Abstract
Complete agenesis of the dorsal pancreas (ADP) is an exceedingly rare congenital anomaly, compatible with life. The first case was reported in 1911 and so far around 100 cases have been reported in the world literature. Majority of the patients with this anomaly are asymptomatic or associated with abdominal pain, hyperglycemia, diabetes mellitus, and acute or chronic pancreatitis. We present a case report of a 34-year-old male with ADP, diagnosed incidentally during radiological evaluation for abdominal pain. Magnetic resonance cholangiopancreatography confirmed the absence of neck, body, and tail of the pancreas along with duct of Santorini and the minor duodenal papilla. Because of its rarity of occurrence, clinical awareness of the ADP can expand the differential diagnosis and improve patient management in pertinent light of the world literature.
Key words: Agenesis, computed tomography, dorsal pancreas, endoscopic retrograde cholangiopancreatography
INTRODUCTION
Complete agenesis of the dorsal pancreas (ADP) is an extremely rare entity, compatible with life into adulthood.[1] ADP is usually asymptomatic in majority of persons but differs with clinical manifestations such as epigastric pain, pancreatitis, hyperglycemia, and diabetes mellitus in a minority group.[2] Ultrasonographic imaging (USG), computed tomography (CT), and magnetic resonance (MR) are the primary investigations to establish the diagnosis of ADP, which can be confirmed by MR cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP), as it depicts the pancreatic ductal morphology.[3] Endoscopic ultrasound (EUS) is relatively a newer technique, which provides high-resolution images of pancreatic parenchyma and ductal system.[4] Majority of the cases were diagnosed incidentally during the workup for an unrelated abdominal problem or in the process of evaluation for abdominal pain. Till now, around 100 cases of dorsal pancreatic agenesis have been reported in the world literature. We present a rare case of complete ADP, accidentally diagnosed in a 34-year-old man during radiological evaluation for nonspecific abdominal pain.
CASE REPORT
A 34-year-old male from the Indian subcontinent was presented with right iliac fossa pain for the past 5 days. There was no history of nausea or vomiting, trauma, drug intake, alcoholism, or any other complaint. The patient had appendicectomy 8 years back for the same complaint. His family history revealed no early infant death or stillbirths or of recurrent infections. Physical examination revealed tenderness over right iliac fossa. Biochemical investigations showed a normal random serum glucose (81 mg/dL; reference value ≤140 mg/dL), near normal serum amylase (83 U/L; reference value 0–140 U/L), and slightly elevated serum pancreatic lipase levels (184 U/L; reference value 0–160 U/L), not consistent with pancreatitis or diabetes mellitus. USG revealed a normal-sized pancreatic head with normal contour and echotexture, with no parenchymal calcification or duct dilatation; however, body and tail of the pancreas could not be visualized, anterior to splenic vein and portal confluence [Figure 1a and b]. Abdominal CT and MR imaging examinations revealed only a partial visualization of the pancreas. The pancreatic head and the uncinate process were visualized with ill-defined margins with peripancreatic fat stranding, but the distal neck, body, and tail of the pancreas were absent [Figure 1c and d]. To confirm the diagnosis, MRCP was performed using thick slab heavily T2-weighted sequences. Thin slab techniques could not be performed as the patient cannot hold breath for longer periods. On MRCP, the dorsal pancreatic duct of Santorini and the minor duodenal papilla could not be visualized. The ventral pancreatic duct of Wirsung and the common bile duct were normal and clearly visualized [Figure 1e]. These findings were compatible with complete ADP, eliminating the need for ERCP. Since the patient was asymptomatic, he was advised to have low-fat diet and frequent blood sugar monitoring.
Figure 1.
