Abstract
This work presents new data concerning the immunohistochemical occurrence of the transient receptor potential vanilloid type‐1 (TRPV1) receptor in the human trigeminal ganglion (TG) and spinal nucleus of subjects at different ontogenetic stages, from prenatal life to postnatal old age. Comparisons are made with the sensory neuropeptides calcitonin gene‐related peptide (CGRP) and substance P (SP). TRPV1‐like immunoreactive (LI) material was detected by western blot in homogenates of TG and medulla oblongata of subjects at prenatal and adult stages of life. Immunohistochemistry showed that expression of the TRPV1 receptor is mostly restricted to the small‐ and medium‐sized TG neurons and to the caudal subdivision of the spinal trigeminal nucleus (Sp5C). The extent of the TRPV1‐LI TG neuronal subpopulation was greater in subjects at early perinatal age than at late perinatal age and in postnatal life. Centrally, the TRPV1 receptor localized to fibre tracts and punctate elements, which were mainly distributed in the spinal tract, lamina I and inner lamina II of the Sp5C, whereas stained cells were rare. The TRPV1 receptor colocalized partially with CGRP and SP in the TG, and was incompletely codistributed with both neuropeptides in the spinal tract and in the superficial laminae of the Sp5C. Substantial differences were noted with respect to the distribution of the TRPV1‐LI structures described in the rat Sp5C and with respect to the temporal expression of the receptor during the development of the rat spinal dorsal horn. The distinctive localization of TRPV1‐LI material supports the concept of the involvement of TRPV1 receptor in the functional activity of the protopathic compartment of the human trigeminal sensory system, i.e. the processing and neurotransmission of thermal and pain stimuli.
Keywords: calcitonin gene‐related peptide, human trigeminal system, immunohistochemistry, substance P, transient receptor potential vanilloid type‐1 receptor, western blot
Introduction
The trigeminal sensory system subserves general sensory perception, i.e. touch, proprioception, temperature and nociception, carried by the 5th cranial nerve from the superficial and deep structures of the head. Most of the primary sensory neurons are located in the trigeminal ganglion (TG), although, exceptionally, a substantial amount of those subserving proprioception reside centrally in the mesencephalic trigeminal nucleus, as well as peripherally in ectopic sites along the oculomotor nerves (Usunoff et al. 1997). On their way to the somatosensory thalamus and cortex, the sensory stimuli are first conveyed centrally to second order neurons in the brainstem trigeminal sensory nuclear column. There, epicritic (discriminative) tactile and proprioceptive sensation from large mechanoreceptor neurons is distributed to the mesencephalic and pontine (principal) nuclei, and the protopathic thermal and painful sensation from medium‐ and small‐sized thermoreceptor and nociceptor neurons is conveyed to the spinal sensory nucleus. In the latter, whilst the rostral part (oral and interpolar subnuclei) seems to be primarily associated with reflexes and appears to participate in pain processing of intraoral structures, the caudal subnucleus (Sp5C) is committed to relaying the protopathic stimuli from the entire trigeminal territory (Paxinos & Mai, 2004; Vanderah & Gould, 2015). TG neurons for protopathic sensibility and the synaptic relay structures within the Sp5C are thus the first elements responsible for normal trigeminal thermal and pain perception. They are a crucial factor in the abnormal ache that characterizes a variety of craniofacial pain syndromes, such as trigeminal neuralgias, headache and burning mouth syndrome (Terrence & Jensen, 2000; Zakrzewska, 2002; Goadsby et al. 2009; Mock & Chugh, 2010), most of which are common, debilitating and disabling.
The transient receptor potential vanilloid type‐1 (TRPV1) cation channel plays a pivotal role in the perception and modulation of pain (Caterina et al. 1997; Szallasi et al. 2007; Chung et al. 2011a; Julius, 2013). The TRPV1 receptor is activated by noxious heat, acidic and basic deviations from homeostatic pH, voltage, endogenous compounds such as endocannabinoids and products of lipoxygenases, and a variety of substances, among which capsaicin and resiniferatoxin are the best known (Szallasi & Blumberg, 1999; Hwang et al. 2000; Bölcskei et al. 2005; Immke & Gavva, 2006; Szallasi et al. 2007; Holzer, 2008; Dhaka et al. 2009). The tissue localization of TRPV1 receptors has been widely shown in primary sensory neurons and their peripheral and central terminals (Caterina et al. 1997; Helliwell et al. 1998; Tominaga et al. 1998; Guo et al. 1999; Michael & Priestley, 1999; Mezey et al. 2000; Matsumoto et al. 2001; Ichikawa & Sugimoto, 2003, 2004; Balaban et al. 2003; Dinh et al. 2004; Damann et al. 2006). In rodent and human trigeminal primary sensory neurons, the release upon TRPV1 receptor activation of the pain‐related neuropeptide transmitters calcitonin gene‐related peptide (CGRP) and substance P (SP) from peripheral (Geppetti et al. 1992; Nicoletti et al. 2008; Fehrenbacher et al. 2009) and central endings (Meng et al. 2009) has been proposed. In turn, the neuropeptides activate their effector cell receptors, leading to neurogenic inflammation and sensitization of nociceptors (Szallasi & Blumberg, 1999; Holzer, 2008). In addition