Abstract
Background:
Hyponatremia has long been recognized as a potentially serious metabolic consequence of tuberculous meningitis (TBM) occurring in 35–65% of children with the disease. The syndrome of inappropriate antidiuretic hormone (SIADH) secretion has for long been believed to be responsible for the majority of cases of hyponatremia in TBM. Cerebral salt wasting syndrome (CSWS) is being increasingly reported as a cause of hyponatremia in some of these children.
Aim:
This study was done to determine the frequency and causes of hyponatremia in children with TBM.
Methods:
Children with newly diagnosed TBM admitted over a 2-year period (January 2009 to December 2010) were included. All patients received anti-tubercular therapy, mannitol for cerebral edema, and steroids. Patients were monitored for body weight, urine output, signs of dehydration, serum electrolytes, blood urea nitrogen, serum creatinine, and urinary sodium. Hyponatremia was diagnosed if the serum sodium was <135 mEq/L. CSWS was diagnosed if there was evidence of excessive urine output, volume depletion, and natriuresis in the presence of hyponatremia. The outcome in terms of survival or death was recorded.
Results:
Twenty-nine of 75 children (38.7%) with TBM developed hyponatremia during their hospital stay. In 19 patients, hyponatremia subsided after the discontinuation of mannitol. Ten patients with persistent hyponatremia had CSWS. There were no patients with SIADH.
Conclusions:
CSWS is an important cause of hyponatremia in children with newly diagnosed TBM. In our patients, it was more commonly seen than SIADH.
Key words: Cerebral salt wasting syndrome, hyponatremia, tuberculosis meningeal
Introduction
As per the World Health Organization Global Tuberculosis Report 2014, an estimated 550,000 new cases of tuberculosis occurred in HIV-negative children in 2013, of which there were 80,000 deaths.[1] Central nervous system (CNS) tuberculosis carries a high mortality and neurological morbidity and disproportionately afflicts children.[2] Hyponatremia occurs in 35–65% of patients with tuberculous meningitis (TBM)[3,4,5] and is an independent predictor of death or severe disability.[6] Hyponatremia may be due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion, cerebral salt wasting syndrome (CSWS), excessive fluid administration in patients with impaired thirst, diuretic therapy such as mannitol, and treatment of transient/permanent diabetes insipidus.
Diagnosing the cause of hyponatremia in patients with acute cerebral insults can be challenging. CSWS is a disease of sodium and water handling that occurs as a result of cerebral disease in the setting of normal renal function. It is characterized by hyponatremia with hypovolemia. The typical features of SIADH, on the other hand, result from excessive retention of water with a concomitant decrease in extracellular fluid osmolality. In TBM, plasma ADH may be secreted as a response to raised intracranial pressure in an effort to raise the mean arterial pressure and maintain cerebral perfusion pressure.
Distinguishing CSWS from SIADH can be difficult but is crucial because treatment of the two is fundamentally different. While fluid restriction is the treatment of choice in SIADH, the treatment of CSWS consists of vigorous sodium and water replacement.
We undertook this study to determine the frequency and causes of hyponatremia in patients with TBM.
Methods
This was a prospective hospital-based cohort study carried out over a 2-year period (January 2009 to December 2010) at the Lokmanya Tilak Municipal Medical College Hospital, Mumbai, India. All newly diagnosed patients of TBM admitted to the pediatric Intensive Care Unit and wards were included. Patients with TBM were excluded from the study if they had received mannitol or 3% saline elsewhere. Patients with TBM readmitted for shunt problems or drug-induced hepatitis were also excluded from the study. A detailed history was taken in all patients including a history of contact with tuberculosis and of receiving Bacille Calmette–Guérin vaccine. Lumbar puncture was done on all patients after fundus examination. Cerebrospinal fluid (CSF) was examined for total and differential cell count and protein and sugar. CSF smear was examined for acid-fast bacilli (AFB). CSF was also sent for AFB and bacterial culture. Mantoux test, chest X-ray, and computerized tomography scan of the brain were done in each patient.
