Abstract
Background:
Urolithiasis or renal stone formation occurs with three times higher frequency in males and decreases with age in parallel with the serum testosterone levels, suggesting a role played by male sex hormones. Androgens appear a promotion action and estrogens an inhibitory action on kidney stone formation in several animal models suggesting a study to be carried out to deduce the role played by serum testosterone in the formation of renal stones.
Aim:
The aim of this study is to define the involvement of serum total testosterone, free testosterone, and dihydrotestosterone in the pathogenesis of urolithiasis in males by comparing the results with healthy males with no present or past history of urolithiasis as controls.
Materials and Methods:
A case–control study was undertaken with 108 participants: 78 males diagnosed with urolithiasis and 30 age-matched healthy males.
Results:
The difference between mean age and body mass index of patients and controls were found to be nonsignificant. The total serum testosterone levels, serum dihydrotestosterone levels, were found to be higher in patients when compared to controls, and the difference was found to be significant. The levels of free testosterone and serum estradiol were also found to be higher in urolithiatic patients.
Conclusion:
The study demonstrates that elevated levels of serum testosterone and serum dihydrotestosterone might be involved in increased incidences of stone formation. The higher levels of estradiol do not seem to be a protective factor in males with urolithiasis with higher serum testosterone levels.
Key words: Elevated serum testosterone, pathogenesis of renal stone formation, urolithiasis
INTRODUCTION
Urolithiasis occurs with greater frequency in males with incidences three times higher compared to females indicating some role played by androgens.[1] Stone formation in renal tissues before puberty is similar between males and females,[2] whereas greater frequency is seen in third to the fourth decade of life when the levels of serum testosterone are also the highest in males.[3] With advancing age, the probability for stone formation also decreases as consistent with the decline in serum testosterone levels with more than 20% of healthy men over 60 years of age presenting with serum levels of hormone below the range for young men.[4] In menopausal females, the frequency for stone formation is considered more compared with premenopausal postulated mainly due to low estrogen levels.[5] The upregulation of androgen receptors in patients with urolithiasis,[6] higher androgen levels in renal stone patients and low urinary oxalate excretion in castrated males further augment the possible role of male sex hormones in the pathogenesis of urolithiasis.[7]
Hormones can exert their effect either through a change in their serum levels or by an alteration in their receptor activity.[8] Testosterone appears to promote stone formation by suppressing osteopontin expression in kidneys and increasing urinary oxalate excretion,[9] whereas reverse action is suggested by estrogen in an animal model of urolithiasis. Watson et al. in 2010 also reported higher serum total testosterone levels in male stone formers compared with a similar cohort without stones indicating that testosterone may be a risk factor for stone formation.[10] Although one study proposed that urolithiasis is associated with low serum testosterone levels in men,[11] the possibility of testosterone involvement in the pathogenesis of renal stones cannot be denied.
So far, the association between serum gonadal steroids and urolithiasis in males has received limited attention and only in the small group of patients. Hence to further investigate the role of testosterone in the pathogenesis of urolithiasis, this study was carried with the aim to find serum levels of male sex hormones in patients with renal stones compared to controls.
MATERIALS AND METHODS
Study subjects
Seventy-eight males with age group 20–52 years diagnosed with urolithiasis and visiting the Urology and Biochemistry Department of Adesh Institute of Medical Sciences and Research and 30 healthy age-matched individuals with no present or past history of urolithiasis were enrolled for the study. The exclusion criteria included hyperthyroidism, primary hyperparathyroidism, intestinal malabsorption, primary hyperoxaluria, recurrent or active urinary tract infections, present or past history of renal transplantation, any liver disease, metabolic syndrome, gout, and renal failure (chronic or acute).
Study protocol
All patients and controls underwent anthropometric and demographic measurements. A detailed history with full clinical examination and written informed consent was obtained from each participant. This study was approved by the Institutional Ethical Committee and Thesis Research Committee.
Specimen collection
A volume of 2 ml of blood samples were obtained before any medical or surgical intervention by venipuncture under all aseptic conditions. Samples were allowed to clot at room temperature. Serum was separated by centrifugation at 3500 × g for 10 min. The separated serum samples were immediately processed for biochemical estimations or stored at -20°C for further use. Out of 78 urolithiatic patients, in 52 stone was removed with operative procedures and collected for further biochemical analysis.