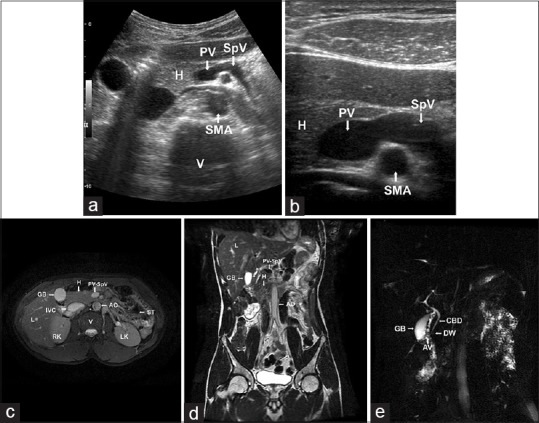
(a and b) Ultrasonographic image in transverse plane showing the head and uncinate process of the pancreas; the neck, body, and tail of the pancreas were absent anterior to portal confluence and splenic vein. H: Head of the pancreas; PV: Portal vein; SpV: Splenic vein; SMA: Superior mesenteric artery; V: Vertebra. (c) Axial T2-weighted magnetic resonance image showing normal head and uncinate process of the pancreas with the absence of neck, body, and tail of the pancreas. (d) Coronal T2-weighted magnetic resonance image showing normal head and uncinate process of the pancreas with the absence of neck, body, and tail of the pancreas. H: Head of the pancreas; PV-SpV: Portal vein-Splenic vein confluence; AO: Abdominal Aorta; IVC: Inferior vena cava; V: Vertebra; RK: Right Kidney; LK: Left Kidney; ST: Air-filled Stomach; L: Liver; GB: Gallbladder. (e) Maximum intensity projection image in magnetic resonance cholangiopancreatography showing common bile duct and duct of Wirsung. The duct of Santorini is not visualized. CBD: Common bile duct; DW: Duct of Wirsung; AV: Ampulla of Vater; GB: Gallbladder
DISCUSSION
Dorsal pancreatic agenesis is an extremely rare congenital anomaly; the first case was reported in 1911 during an autopsy.[5] Around 100 cases has been reported since 1911 till date.[6] ADP may be either complete or partial. In the complete agenesis, the neck, body, tail, duct of Santorini and minor duodenal papilla are absent. However, in partial agenesis, the body, duct of Santorini and minor duodenal papilla are retained. Partial agenesis of the pancreas or pancreatic hypoplasia occurs approximately 1–2/10,000 patients and is often associated with other congenital malformations.[7] Abnormal embryogenesis can lead to the developmental failure of the dorsal pancreas, resulting in complete ADP.[1,2,5] ADP primarily originates from a defect in the early embryogenesis which may usually be associated with other congenital anomalies such as heterotaxy, polysplenia syndrome, ectopic spleen, bowel malrotation, horseshoe kidney, coarctation of the aorta, Fallot's tetralogy, and ventricular septal defects.[8] Primary dysgenesis and ischemic injury to the developing pancreas could also play a role in the etiology of development of ADP. Familial transmission has been reported in the literature.[8] Moreover, the retinoic acid (Raldh) and hedgehog (Hh) gene signaling pathways have also been shown to play a role in the pathogenesis of ADP.[9]
Most of the patients with ADP are asymptomatic; however, if symptomatic, then majority of cases present with epigastric pain. In most cases, the pain is localized to epigastric region, which is aggravated following meals.[6,10] About 50% of the affected individuals present with diabetes mellitus. The abdominal pain is more common in partial agenesis while diabetes mellitus is seen commonly in complete agenesis. In the long duration, the majority of ADP patients developed recurrent or intermittent pancreatitis. Some patients also reported steatorrhea and signs of pancreatic exocrine failure.[11] The differential diagnosis of ADP includes that of pseudoagenesis, carcinoma of the head of pancreas, pancreas divisum, pancreatic pseudolipodystrophy, pancreatic tumors, and distal pancreatic lipomatosis.[7,12] With the recent advancement in the newer imaging techniques, the diagnosis of ADP seems to be a total reality. USG is the primary imaging study, but USG finding looks to be suspicious in some patients due to organ screen and the overlying bowel gas shadows. Three-dimensional (3D) reconstruction CT is a better technique to diagnose ADP because the blood supply to viscera can be visualized in this method. In the absence of dorsal pancreas, the distal pancreatic bed is filled with stomach or intestine (dependent stomach or dependent intestine signs), which abut splenic vein.[12] MRCP is the choice of imaging technique, for confirmation of ADP as it is a noninvasive procedure. Its diagnostic accuracy can be improved with 3D reconstruction or dynamically with secretin injection.[10,12,13] ERCP is considered to be the gold standard investigation for detailed description and evaluation of the biliary and pancreatic tree because of its superior spatial resolution. However, it is an invasive procedure, carrying radiation exposure and with morbidity risk due to pancreatitis caused by catheterization of the minor duodenal papillae. The use of S-MRCP (with secretin injection) allows for a greater diagnostic accuracy, has no associated complications, and avoids risks of ERCP.[5] EUS is a relatively new minimally invasive imaging technique which provides direct visualization of the total pancreatic parenchyma and the pancreatic ductal system. It also provides an opportunity to take fine needle aspiration cytology and may be as good as ERCP.[14]
In our case study, ADP was diagnosed during evaluation of the patient for right iliac fossa tenderness. A diagnosis of stump appendicitis was made and he was managed conservatively, with antibiotics and analgesics, and he was free of symptoms within a fortnight. Given by its rare occurrence, our case report with its unusual presentation adds up to the existing knowledge in terms of better understanding of the disease process and patient's management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors sincerely acknowledge Dr. Ajith TA, Dr. Sugathan KR, Smt. Sindhu CD, Mr. Mathew PP, and Mr. Suresh PV of Amala Institute of Medical Sciences, Thrissur, Kerala, India, for their wholehearted cooperation and timely help rendered during the preparation of this case report.