Diagnosis
Patients were classified as definite, probable, or possible cases of TBM as per the uniform case definition for use in clinical research proposed by Marais et al.[7] TBM was staged using the modified criteria of the British Medical Research Council[8] to determine the severity of TBM: Stage 1 TBM (Glasgow coma scale [GCS] 15 prodromal with no neurologic symptoms), Stage 2 TBM (GCS 11–14 or GCS of 15 with focal neurologic deficit), and Stage 3 TBM (GCS <11).[8]
Treatment
We started antitubercular therapy in all patients with isoniazide, rifampicin, pyrazinamide, and ethambutol as per the National Guidelines for Diagnosis and Treatment of Pediatric Tuberculosis.[9] All patients received prednisolone orally at a dose of 2 mg/kg/day in divided doses for 4–6 weeks, followed by a tapering dose over the next 2 weeks. Intravenous mannitol 12.5% was used for the treatment of cerebral edema. As per the protocol, all patients received intravenous 0.9% saline with 5% dextrose (with added potassium) as the maintenance fluid.
In addition to neurological status, all patients were monitored for body weight, urine output, signs of dehydration, serum electrolytes, blood urea nitrogen (BUN), serum creatinine, and urinary sodium. A central venous line was placed if volume status could not be determined clinically (in 22 of the 75 patients). Hyponatremia was diagnosed if the serum sodium was <135 mEq/L.
In patients with hyponatremia, mannitol was discontinued if in use. A diagnosis of CSWS was made if there was persistent hyponatremia, 48 h after discontinuation of mannitol, with clinical signs of dehydration or central venous pressure (CVP) <5 cm of water, low blood pressure, urine output >4 ml/kg/h, and urinary sodium >40 mmol/L. SIADH was diagnosed if there was hyponatremia with no signs of dehydration or CVP >6 cm of water, normal blood pressure, urine output 2 ml/kg/h or less, and urinary sodium >40 mmol/L.
In patients with CSWS, we restored intravascular volume with normal saline. Hyponatremia was corrected using 3% saline. In patients with hyponatremia, serum sodium was monitored six hourly (or more frequently if indicated). Any subsequent value of serum sodium of <135 mEq/L was corrected again with 3% saline. Fludrocortisone was started at a dose of 0.1–0.2 mg twice a day in patients requiring more than one correction per day. It was weaned and stopped over 3 days if serum sodium was normal and urine output had dropped to 2 ml/kg/h or less. Potassium supplements were given to patients on fludrocortisone who developed hypokalemia. All patients were followed up till discharge or death.
Demographic data, clinical and laboratory features, course, and outcome (survival or death) were entered on a case record form. Patients were included in the study after taking signed informed consent from the parent or guardian. The study was approved by the Institutional Ethics Committee.
Results
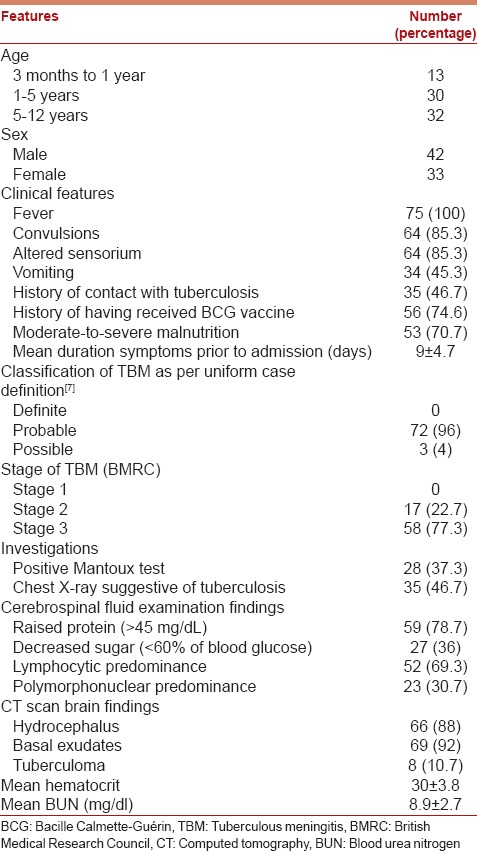
Seventy-five of 102 patients with TBM seen during the study period fulfilled the inclusion criteria. Table 1 gives the clinical profile of these children: 57.33% were <5 years of age. Though CSF was stained for AFB, and AFB culture was done in all patients, there were no positive smears or cultures. Bacterial cultures of CSF were sterile in all the patients. Facilities for nucleic acid amplification test were not available in our hospital. Hence, as per the “uniform case definition of TBM for use in clinical research” proposed by Marais et al.,[7] we had no “definite” cases of TBM. No patients with Stage 1 TBM were seen. Ours is a tertiary referral hospital and hence we tend to see patients with more severe disease. This may also reflect a delay in diagnosis. There was no HIV-positive patient in this cohort.
