years) who, in theory, should be at greater risk of having recently contracted RV gastroenteritis 19, the control group showed a significantly higher IgG immunological response to RV in comparison to the CD patients.
Although our CD patients had a very high anti‐tTG serum concentration (more than five times the cut‐off limit), there was no reactivity against the synthetic peptide 476–487 (ptTG) of the human enzyme type 2 transglutaminase (derived from its C‐terminal region), which has a modest sequence homology with the RV VP7 peptide. This is not surprising, as it was demonstrated previously that the main epitopes targeted by anti‐tTG antibodies of CD patients are clustered in the N‐terminal part of the enzyme and seem to exert a pathogenic role 20, 21. Taken together, these observations argue against the proposed mechanism of molecular mimicry between the VP7 viral protein and the tTG self‐antigen 476–487 as a triggering factor for autoimmunity in CD. Our study shows that a high reactivity against the RV particles is restricted not only among coeliac serum samples, but involves several serum samples of healthy and sick subjects without any statistically significant differences. This observation is in line with data obtained by cloning human monoclonal antibodies against RV particles 22 from intestinal plasma cells of both adult treated and untreated CD patients and adult non‐CD patients, in whom all the intestinal specimens presented specific plasma cells against RV particles with no reactivity to the human tissue transglutaminase by ELISA. A precise analysis of all the anti‐RV monoclonal antibodies displayed no difference either in the specificity of RV antigen recognition or in the use of the VH‐region gene (over‐representation of the VH4‐39 gene) to build these antibodies. The anti‐RV antibody V‐region gene sequences showed high levels of somatic mutations, suggesting they were derived from ongoing viral antigen stimulation in all these subjects. A recent prospective study did not demonstrate significant differences in the number of infectious enteritis with diarrhoea between genetically predisposed newborns at risk for CD (i.e. children with first‐degree relative with CD) who later developed an overt CD or CD‐related autoimmunity and an age‐matched control group 23. Moreover, a Norwegian large‐scale prospective cohort study did not find a significant association between parent‐reported gastrointestinal infections during the first 18 months of life and the risk of later CD 24. Based on these clinical and epidemiological data, infectious enteritis seems to play no role in CD development. A prospective serological study, based on the measurement of anti‐RV antibodies in children positive for CD‐related HLA, found that increased frequency of RV serologically defined infections may predict an increased risk of CD‐related autoimmunity in some patients studied 25. While this latest study questions the molecular mimicry as a mechanism for the development of CD autoimmunity, it seems to sustain recurrent RV infections as a possible co‐factor in CD pathogenesis. The role of RV infection as a triggering factor for the onset of autoimmunity was reported in type 1 diabetes (T1D) 26. In this case, RV enteritis has been associated with temporary increases of serum T1D‐associated autoantibodies in genetically predisposed infants younger than 3 months and only if fed with cow's milk. In addition, no significant synergic effect of early exposure to cow's milk and RV infection during infancy was observed in the development of overt clinical T1D in these subjects 26. In T1D, RV infection in combination with other environmental factors may pose a risk to the development of T1D‐related autoimmunity, but not for the onset of overt disease. However, despite the increase of T1D‐associated autoantibodies they were not identified to be reactive with any viral antigen to support a mechanism of molecular mimicry 27. In gluten genetically predisposed children, who are receiving a gluten‐containing diet, the serum anti‐tTG can be produced temporarily during viral infection diseases without affecting the onset of the disease 28, 29. Although these autoantibodies can exert some biological effects on inflamed tissues 28, they are a gluten‐independent phenomenon which may be determined by a viral polyclonal activation of B cells 30.
The strength of our study lies in its having monitored immunological reactivity to native and well‐characterized viral antigens in the sera of both coeliac patients and controls at a young age, very close to an RV infection, without finding any difference in the two study groups. However, further studies with a longer follow‐up of the immune response to RV are clearly needed before we can conclude that RV infections do not play a role in the pathogenesis of CD.
In conclusion, the data we have gathered, based on a large number of CD and non‐CD subjects and on precise determination of the presence of anti‐RV antibodies, fail to support the hypothesis of CD autoantibodies induced by mechanisms related to molecular mimicry between the RV‐VP7 peptide and the human tissue type 2 transglutaminase protein.
