Abstract
Dyke–Davidoff–Masson syndrome (DDMS) is an uncommon condition, in which the diagnosis is mainly done by various clinical presentations along with positive radiological findings. Patients have facial asymmetry, seizures, learning difficulties, and contralateral hemiparesis. The radiological discoveries of the same incorporate cerebral hemiatrophy with homolateral hypertrophy of the skull and sinuses. Here, we report a case of a 10-year-old female child who presented with a single episode of convulsion, mental retardation, and contralateral hemiparesis. Magnetic resonance imaging of the brain showed unilateral atrophy of the left cerebral hemisphere with dilatation of ipsilateral lateral ventricle and ipsilateral sulcal prominence. These findings were suggestive of the diagnosis of DDMS.
Key words: Cerebral hemiatrophy, Dyke–Davidoff–Masson syndrome, hemiparesis
Introduction
Dyke–Davidoff–Masson syndrome (DDMS) is described as skull radiographic and pneumatoencephalographic changes in their series of nine patients whose clinical characteristics included hemiparesis, seizures, facial asymmetry, and mental retardation which was proposed in 1933 by Dyke et al.[1,2] DDMS is occasionally seen in clinical practice. It has been reported that DDMS is caused by cerebral insult that may occur in utero when the maturation of calvarium has not been completed, or during early life due to brain damage (usually traumatic). The clinical findings may be of variable degree according to the extent of the brain injury.[3] Here, we describe a 10-year-old female patient who presented to us with a single episode of focal convulsion, mental retardation, hemiparesis, and characteristic radiologic findings suggestive of DDMS.
Case Report
A 10-year-old female child was brought for deteriorated mental status and a history of single episode of the right focal convulsion with secondary generalization 2½ years back. There was no history of head trauma. The convulsion was followed by decreased movement of the right side of the body and drooling of saliva from the right side occasionally, for which the patient was given some native medication.
Birth history was uneventful. However, her sibling had a history of generalized tonic-clonic convulsions, for which she was taking some antiepileptic drug for 2 years. The patient did not attend school. On examination, the child had very poor cognitive function and was undernourished. Vitals were normal. Positive findings were – microcephaly – severe mental retardation with an intelligence quotient of 40, right-sided facial palsy, and sandal gap in both the lower limbs. Sandal gap has not been a positive finding in any of the other reported cases of DDMS. Examination of the central nervous system revealed hemiparesis on the right side. Examination of other systems was unremarkable.
Complete blood count revealed moderate anemia. Renal and liver function tests were normal. Erythrocyte sedimentation rate was elevated at 70 mm/h. An X-ray of paranasal sinuses was done which was normal. Magnetic resonance imaging (MRI) was done which suggested unilateral atrophy of the left cerebral hemisphere with dilatation of ipsilateral lateral ventricle and ipsilateral sulcul prominence [Figures 1 and 2]. There was subtle thickening of the left hemicranium noted, measuring 4.0 mm on the right side and 6–7 mm on the left temporal lobes. There was midline shift of 4 mm ipsilaterally. There was also mild atrophy of the left cerebral peduncle. These clinical and radiological findings are suggestive of DDMS.
Figure 1.
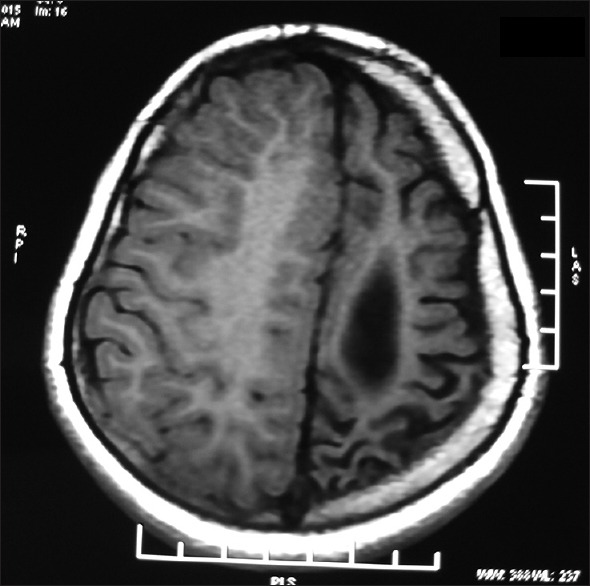
Magnetic resonance imaging of the brain showing diffuse atrophy of the left cerebral hemisphere with dilatation of the left lateral ventricle and prominence of sulci over the left cerebral hemisphere. There is also compensatory thickening of the skull vault
Figure 2.