Table 1.
Profile of children admitted with newly diagnosed tuberculous meningitis
Twenty-nine patients developed hyponatremia within 1–7 days of admission. Table 2 gives the etiology of hyponatremia in these patients. In children with hyponatremia, the lowest values recorded for serum sodium ranged from 114 to 125 mEq/L. In patients with CSWS, though there was much fluctuation in weights on a day-to-day basis, there was a net weight gain ranging from 7% to 13% between weights at onset and after resolution of hyponatremia.
Table 2.
Etiology of hyponatremia in children admitted with newly diagnosed tuberculous meningitis (n=29)
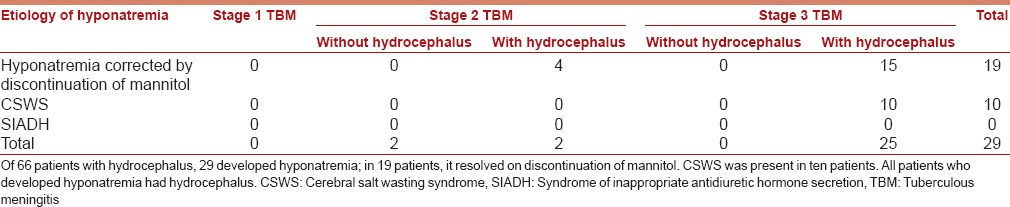
Twenty-nine of 66 patients with hydrocephalus developed hyponatremia. All patients with hyponatremia had had Stage 2 or 3 TBM with hydrocephalus. In 19 patients, hyponatremia resolved within 48 h of discontinuation of mannitol. Ten patients with persistent hyponatremia (beyond 48 h of discontinuation of mannitol) had CSWS. There were no patients with SIADH.
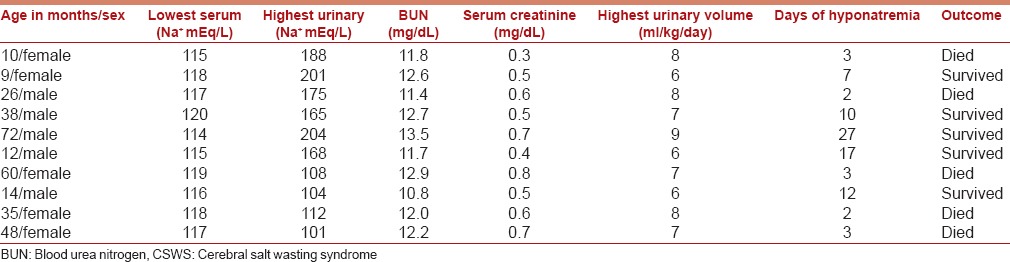
The laboratory features of children with CSWS are summarized in Table 3. Clinical features in patients with CSWS (n = 10) were low blood pressure (n = 10), prolonged capillary refill time (n = 5), and loss of skin turgor (n = 8). CVP was decreased in 6 of 22 patients, in whom CVP monitoring was done, all with CSWS.
Table 3.
Features of children with cerebral salt wasting syndrome
Discussion
Twenty-nine of seventy-five patients with TBM and hydrocephalus developed hyponatremia. Nineteen of these responded to discontinuation of mannitol with serum sodium returning to normal within 48 h. Ten of our twenty-nine patients had persistent hyponatremia which was secondary to CSWS. All of these patients had hydrocephalus and required emergency shunt surgery within 2–4 days of admission. There was no patient with SIADH. Mortality in patients with hyponatremia was 51.72% as compared with 13.04% in patients without hyponatremia (relative risk of 3.97).