Author contributions
Study concept and design: T. N., A. V., O. R. B., F. Z., G. D. L. and F. A. Interpretation of data: T. N., O. R. B. and F. Z. Sample collection: S. M., F. Z., S. Q., L. D. L. and S. V. ELISA test: V. S. Production and purification of RV particles: G. D. L., F. A., F. Z. and V. S. Statistical analysis: F. Z. and S. Q. Drafting of the manuscript: F. Z., T. N. and O. R. B. All authors critically read, commented on and approved the final version of the manuscript.
Disclosure
These authors declare no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Western blot of isolated viral particles. The isolation of purified two types of rotavirus (RV) particles, triple‐layered particles (TLPs) and double‐layered particles (DLPs), were loaded in each lane of a polyacrylamide gel. After separation under reducing conditions, the proteins VP1, VP2 and VP7 were revealed using the chemiluminescent system. Both DLPs and TLPs contain the two structural viral proteins VP1 and VP2 while only TLPs contain the neutralizing protein VP7, the main component of the outer layer, totally absent in DLPs. T = TLPs; D = DLPs; M = marker.
Fig. S2. Agarose gel electrophoresis of viral particles in MOPS [3‐(N‐morpholino)propanesulphonic acid] buffer. Purified rotavirus (RV) triple‐layered particles (TLPs) and double‐layered particles (DLPs) were run in non‐denaturing agarose gel to verify the retention of their integrity after dilution in phosphate‐buffered saline (PBS) and PIPES [piperazine‐N,N′‐bis(2‐ethanesulphonic acid] buffers. The particles were visualized by ethidium bromide staining of their RNA genome content. T = TLPs; D = DLPs.
Acknowledgements
This study was supported by grant from IRCCS Burlo Garofolo: RC 05/14 to T. N.
References
- 1. Troncone R, Auricchio S. Rotavirus and celiac disease: clues to the pathogenesis and perspectives on prevention. J Pediatr Gastroenterol Nutr 2007; 44:527–8. [DOI] [PubMed] [Google Scholar]
- 2. Wucherpfenning KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 2001; 108:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000; 13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ang CW, Jacobs BC, Laman JD. The Guillain–Barré: a true case of molecular mimicry. Trends Immunol 2004; 25:61–6. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed SS, Steinman L. Mechanistic insights into influenza vaccine‐associated narcolepsy. Hum Vaccin Immunother 2016; 31:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed SS, Volkmuth W, Duca J. Antibodies to influenza nucleoprotein cross‐react with human hypocretin receptor 2. Sci Transl Med 2015; 7:294ra105. [DOI] [PubMed] [Google Scholar]
- 7. Kagnoff M, Austin R, Hubert J, Bernardin J, Kasarda D. Possible role for a human adenovirus in the pathogenesis of celiac disease. J Exp Med 1984; 160:1544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howdle P, Blair Zajdel M, Smart C, Trejdosiewicz L, Blair G, Losowky M. Lack of a serologic response to an E1b protein of adenovirus 12 in coeliac disease. Scand J Gastroenterol 1989; 24:282–6. [DOI] [PubMed] [Google Scholar]
- 9. Lawler M, Humphries P, O'Farrelly C et al Adenovirus 12 E1A gene detection by polymerase chain reaction in both the normal and coeliac duodenum. Gut 1994; 35:1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zanoni G, Navone R, Lunardi C et al In celiac disease, a subset of autoantibodies against transglutaminase binds toll‐like receptor 4 and induces activation of monocytes. PLOS Med 2006; 3:e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dolcino M, Zanoni G, Bason C et al A subset of anti‐rotavirus antibodies directed against the viral protein VP7 predicts the onset of celiac disease and induces typical features of the disease in the intestinal epithelial cell line T84. Immunol Res 2013; 56:465–76. [DOI] [PubMed] [Google Scholar]
- 12. Plot L, Amital H, Barzilai O, Ram M, Bizzarro N, Shoenfeld Y. Infections may have a protective role in the etiopathogenesis of celiac disease. Ann NY Acad Sci 2009; 1173:670–4. [DOI] [PubMed] [Google Scholar]
- 13. Welander A, Tjenberg A, Montgomery S, Ludvigsson J, Ludvigsson JF. Infectious disease and risk of later celiac disease in childhood. Pediatrics 2010; 125:e530–6. [DOI] [PubMed] [Google Scholar]
