Abstract
Background:
Hepatitis C virus (HCV) infection is common worldwide. The treatment typically involves a combination of interferon-alpha (IFN-α) and ribavirin (RBV) therapy; however, the use of IFN-α is well documented to be associated with thyroid disease, the most common autoimmune disorder associated with IFN-α.
Aim:
The aim of the present study was to know the prevalence of thyroid abnormality in the HCV-positive patients on IFN and antiviral therapy.
Materials and Methods:
Fifty known HCV positive patients were enrolled for the study. All the patients were on IFN (3 million unit subcutaneously 3 times/week) and antiviral therapy (oral RBV 1000–1200 mg/day). Thyroid function tests were performed first at the start of treatment and then after 12 weeks of treatment.
Results:
13 (26%) of the patients were found to develop hypothyroidism, and 1 (2%) patient developed hyperthyroidism in the course of 12 weeks therapy.
Conclusion:
HCV patients on IFN and antiviral therapy have an effect on the thyroid gland, so these patients should be regularly screened for thyroid disorders and appropriately treated to maintain euthyroid status.
Key words: Hepatitis C virus, hypothyroid, interferon therapy
INTRODUCTION
Hepatitis C virus (HCV) infection is one of the major epidemics afflicting young adults.[1] In some countries, HCV remains the most common chronic blood-born infection.[2,3] The effective management of the new cases is critically important because, without treatment, patients will develop chronic HCV infection, HCV-related cirrhosis, liver failure, and hepatocellular carcinoma. The treatment typically involves combined interferon-alpha (IFN-α) and ribavirin (RBV) therapy. This is an effective therapy with a “cure” rate of up to 70% depending on genotype as judged by the negative HCV ribonucleic acid (RNA) polymerase chain reaction detection.[4] IFNs are integral players in immunity, and a number of immune-mediated complications can arise during IFN therapy.[5] IFN-α can commonly induce thyroiditis, which is classified as either autoimmune or nonautoimmune IFN-induced thyroiditis (IIT). Subclinical thyroiditis occurs in 20–40% of and clinical thyroiditis in 5–10% of patients.[6] Autoimmune IIT manifests as Hashimoto's thyroiditis, which is defined by an emergence of or worsening of antithyroid antibody levels with or without hypothyroidism. In rare cases, autoimmune IIT also manifests as Graves' disease, which is defined by antithyroid antibodies with hyperthyroidism. Nonautoimmune IIT presents as destructive thyroiditis and hypothyroidism.[7]
IFN-α is a Type I IFN that has been widely used as a therapeutic agent mostly, for infectious and malignant diseases.[7] IFN-α binds to IFN receptors and activates various signaling pathways, including the JAK-STAT pathway, and the MAP kinase pathway leading to transcription of target proteins which mediate its immune and anti-tumor effects.[8,9,10] One of the most remarkable successes of IFN-α as a therapeutic agent has been in the treatment of chronic hepatitis C, where the combination of IFN-α and RBV induces remission in up to 50% of patients.[11] However, IFN-α therapy can cause numerous and wide-ranging side effects, including severe complications that can result in morbidity and discontinuation of therapy.[12] This study aimed at measuring the prevalence of Thyroid disease in HCV patients on treatment with IFN and antiviral drug RBV.
MATERIALS AND METHODS
This study was conducted in Departments of Biochemistry and Medicine of a Tertiary Care Hospital attached to a Medical College in North India. Fifty patients already diagnosed with HCV infection and on IFN therapy (3 MIU thrice a week dose) and antiviral therapy with RBV 1000–1200 mg daily were included in the study. Purposive sampling was performed depending on the number of HCV patients coming to medicine outpatient department. The study was conducted with prior approval by Institutional Ethics Committee.
Patients already on any kind of thyroid treatment; co-infection with human immunodeficiency virus or hepatitis B virus; pregnancy and lactation; concomitant serious medical illnesses such as malignancy, severe cardiopulmonary disease, or uncontrolled diabetes mellitus were excluded from the study. Similarly, patients with a history of any other drug affecting thyroid function (such as lithium, amiodarone and iodine preparations), patients with a history of radiation that can alter the levels of thyroid hormone were excluded.