Hyponatremia in the neurologic patients may be caused by various factors such as use of diuretics and hypotonic solutions and comorbid factors such as diarrhea. These should be promptly identified and addressed as soon as possible. None of our patients had diarrhea. As per the protocol, we do not use hypotonic fluids for maintenance therapy in any patient. All patients received 0.9% saline with 5% dextrose (with added potassium).
Mannitol is an osmotic diuretic and causes hyponatremia secondary to an increase in plasma osmolality leading to extracellular fluid expansion, inhibition of renal sodium absorption and natriuresis. CSWS or SIADH was considered in the diagnosis only 48 h after discontinuation of mannitol, if there was persistent hyponatremia.
Two main mechanisms of hyponatremia, SIADH and CSW syndrome, account for hyponatremia in patients with neurologic diseases. In 1957, Schwartz et al. described the SIADH secretion.[10] They observed patients with chronic hyponatremia and urinary wasting of sodium and speculated that the cause was release of ADH in amounts inappropriate to the prevailing low-circulating sodium level. Seven years earlier, Peters et al. had coined the term CSWS to denote patients with intracranial disease, who developed extracellular fluid volume depletion due to a renal sodium transport abnormality.[11] However, CSWS as a cause of hyponatremia was abandoned after 1957 in favor of the newly described SIADH. It has become evident, however, that inappropriate antidiuresis is less common than assumed from previous studies.[12,13,14,15,16]
The mechanisms by which intracranial disorders lead to renal salt wasting are poorly understood. Two possible mechanisms have been suggested.[12,13,14] The first mechanism suggested is that impaired sympathetic neural input causes a reduction in proximal sodium and urate re-absorption and the impaired release of renin and aldosterone. The second theory is that there is release of a circulating factor - B-type natriuretic peptide, once known as “brain natriuretic peptide” that impairs renal tubular sodium re-absorption in patients with brain injury. More recent observations suggest that adrenomedullin, a further natriuretic factor, might mediate renal salt wasting.[17]
Sodium imbalance has been reported in 21–66% of the patients with acute CNS infections.[4,5,18,19,20,21] Earlier authors have studied hyponatremia in patients with TBM and attributed it to SIADH.[4,18] However, their studies did not consider the effective blood volume and urine output, and hence CSWS was not considered at all. Ten of our twenty-nine patients with hyponatremia had CSWS. All of these patients were admitted in Stage 3 TBM and all had hydrocephalus requiring shunt surgery. There were no patients with SIADH. There are numerous case reports which suggest that CSWS is a more common cause of hyponatremia than SIADH in patients with TBM.[22,23,24,25,26,27]
It is important to distinguish between CSWS and SIADH since the treatment regimens in the two are quite different. CSWS has to be treated with vigorous fluid and sodium replacement, whereas in SIADH, fluid restriction is required. Both disorders are commonly associated with neurologic diseases and there may be overlapping laboratory findings. Volume status tends to be normal or slightly increased in SIADH, whereas it is decreased in CSWS.
Laboratory findings reflecting hemoconcentration such as elevated hematocrit and BUN levels can provide further support for the diagnosis of CSWS. Fifty-three of our seventy-five (70.6%) patients with TBM had moderate-to-severe malnutrition and this could explain the mean BUN of 8.9 ± 2.4 mg/dL. The mean BUN in patients with CSWS was 12.16 ± 0.79 mg/dL, and though higher, it was still in the normal range. Since the premorbid values of BUN in these patients were not available, it was difficult to determine whether they reflected hemoconcentration in a given patient. Hence, the only helpful measures in differentiating SIADH from CSWS in our study were the volume status and urinary output.