Biochemical analysis
Serum free testosterone, serum total testosterone, serum dihydrotestosteone, serum estradiol were estimated on AIA-360, fully automatic analyzer Tosoh, Japan with kits from Alere, Tosoh, Japan. Stones collected from urolithiatic patients were crushed to homogeneous powered form and subjected to battery of biochemical tests for the presence of calcium, magnesium, phosphates, oxalates, cystine, and uric acid.
Statistical analysis
All statistical analysis in the study was performed using SPSS for Windows, Version 16.0. Chicago, SPSS Inc. The results of laboratory tests in the study and control groups were summarized as a mean ± standard deviation. Comparison between subjects (both participating groups) was carried out using Student's unpaired t-test. 95% confidence interval was taken into consideration and P < 0.05 was considered statistically significant.
RESULTS
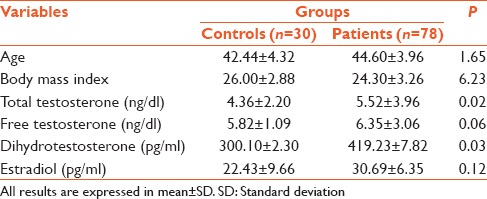
As shown in Table 1, the difference between mean age and body mass index (BMI) of patients (44.60 ± 3.96, 24.30 ± 3.26) and controls (42.44 ± 4.32, 26.00 ± 2.88) were found to be nonsignificant (P = 1.65, 6.23), respectively. The total serum testosterone levels, serum dihydrotestosterone levels, were found to be higher in patients (5.52 ± 3.96, 419.23 ± 7.82), when compared to controls (4.36 ± 2.20, 300.10 ± 2.30) and the difference was found to be significant (P = 0.02, P = 0.03). The levels of free testosterone and serum estradiol were although found to be higher in patients (6.35 ± 3.06), but the difference was not statistically significant (P = 0.06, P = 0.12).
Table 1.
Comparison of gonadal sex hormones in controls and urolithiatic patients
For stone analysis of 52 patients, in most of the patients, calcium oxalate stone (n = 39 patients, 75%) and mixed stones of calcium oxalate and calcium phosphate (n = 5, 9.6%) were found followed by uric acid and struvite [Table 2].
Table 2.
Distribution of types of stone in urolithiatic patients (n=52)

DISCUSSION
In our age-matched case–control study, no association was found between frequency of urolithiasis and age or BMI. It is reported that obesity and weight gain increase the risk for kidney stone formation.[12] However, the prevalence of stone disease may not increase in concert with increasingly obese BMI values when the BMI values are below the threshold of 30 kg/m2.[13] Consistent with these reports, the values of BMI in both the patient population and control group was found to be below 30 kg/m2.
Serum testosterone was found to be higher in patients with urolithiasis, consistent with the earlier conclusions of Naghii et al.[7] and Watson et al.[10] that serum total testosterone levels tend to be in higher range in urolithiatic patients than controls. In animal models of urolithiasis also extensive crystal deposition in renal tissues have been reported in male rats, testosterone administrated male and female rats, whereas few crystal deposits are reported in intact females, castrated females, castrated males and estradiol administrated males indicating that testosterone is a promoter and estradiol is an inhibitor of kidney crystal deposition because of their effect on plasma and urinary oxalate metabolism.[14,15,16]
Testosterone is reported to promote renal stone formation by suppressing renal osteopontin expression and increasing urinary oxalate excretion.