All patients were evaluated clinically, hematologically, biochemically, and serologically at baseline. Routine biochemical (liver function test and renal function test) and hematological tests were performed using automated techniques. HCV antibodies were detected using third generation commercial enzyme-linked immune sorbent assay. The HCV RNA load was measured. Thyroid function tests, including serum thyrotropin (thyroid-stimulating hormone [TSH]), and free thyroxine (FT4), and free triiodothyronine (FT3) were performed by an ultrasensitive immune chemiluminescent noncompetitive assay (Access 2, Beckman Coulter). Thyroid disease was defined as any value of these markers (TSH, FT3, and FT4,) which was greater or less than the normal values. The reference range (RR) for TSH was 0.35–5.5 uIU/ml, FT4 0.61–1.12 ng/dl and FT3 was 2.5–3.90 pg/ml.
Statistical analysis
Statistical analysis was performed using SPSS software 20 (IBM). Quantitative variables were expressed as mean ± standard deviation as the data was normally distributed. The Karl Pearson coefficient was calculated and P < 0.05 was considered significant.
RESULTS
The mean age of patients was 45 ± 10 years. Mean baseline alanine transaminase levels were 147 ± 52 U/L [Table 1].
Table 1.
General baseline characteristics of patients

A total of 13 patients (26%) developed hypothyroidism after 12 weeks of treatment. Thyroid peroxidase antibodies were positive in 48% of these patients. One patient was diagnosed as hyperthyroid [Figure 1].
Figure 1.
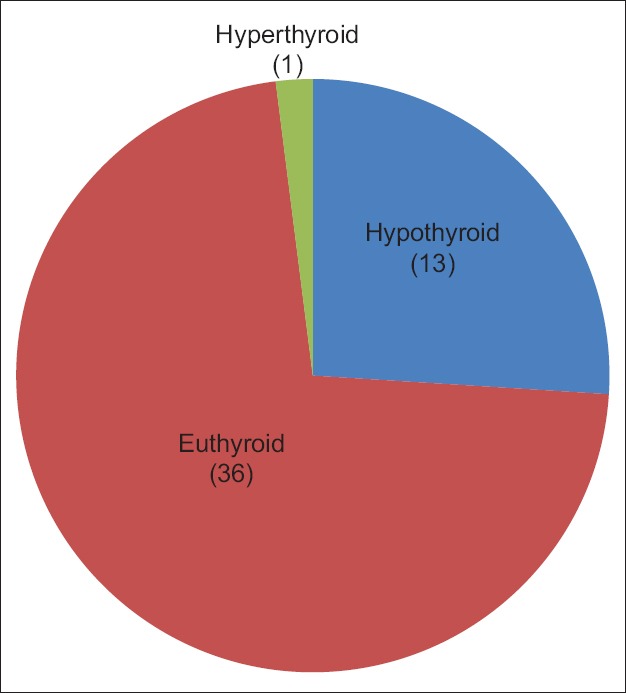
Thyroid disorders in hepatitis C virus patients after 12 weeks interferon and antiviral therapy
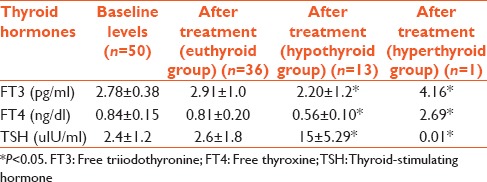
Thyroid profile was measured at baseline before the start of therapy, then at 12 weeks of treatment. The mean TSH levels were significantly higher in 26% hypothyroid patients as compared to euthyroid patients (P < 0.05) [Table 2].
Table 2.
Thyroid profile in patients on interferon and antiviral therapy for 12 weeks
DISCUSSION
Hepatitis C is a disease that is disseminated worldwide, and chronic infections affect up to 80% of the infected subjects.[13] Consequently, IFN-α therapy has frequently been used. IFN-α is one of a group of cytokines with antiviral, antiproliferative, and immunomodulatory properties. In cases of hepatitis C, the presence of moderate to severe necroinflammatory activity and/or moderate to severe fibrosis (as assessed by a liver biopsy) represent formal indications of IFN-α use. The drug is available in both conventional and pegylated forms and is frequently used in association with RBV an antiviral drug.[11,14] The treatment is continuous and lasts from 6 to 12 months, and retreatment may be necessary.