The overall mortality was 28% in our study. Mortality in children with hyponatremia was 51.72% as against 13.04% in those without hyponatremia. Hyponatremia has been reported to be associated with a 1.5-fold higher in-hospital mortality rate than patients without hyponatremia.[28]
In the past, restriction of fluids was recommended in patients with CNS disease. This was based on the belief that SIADH was the cause of hyponatremia and this could be prevented or corrected by fluid restriction.[4,19,29] In bacterial/TBM, the prevalence of hyponatremia, hypo-osmolality, and SIADH has varied widely, and nonosmotic stimuli for the secretion of ADH such as raised intracranial pressure and hypovolemia have been shown to be present in most patients.[4,5,19] There is no evidence that fluid restriction reduces the cerebral edema in meningitis. Fluid therapy in acute CNS infections should aim at avoiding hypo-osmolality based on the assumptions that ADH is increased by nonosmotic stimuli, and elevated ADH is less detrimental for cerebral edema than severe hypo-osmolality.[29] Hypovolemia should also be addressed aggressively since it can result in decreased cerebral perfusion in these patients.[30]
None of our patients had a confirmed diagnosis of TBM, and this is a serious limitation of this study. Furthermore, the number of patients with CSWS was small, thus limiting generalization. In addition, we had no long-term follow-up, especially neurological evaluation in survivors.
Conclusion
However, hyponatremia is a serious metabolic consequence of TBM. CSWS and not SIADH was seen in our patients. It is important to monitor urine output, serum and urine electrolytes (especially sodium) in all patients with TBM. Distinguishing between CSWS and SIADH is critical in these patients since treatment of the two conditions is radically different.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Sandhya Kamath, Dean of our institution, for permitting us to send this manuscript for publication.
References
- 1.Global Tuberculosis Report. 2013. [Last accessed on 2013 Dec 12]. Available from: http://www.who.int/tb/publications/global_report/en/
- 2.Girgis NI, Sultan Y, Farid Z, Mansour MM, Erian MW, Hanna LS, et al. Tuberculosis meningitis, Abbassia Fever Hospital-naval medical research unit no 3-Cairo, Egypt, from 1976 to 1996. Am J Trop Med Hyg. 1998;58:28–34. doi: 10.4269/ajtmh.1998.58.28. [DOI] [PubMed] [Google Scholar]
- 3.Christopher R, Gourie-Devi M. The syndrome of inappropriate antidiuretic hormone secretion in tuberculous meningitis. J Assoc Physicians India. 1997;45:933–5. [Google Scholar]
- 4.Singh BS, Patwari AK, Deb M. Serum sodium and osmolal changes in tuberculous meningitis. Indian Pediatr. 1994;31:1345–50. [PubMed] [Google Scholar]
- 5.Narotam PK, Kemp M, Buck R, Gouws E, van Dellen JR, Bhoola KD. Hyponatremic natriuretic syndrome in tuberculous meningitis: The probable role of atrial natriuretic peptide. Neurosurgery. 1994;34:982–8. doi: 10.1227/00006123-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gujjar AR. Sodium dysregulation and infections of central nervous system. Ann Indian Acad Neurol. 2003;6:253–8. [Google Scholar]
- 7.Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 8.British Medical Research Council. Streptomycin treatment of tuberculous meningitis. Br Med J. 1948;1:582–97. [Google Scholar]
- 9.National Guidelines for Diagnosis and Treatment of Pediatric Tuberculosis. [Last accessed on 2010 Dec 05]. Available from: http://www.tbcindia.nic.in/pdfs/PediatricGuidelinesFinal.pdf .