REFERENCES
- 1.Schulte SJ. Embryology, normal variation, and congenital anomalies of pancreas. In: Freeny PC, Stevenson OW, editors. Margulis and Burhenne's Alimentary Tract Radiology. 5th ed. St. Louis: Mosby-Yearbook; 1994. pp. 1039–49. [Google Scholar]
- 2.Fukuoka K, Ajiki T, Yamamoto M, Fujiwara H, Onoyama H, Fujita T, et al. Complete agenesis of the dorsal pancreas. J Hepatobiliary Pancreat Surg. 1999;6:94–7. doi: 10.1007/s005340050090. [DOI] [PubMed] [Google Scholar]
- 3.Macari M, Giovanniello G, Blair L, Krinsky G. Diagnosis of agenesis of the dorsal pancreas with MR pancreatography. AJR Am J Roentgenol. 1998;170:144–6. doi: 10.2214/ajr.170.1.9423620. [DOI] [PubMed] [Google Scholar]
- 4.Gold RP. Agenesis and pseudo-agenesis of the dorsal pancreas. Abdom Imaging. 1993;18:141–4. doi: 10.1007/BF00198051. [DOI] [PubMed] [Google Scholar]
- 5.Schnedl WJ, Piswanger-Soelkner C, Wallner SJ, Reittner P, Krause R, Lipp RW, et al. Agenesis of the dorsal pancreas and associated diseases. Dig Dis Sci. 2009;54:481–7. doi: 10.1007/s10620-008-0370-3. [DOI] [PubMed] [Google Scholar]
- 6.Mohapatra M, Mishra S, Dalai PC, Acharya SD, Nahak B, Ibrarullah M, et al. Imaging findings in agenesis of the dorsal pancreas. Report of three cases. JOP. 2012;13:108–14. [PubMed] [Google Scholar]
- 7.Rastogi R, Kumar R, Bhargava S, Rastogi V. Isolated pancreatic hypoplasia: A rare but significant radiological finding. Saudi J Gastroenterol. 2009;15:289–90. doi: 10.4103/1319-3767.56090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Vyas K, Agrahari NS, Kundu J. Complete agenesis of dorsal pancreas – A rare congenital anomaly: Case presentation with imaging findings and review of literature. Pancreat Disord Ther. 2015;5:150. doi: 10.4314/mmj.v27i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittenhouse DW, Kennedy EP, Mascaro AA, Brumbaugh JL, Stein LH, Rosenberger LH, et al. The novel triad of dorsal agenesis of the pancreas with concurrent pancreatic ductal adenocarcinoma and nonalcoholic chronic calcific pancreatitis: A case series and review of the literature. J Gastrointest Surg. 2011;15:1643–9. doi: 10.1007/s11605-011-1542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uygur-Bayramiçli O, Dabak R, Kiliçoglu G, Dolapçioglu C, Oztas D. Dorsal pancreatic agenesis. JOP. 2007;8:450–2. [PubMed] [Google Scholar]
- 11.Fadell M, II, Elsamaloty H, Barak R. Agenesis of the dorsal pancreas. A case report. Appl Radiol. 2010;39:62–3. [Google Scholar]
- 12.Karcaaltincaba M. CT differentiation of distal pancreas fat replacement and distal pancreas agenesis. Surg Radiol Anat. 2006;28:637–41. doi: 10.1007/s00276-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 13.Nicaise N, Pellet O, Metens T, Devière J, Braudé P, Struyven J, et al. Magnetic resonance cholangiopancreatography: Interest of IV secretin administration in the evaluation of pancreatic ducts. Eur Radiol. 1998;8:16–22. doi: 10.1007/s003300050330. [DOI] [PubMed] [Google Scholar]
- 14.Kahl S, Glasbrenner B, Zimmermann S, Malfertheiner P. Endoscopic ultrasound in pancreatic diseases. Dig Dis. 2002;20:120–6. doi: 10.1159/000067481. [DOI] [PubMed] [Google Scholar]