[9] It tends to increase the hepatic levels of glycolic acid oxidase (GAO), an enzyme in metabolic pathway for urinary oxalate synthesis resulting in hyperoxaluria;[17] therefore, it is postulated that elevated levels of testosterone in urolithiatic patients may lead to increase in synthesis of GAO, resulting in increased excretion of oxalate in urine and so more incidences of urinary calcium oxalate stone formation. This was also evident in present patient population with more number of patients found with stones of calcium oxalate and mixed stones of calcium oxalate and phosphate confirming disturbed oxalate metabolism. Although in India more incidences of calcium oxalate stones are reported,[18] androgen screening should be ensured in these urolithiatic patients to reveal the one of underlying cause. This is again consistent with the findings that stone forming men excrete more calcium and oxalate (two important promoters of lithogenesis), while less citrate (an important inhibitor of lithogenesis) than women.[19,20] In one study, the low serum testosterone was observed in urolithiatic patients with metabolic syndrome,[11] but in this study, the urolithiatic patient with metabolic syndrome were excluded, so giving birth to hypothesis that metabolic syndrome along with urolithiasis may lead to lowering of serum testosterone levels. Similarly in another study, although no significant difference was found between serum levels of testosterone between the active stone formers (ASF) and control groups, serum testosterone was related to higher urinary excretion of uric acid in ASF patients and to higher urinary excretion of oxalate in the control group raising the possibility that testosterone may be involved in the pathogenesis of renal stones through higher urinary uric acid and oxalate.[8]
The free testosterone is considered as the biologically active form of hormone interacting at target tissue receptors,[21] whereas dihydrotestosterone, a more potent form produced from testosterone by cytosomal enzyme 5α-reductase, was found to be partially responsible for exaggerated hyperoxaluria in rat ethylene glycol model of urolithiasis.[22] In this study, increased levels of free testosterone and dihydrotestosterone were reported and elevation in the later was significant indicating the possible effect of more potent form of testosterone on stone formation in urolithiatic patients. Similar results were obtained by Nath et al. with higher serum free and total testosterone and 24 h of urinary oxalate in male stone formers with a positive correlation between serum testosterone and urinary oxalate.[23] In another case report also, the results of twice repeated estimation of testosterone, free testosterone, dihydrotestosterone, estradiol, and sex hormone binding globulin revealed hyperandrogenicity, indicating the association between serum testosterone and urolithiasis.[24]
The higher serum level of estradiol is considered a protective factor from stone formation as indicated in naturally postmenopausal women who suffer less from kidney calcium oxalate stones.[25] The higher levels of estradiol in this study indicated a higher rate of conversion of total testosterone into estradiol, but still they fail to protect from urolithiasis. The possible explanation may be the only decreased levels of testosterone along with higher estradiol acts as a protective measure for urolithiasis, which was not observed in this patient group. This is again supported by the findings that in women with polycystic ovary syndrome, hyperandrogenism triggers the urinary stone formation and is known to be a risk factor in the formation of urinary stone disease.[26]
In this study, elevated levels of serum total testosterone, free testosterone and dihydrotestosterone indicate a positive correlation between the frequency of stone formation and male sex hormones, indicating that these hormones might play a pivotal role in pathogenesis of stone formation either by up regulation of receptors, increased activity of these enzymes or by some other mechanism yet to be discovered. The exact mechanism for the possible role of elevated testosterone as one of the causative agent for urolithiasis is still to be explained, but the therapeutic approached can be aimed to decrease the levels of testosterone or its bioavailability to relieve the agony of urolithiatic patients.