- 10.Schwartz WB, Bennett W, Curelop S, Bartter FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957;23:529–42. doi: 10.1016/0002-9343(57)90224-3. [DOI] [PubMed] [Google Scholar]
- 11.Peters JP, Welt LG, Sims EA, Orloff J, Needham J. A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians. 1950;63:57–64. [PubMed] [Google Scholar]
- 12.Albanese A, Hindmarsh P, Stanhope R. Management of hyponatraemia in patients with acute cerebral insults. Arch Dis Child. 2001;85:246–51. doi: 10.1136/adc.85.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brimioulle S, Orellana-Jimenez C, Aminian A, Vincent JL. Hyponatremia in neurological patients: Cerebral salt wasting versus inappropriate antidiuretic hormone secretion. Intensive Care Med. 2008;34:125–31. doi: 10.1007/s00134-007-0905-7. [DOI] [PubMed] [Google Scholar]
- 14.Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182–7. doi: 10.1016/s1043-2760(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 15.Powell KR, Sugarman LI, Eskenazi AE, Woodin KA, Kays MA, McCormick KL, et al. Normalization of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J Pediatr. 1990;117:515–22. doi: 10.1016/s0022-3476(05)80682-1. [DOI] [PubMed] [Google Scholar]
- 16.Bettinelli A, Longoni L, Tammaro F, Faré PB, Garzoni L, Bianchetti MG. Renal salt-wasting syndrome in children with intracranial disorders. Pediatr Nephrol. 2012;27:733–9. doi: 10.1007/s00467-011-2093-5. [DOI] [PubMed] [Google Scholar]
- 17.Yee AH, Burns JD, Wijdicks EF. Cerebral salt wasting: Pathophysiology, diagnosis, and treatment. Neurosurg Clin N Am. 2010;21:339–52. doi: 10.1016/j.nec.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Menon RK, Sharma V, Siddiqui HH, Bhide NK, Ghai OP. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in children with meningoencephalitis. Indian J Med Res. 1983;77:373–6. [PubMed] [Google Scholar]
- 19.Patwari AK, Singh BS, Manorama DE. Inappropriate secretion of antidiuretic hormone in acute bacterial meningitis. Ann Trop Paediatr. 1995;15:179–83. doi: 10.1080/02724936.1995.11747769. [DOI] [PubMed] [Google Scholar]
- 20.von Vigier RO, Colombo SM, Stoffel PB, Meregalli P, Truttmann AC, Bianchetti MG. Circulating sodium in acute meningitis. Am J Nephrol. 2001;21:87–90. doi: 10.1159/000046229. [DOI] [PubMed] [Google Scholar]
- 21.Karandanis D, Shulman JA. Recent survey of infectious meningitis in adults: Review of laboratory findings in bacterial, tuberculous, and aseptic meningitis. South Med J. 1976;69:449–57. doi: 10.1097/00007611-197604000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Celik US, Alabaz D, Yildizdas D, Alhan E, Kocabas E, Ulutan S. Cerebral salt wasting in tuberculous meningitis: Treatment with fludrocortisone. Ann Trop Paediatr. 2005;25:297–302. doi: 10.1179/146532805X72458. [DOI] [PubMed] [Google Scholar]
- 23.Ravishankar B, Mangala, Prakash GK, Shetty KJ, Ballal HS. Cerebral salt wasting syndrome in a patient with tuberculous meningitis. J Assoc Physicians India. 2006;54:403–4. [PubMed] [Google Scholar]
- 24.Huang SM, Chen CC, Chiu PC, Cheng MF, Chiu CL, Hsieh KS. Tuberculous meningitis complicated with hydrocephalus and cerebral salt wasting syndrome in a three-year-old boy. Pediatr Infect Dis J. 2004;23:884–6. doi: 10.1097/01.inf.0000137567.04914.47. [DOI] [PubMed] [Google Scholar]
- 25.Dass R, Nagaraj R, Murlidharan J, Singhi S. Hyponatraemia and hypovolemic shock with tuberculous meningitis. Indian J Pediatr. 2003;70:995–7. doi: 10.1007/BF02723828. [DOI] [PubMed] [Google Scholar]
- 26.Loo KL, Ramachandran R, Abdullah BJ, Chow SK, Goh EM, Yeap SS. Cerebral infarction and cerebral salt wasting syndrome in a patient with tuberculous meningoencephalitis. Southeast Asian J Trop Med Public Health. 2003;34:636–40. [PubMed] [Google Scholar]
- 27.Nagotkar L, Shanbag P, Dasarwar N. Cerebral salt wasting syndrome following neurosurgical intervention in tuberculous meningitis. Indian Pediatr. 2008;45:598–601. [PubMed] [Google Scholar]
- 28.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–65. doi: 10.1016/j.amjmed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laine J, Holmberg C, Anttila M, Peltola H, Perheentupa J. Types of fluid disorder in children with bacterial meningitis. Acta Paediatr Scand. 1991;80:1031–6. doi: 10.1111/j.1651-2227.1991.tb11779.x. [DOI] [PubMed] [Google Scholar]
- 30.Møller K, Larsen FS, Bie P, Skinhøj P. The syndrome of inappropriate secretion of antidiuretic hormone and fluid restriction in meningitis – How strong is the evidence? Scand J Infect Dis. 2001;33:13–26. doi: 10.1080/003655401750064022. [DOI] [PubMed] [Google Scholar]