- 14. Rostami‐Nejad M, Rostami K, Sanaei M et al Rotavirus and coeliac autoimmunity among adults with non‐specific gastrointestinal symptoms. Saudi Med J 2010; 31:891–4. [PubMed] [Google Scholar]
- 15. Husby S, Koletzko S, Korponay‐Szabó I et al European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54:136–60. [DOI] [PubMed] [Google Scholar]
- 16. Pillon R, Ziberna F, Badina L et al Prevalence of celiac disease in patients with severe food allergy. Allergy 2015; 70:1346–9. [DOI] [PubMed] [Google Scholar]
- 17. Patton J, Chizhikov V, Taraporewala Z, Chen D. Virus replication In: Gray J, Desselberger U, eds. Rotaviruses: methods and protocols. Totowa, NJ: Humana Press, 2000:33–66. [DOI] [PubMed] [Google Scholar]
- 18. Gomara M, Haro I. Synthetic peptides for the immunodiagnosis of human disease. Curr Med Chem 2007; 14:531–46. [DOI] [PubMed] [Google Scholar]
- 19. Giaquinto C, Van Damme P. Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis 2010; 42:142–7. [DOI] [PubMed] [Google Scholar]
- 20. Iversen R, Di Niro R, Stamnaes J, Lundin K, Wilson P, Sollid L. Transglutaminase 2‐specific autoantibodies in celiac disease target clustered, N‐terminal epitopes not displayed on the surface of cells. J Immunol 2013; 190:5981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Hnida K, Graewert M et al Structural basis for antigen recognition by transglutaminase 2‐specific autoantibodies in celiac disease. J Biol Chem 2015; 290:21365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Niro R, Mesin L, Raki M et al Rapid generation of Rotavirus‐specific human monoclonal antibodies from small‐intestinal mucosa. J Immunol 2010; 185:5377–83. [DOI] [PubMed] [Google Scholar]
- 23. Lionetti E, Castellaneta S, Francavilla R et al Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014; 371:1295–303. [DOI] [PubMed] [Google Scholar]
- 24. Marild K, Kahars C, Tapia G et al Infections and risk of celiac disease in childhood: a prospective nationwide cohort study. Am J Gastronterol 2015; 110:1475–84. [DOI] [PubMed] [Google Scholar]
- 25. Stene L, Honeyman M, Hoffenberg E et al Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006; 101:2333–40. [DOI] [PubMed] [Google Scholar]
- 26. Lempainen J, Tauriainen S, Vaarala O et al Interaction of enterovirus infection and cow's milk‐based formula nutrition in type 1 diabetes‐associated autoimmunity. Diabetes Metab Res Rev 2012; 28:177–85. [DOI] [PubMed] [Google Scholar]
- 27. Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allerg Immunol 2014; 47:174–92. [DOI] [PubMed] [Google Scholar]
- 28. Ferrara F, Quaglia S, Caputo I et al Anti‐transglutaminase antibodies in non‐coeliac children suffering from infectious diseases. Clin Exp Immunol 2010; 159:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Leo L, Quaglia S, Ziberna F et al Serum anti‐tissue transglutaminase antibodies detected during febrile illness may not be produced by the intestinal mucosa. J Pediatr 2015; 166:761–3. [DOI] [PubMed] [Google Scholar]
- 30. Blutt S, Crawford S, Warfield K, Lewis D, Estes M, Conner ME. The VP7 outer capsid protein of rotavirus induces polyclonal B‐cell activation. J Virol 2004; 78:6974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Western blot of isolated viral particles. The isolation of purified two types of rotavirus (RV) particles, triple‐layered particles (TLPs) and double‐layered particles (DLPs), were loaded in each lane of a polyacrylamide gel. After separation under reducing conditions, the proteins VP1, VP2 and VP7 were revealed using the chemiluminescent system. Both DLPs and TLPs contain the two structural viral proteins VP1 and VP2 while only TLPs contain the neutralizing protein VP7, the main component of the outer layer, totally absent in DLPs. T = TLPs; D = DLPs; M = marker.
Fig. S2. Agarose gel electrophoresis of viral particles in MOPS [3‐(N‐morpholino)propanesulphonic acid] buffer. Purified rotavirus (RV) triple‐layered particles (TLPs) and double‐layered particles (DLPs) were run in non‐denaturing agarose gel to verify the retention of their integrity after dilution in phosphate‐buffered saline (PBS) and PIPES [piperazine‐N,N′‐bis(2‐ethanesulphonic acid] buffers. The particles were visualized by ethidium bromide staining of their RNA genome content. T = TLPs; D = DLPs.