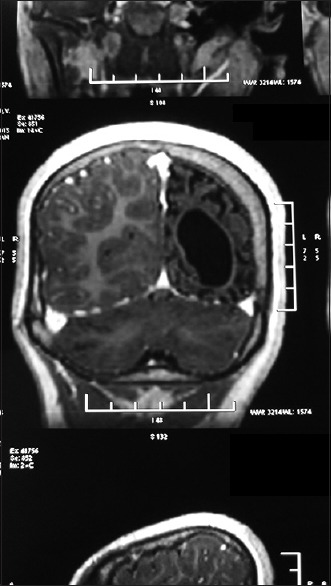
Magnetic resonance imaging of the brain showing diffuse atrophy of the left cerebral hemisphere with dilatation of the left lateral ventricle with compensatory thickening of the skull vault
Discussion
It has been reported that DDMS is caused by cerebral insult that may occur in utero when the maturation of calvarium has not been completed, or during early life due to brain damage (usually traumatic).[3] Prenatal causes include congenital abnormalities, cerebral infarction, vascular malformations, infections, and gestational vascular occlusion, primarily involving the middle cerebral vascular territory. Birth trauma, hypoxia, intracranial hemorrhage, tumors, infections, and prolonged febrile seizures after birth are important peri- and post-natal causes. It can also be due to decreased carotid artery blood flow due to coarctation of aorta.[4]
There are two types of DDMS – congenital (infantile) and acquired.[5] In congenital hemiatrophy, when the insult occurs in utero, there is a shift of midline structures toward the side of the disease, and the sulcal prominence replacing the gliotic tissue is absent. This feature differentiates it from cerebral hemiatrophy which occurs in early life. The atrophied cerebral hemisphere will have prominent sulcal spaces if the insult occurs after birth or after the end of sulcation.[6]
The clinical findings in DDMS may be of variable degree according to the extent of the brain injury: Seizures, facial asymmetry, contralateral hemiplegia or hemiparesis, mental retardation, and rarely, patients can also present with sensory symptoms and psychiatric disorders such as schizophrenia.[4,6] On the other hand, as indicated by Sharma et al., mental retardation is not generally present, and seizures may show up months or years after the onset of hemiparesis. In the patient mentioned above, single episode of seizure was trailed by hemiparesis and mental deterioration.
When DDMS develops early in life (during the first 2 years), certain cranial changes such as ipsilateral hypertrophy of the skull and enlargement of sinuses occur, the elevations of the greater wing of sphenoid and the petrous ridge on the affected side and ipsilateral falcine displacement. The compensatory cranial changes occur to take up the relative vacuum created by the atrophied or hypoplastic cerebral hemisphere.[7] Hageman et al. proposed the terms cerebral hemihypoplasia or unilateral cerebral hypoplasia for the primary (congenital) cerebral atrophy owing to the fact that there is a lack of cerebral development rather than atrophy.[8]
An exhaustive clinical history and computed tomography (CT) or MRI gives the right diagnosis. In addition to CT findings described above, MRI demonstrates the gray-white matter loss with hyperintensities on T2-weighted images (diffuse cortical and subcortical atrophy) and asymmetry of the basal ganglia.[6] MRI has the ability to bring light changes in the cerebral hemispheres as well as highlighting bony structures, thus differentiating between congenital and acquired types of DDMS.[7]
Important differential diagnosis includes – Sturge–Weber Syndrome, basal cell germinoma, Fishman syndrome, Silver–Russell syndrome, linear nevus syndrome, and Rasmussen encephalitis. Rasmussen encephalitis does not show calvarial changes, and Sturge–Weber syndrome additionally shows enhancing pial angiomas and cortical calcifications.[9]
The treatment is symptomatic, and should target convulsion, hemiplegia, hemiparesis, and learning difficulties. Prognosis is better if hemiparesis occurs after the age of 2 years and without prolonged or repetitive seizures. Children with intractable disabling and hemiplegia are the potential candidates for hemispherectomy with a success rate of 85% in carefully selected cases.[9,10]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dyke CG, Davidoff LM, Masson CB. Cerebral hemiatrophy and homolateral hypertrophy of the skull and sinuses. Surg Gynecol Obstet. 1933;57:588–600. [Google Scholar]
- 2.Yerdelen D, Zafer F. Dyke–Davidoff–Masson syndrome. Neurosurg Quarterly. 2009;19:59–61. [Google Scholar]
- 3.Goyal J, Shah V, Rao S, Jindal N. Dyke–Davidoff–Masson syndrome in children. Internet J Pediatr Neonatol. 2009;10:101–7. [Google Scholar]
- 4.Sharma S, Goyal D, Negi A, Sood RG, Jhobta A, Surya M. Dyke–Davidoff–Masson syndrome. Indian J Radiol Imaging. 2006;16:165–6. [Google Scholar]
- 5.Pendse NA, Bapna P, Menghani V, Diwan A. Dyke-Davidoff-Masson syndrome (DDMS) Indian J Pediatr. 2004;71:943. doi: 10.1007/BF02830843. [DOI] [PubMed] [Google Scholar]
- 6.Shetty DS, Lakhkar BN, John JR. Dyke-Davidoff-Masson syndrome. Neurol India. 2003;51:136. [PubMed] [Google Scholar]
- 7.Singh P, Saggar K, Ahluwalia A. Dyke-Davidoff-Masson syndrome: Classical imaging findings. J Pediatr Neurosci. 2010;5:124–5. doi: 10.4103/1817-1745.76108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hageman G, Gooskens RH, Willemse J. A cerebral cause of arthrogryposis: Unilateral cerebral hypoplasia. Clin Neurol Neurosurg. 1985;87:119–22. doi: 10.1016/0303-8467(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 9.Abdel Razek AA, Kandell AY, Elsorogy LG, Elmongy A, Basett AA. Disorders of cortical formation: MR imaging features. AJNR Am J Neuroradiol. 2009;30:4–11. doi: 10.3174/ajnr.A1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narain NP, Kumar R, Narain B. Dyke-Davidoff-Masson syndrome. Indian Pediatr. 2008;45:927–8. [PubMed] [Google Scholar]