The use of IFN-α is well documented to be associated with thyroid disorder, the commonest autoimmune disorder associated with IFN-α therapy. Various pathophysiological processes that may be involved are – IFN therapy can precipitate immune-mediated abnormalities de novo or can exacerbate an existing autoimmune tendency;[15] IFN-α is thought to have a direct inhibitory effect on thyrocytes preventing hormonogenesis and secretion; Immunostimulation in the presence of hepatitis C infection. This is thought to include activation of lymphocytes and natural killer cells, increased production of tumor necrosis factor, IFN-α, Interleukins and other cytokines and increased production of immunoglobulins, all lead to the development of thyroid auto-antibodies with complete destruction and consequently permanent hypothyroidism in genetically susceptible individuals.[16]
When IFN-α is administered exogenously, another layer of complexity is added. It is possible but purely speculative that exogenous IFN-α synergizes with the endogenous source, thus exaggerating the effect on the thyroid thus causing additional hypothyroidism. Hashimoto's thyroiditis is diagnosed in up to 40% of patients, and hypothyroidism can be triggered by IFN-α.[17] Hypothyroidism frequently escapes diagnosis due to the overlap of its symptoms with those induced by IFN-α itself, such as fatigue, somnolence, and depression. Destructive thyroiditis represents a form of nonautoimmune IIT characterized by self-limited thyrotoxicosis with a triphasic evolution similar to that of subacute thyroiditis Destructive thyroiditis may recur upon re-treatment, and it is not necessary to discontinue IFN-α use.[6] The patient who has developed thyrotoxicosis in the study may be because of destructive thyroiditis.
Case reports and follow-up studies of large cohorts of patients on IFN therapy have confirmed that immune-mediated complications are uncommon but can occur in a number of different organ systems. IFN-α production is induced by specific autoantibody–nuclear antigen immune complexes and has a key role in the development and maintenance of autoimmunity. IFN therapy can precipitate immune-mediated abnormalities de novo or can exacerbate an existing autoimmune tendency. This is manifest in the rise in titer of existing antibodies and in the development of clinical disease in patients with preexisting antibodies.[5] IFN-α and RBV therapy induce thyroid dysfunction in chronic hepatitis C patients. There is no association between severity of disease and response to therapy with IFN-induced thyroid dysfunction. In a study 20 treated patients (18.69%) developed thyroid dysfunction with a relative risk (RR) of 11.25 and the attributable risk of 91%.[18] There is a wide range in the incidence of newly developed thyroid dysfunctions and thyroid antibodies in IFN-treated HCV patients. IFN a therapy alone or in combination with other drugs has different effects on the incidence of thyroid dysfunctions.[19]
CONCLUSION
HCV patients on IFN and antiviral therapy have an effect on the thyroid gland, so these patients should be regularly screened for thyroid disorders and appropriately treated to maintain euthyroid status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to residents and technical staff of Department of Biochemistry, GGS Medical College, Faridkot, Punjab, India.
REFERENCES
- 1.Yohannes K, Roche P, Blumer C, Spencer J, Milton A, Bunn C, et al. Australia's Notifiable Diseases Status, 2002 Annual Report of the National Notifiable Diseases Surveillance System. CDI. 2004;28(1):27–9. [PubMed] [Google Scholar]
- 2.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 3.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 4.Sievert W. An overview of antiviral therapy for chronic hepatitis C infection. In: Crofts N, Dore G, Locarnini S, editors. Hepatitis C: An Australian Perspective. Melbourne: IP Communications; 2001. pp. 140–54. [Google Scholar]
- 5.Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Curr Opin Rheumatol. 2007;19:61–6. doi: 10.1097/BOR.0b013e328010c547. [DOI] [PubMed] [Google Scholar]
- 6.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58:2489–99. [PubMed] [Google Scholar]
- 8.Parmar S, Platanias LC. Interferons: Mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–9. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Jonasch E, Haluska FG. Interferon in oncological practice: Review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 10.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenhaver DH, Niesel DW, Klimpel GR, et al. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–83. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 11.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 12.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711–9. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Ghinoi A, Rotondi M, et al. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16:563–72. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 14.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, et al. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158:1445–8. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 16.Chehadeh W, Weill J, Vantyghem MC, Alm G, Lefèbvre J, Wattré P, et al. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: Relationship with coxsackievirus B infection. J Infect Dis. 2000;181:1929–39. doi: 10.1086/315516. [DOI] [PubMed] [Google Scholar]
- 17.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: Toward a new classification. Hepatology. 2006;43:661–72. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 18.Nadeem A, Aslam M. Association of interferon-alpha and ribavirin-induced thyroid dysfunction with severity of disease and response to treatment in Pakistani Asian patients of chronic hepatitis C. Hepat Res Treat. 2012;2012:864315. doi: 10.1155/2012/864315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair Kesavachandran C, Haamann F, Nienhaus A. Frequency of thyroid dysfunctions during interferon alpha treatment of single and combination therapy in hepatitis C virus-infected patients: A systematic review based analysis. PLoS One. 2013;8:e55364. doi: 10.1371/journal.pone.0055364. [DOI] [PMC free article] [PubMed] [Google Scholar]