CONCLUSION
Increase in serum testosterone appears to promote the formation of renal stone in a mechanism yet to be elucidated clearly. The increase in serum estradiol levels does not appear to have a protective role in urolithiatic males.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007;34:287–93. doi: 10.1016/j.ucl.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohri K, Kodama M, Ishikawa Y, Katayama Y, Takada M, Katoh Y, et al. Relationship between metabolic acidosis and calcium phosphate urinary stone formation in women. Int Urol Nephrol. 1991;23:307–16. doi: 10.1007/BF02549600. [DOI] [PubMed] [Google Scholar]
- 3.Travison TG, Araujo AB, Hall SA, McKinlay JB. Temporal trends in testosterone levels and treatment in older men. Curr Opin Endocrinol Diabetes Obes. 2009;16:211–7. doi: 10.1097/med.0b013e32832b6348. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Yamaguchi S, Kakizaki H, Yachiku S. Influence of estrus status on urinary chemical parameters related to urolithiasis. Urol Res. 2005;33:476–80. doi: 10.1007/s00240-005-0511-5. [DOI] [PubMed] [Google Scholar]
- 6.Li JY, Zhou T, Gao X, Xu C, Sun Y, Peng Y, et al. Testosterone and androgen receptor in human nephrolithiasis. J Urol. 2010;184:2360–3. doi: 10.1016/j.juro.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Naghii MR, Mofid M, Hedayati M, Khalagi K. Antioxidants inhibition of high plasma androgenic markers in the pathogenesis of ethylene glycol (EG)-induced nephrolithiasis in Wistar rats. Urolithiasis. 2014;42:97–103. doi: 10.1007/s00240-013-0620-5. [DOI] [PubMed] [Google Scholar]
- 8.Shakhssalim N, Gilani KR, Parvin M, Torbati PM, Kashi AH, Azadvari M, et al. An assessment of parathyroid hormone, calcitonin, 1,25 (OH) 2 vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol Res. 2011;39:1–7. doi: 10.1007/s00240-010-0276-3. [DOI] [PubMed] [Google Scholar]
- 9.Yagisawa T, Ito F, Osaka Y, Amano H, Kobayashi C, Toma H. The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J Urol. 2001;166:1078–82. [PubMed] [Google Scholar]
- 10.Watson JM, Shrewsberry AB, Taghechian S, Goodman M, Pattaras JG, Ritenour CW, et al. Serum testosterone may be associated with calcium oxalate urolithogenesis. J Endourol. 2010;24:1183–7. doi: 10.1089/end.2010.0113. [DOI] [PubMed] [Google Scholar]
- 11.Otunctemur A, Ozbek E, Cakir SS, Dursun M, Polat EC, Ozcan L, et al. Urolithiasis is associated with low serum testosterone levels in men. Arch Ital Urol Androl. 2015;87:83–6. doi: 10.4081/aiua.2015.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 13.Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The association of increasing body mass index and kidney stone disease. J Urol. 2010;183:571–5. doi: 10.1016/j.juro.2009.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshihara H, Yamaguchi S, Yachiku S. Effect of sex hormones on oxalate-synthesizing enzymes in male and female rat livers. J Urol. 1999;161:668–73. [PubMed] [Google Scholar]
- 15.Yoshioka I, Tsujihata M, Momohara C, Akanae W, Nonomura N, Okuyama A. Effect of sex hormones on crystal formation in a stone-forming rat model. Urology. 2010;75:907–13. doi: 10.1016/j.urology.2009.09.094. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Chandhoke PS, Grampsas SA. Role of sex hormones in experimental calcium oxalate nephrolithiasis. J Am Soc Nephrol. 1999;10(Suppl 14):S376–80. [PubMed] [Google Scholar]
- 17.Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–6. [PubMed] [Google Scholar]
- 18.Trinchieri A. Epidemiology of urolithiasis: An update. Clin Cases Miner Bone Metab. 2008;5:101–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int. 1986;30:85–90. doi: 10.1038/ki.1986.155. [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–8. doi: 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 21.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab. 2006;91:4326–34. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Glass MA, Chandhoke PS. Effect of castration and finasteride on urinary oxalate excretion in male rats. Urol Res. 1998;26:71–5. doi: 10.1007/s002400050026. [DOI] [PubMed] [Google Scholar]
- 23.Nath SJ, Sarma D, Bagchi PK, Baruah SK, Puthenveetil RT, Baruah SJ. The role of serum testosterone as a lithogenic factor and its correlation with stone and urine composition amongst male stone formers. UroToday Int J. 2013;6 [Art 37] [Google Scholar]
- 24.Naghii MR, Hedayati M. Determinant role of gonadal sex hormones in the pathogenesis of urolithiasis in a male subject – A document for male predominancy (case study) Endocr Regul. 2010;44:143–6. doi: 10.4149/endo_2010_04_143. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Mai Z, Ou L, Duan X, Zeng G. Serum estradiol and testosterone levels in kidney stones disease with and without calcium oxalate components in naturally postmenopausal women. PLoS One. 2013;8:e75513. doi: 10.1371/journal.pone.0075513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaygusuz I, Karatas OF, Kafali H, Cimentepe E, Unal D. Is polycystic ovarian syndrome a risk factor for urolithiasis? Urolithiasis. 2013;41:361–2. doi: 10.1007/s00240-013-0564-9. [DOI] [PubMed] [Google Scholar]