to its significance at the peripheral nerve terminals, the activity of the TRPV1 receptor appears to be highly relevant for the sensory neuromodulation at the central first synaptic level of the pain pathways (Kim et al. 2014 and references therein). The aberrant activation of the TRPV1 receptor has been implicated in different trigeminal neuropathological conditions, such as nerve injury (Urano et al. 2012; Zakir et al. 2012) and pulpal inflammation (Park et al. 2006; Chung et al. 2011b). In humans, the local injection of capsaicin has been shown to cause sensitization of the forehead cutaneous afferents (Gazerani et al. 2005, 2009), release of CGRP from dental pulp (Fehrenbacher et al. 2009) and pain in the masseter muscle (Sohn et al. 2000), and to have a preventive effect in the treatment of headache (Cianchetti, 2010; Benemei et al. 2013). The TRPV1 receptor has thus received much attention as a target for the development of new therapeutic strategies in pain management (Wong & Gavva, 2009; Kim et al. 2010; Trevisani & Szallasi, 2010). However, it has been shown that substantial regional and species differences exist in the relative proportion and neurochemical features of primary sensory neurons that express the TRPV1 receptor (Price & Flores, 2007). This warns against the extrapolation of data obtained from experimental animals and requires the acquisition of specific knowledge of human regional chemical neuroanatomy. Regarding the localization of the TRPV1 receptor in the human trigeminal somatosensory system, the available information pertains, to the best of our knowledge, to the TG neurons (Hou et al. 2002) and to certain territories of their peripheral innervation (Morgan et al. 2005; Yilmaz et al. 2007; Del Fiacco et al. 2015). With a view to contributing to the characterization of the capsaicin‐sensitive component of the human trigeminal sensory system, we present new data here concerning the immunohistochemical occurrence of the TRPV1 receptor in autoptic specimens of the human TG and spinal trigeminal nucleus, and we compare this with that of CGRP and SP. The availability of specimens from subjects at different ontogenetic life stages allows us to extend the analysis from prenatal life to old age.
Materials and methods
Sampling
Specimens of TG and caudal brainstem were obtained at autopsy from pre‐ and full‐term newborns, one child subject and adult subjects with no history of neuropathology (Table 1). These specimens were immediately reduced and processed for either western blot or immunohistochemical analysis. The whole process of sampling and handling of human specimens was performed anonymously according to the standardized procedure for autopsy samples of the Section of Forensic Medicine of the Department of Public Health, Clinical and Molecular Medicine, University of Cagliari, Italy. This complies with the principles stated in the Declaration of Helsinki and with the guidelines of the local Ethics Committee (EC) of the National Health System. The EC has formally stated the moral principles to which the present study adheres.
Table 1.
List of specimens
| Case | Age | Gender | Causa mortis | Postmortem interval, h | Method |
|---|---|---|---|---|---|
| 1 | 23 w.g. | F | Premature rupture of membranes | 24 | WB, IHC |
| 2 | 34 w.g. | F | Respiratory insufficiency | 34 | WB, IHC |
| 3 | 35 w.g. | M | Intestinal malformation | 48 | WB, IHC |
| 4 | 40 w.g. | M | Cardiorespiratory failure | 37 | IHC |
| 5 | 42 w.g. | M | Disseminated intravascular coagulation | 36 | IHC |
| 6 | 10 y | M | Cardiorespiratory failure | 81 | IHC |
| 7 | 53 y | M | Ventricular fibrillation | 45 | IHC |
| 8 | 60 y | F | Intestinal occlusion and acute cardiac insufficiency | 30 | IHC |
| 9 | 62 y | F | Myocardial infarction | 36 | WB, IHC |
| 10 | 81 y | M | Ruptured abdominal aortic aneurysm | 39 | WB, IHC |
d, days; F, female; h, hours; IHC, immunohistochemistry; M, male; WB, western blot; w.g., weeks of gestation; y, years.
Western blot
Tissue blocks of TG and caudal medulla oblongata were immediately stored at −80 °C until required. Tissue homogenates were prepared in 10 volumes of water containing 2% sodium dodecyl sulphate (SDS). Total protein concentrations were determined using the Lowry method of protein assay (Lowry et al. 1951) with bovine serum albumin as standard. Proteins for each tissue homogenate (40 μg), diluted 1 : 1 in loading buffer, were heated to 95 °C for 7 min and separated by SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) using a 10% polyacrylamide resolving gel. Internal molecular weight (mw) standards (Kaleidoscope Prestained Standards, Bio‐Rad, Hercules, CA, USA) were run in tandem. Two gels at a time were run for Coomassie staining and immunoblotting, respectively. Proteins for immunoblotting were electrophoretically transferred on a polyvinylidene fluoride membrane (Bio‐Rad) using the Mini Trans Blot Cell (Bio‐Rad). Blots were blocked by immersion in 20 mm Tris base and 137 mm sodium chloride (TBS) containing 5% milk powder and 0.1% Tween 20 (TBS‐T), for 60 min at room temperature and incubated for two nights at 4 °C with the primary antibody. The following commercially available rabbit polyclonal antisera against TRPV1 were tested: Thermo Scientific Pierce‐Fisher (TS), raised against a synthetic peptide corresponding to residues T(7)DLGAAADPLQKDTC(21) of the human protein; Neuromics (N), raised against the sequence RASLDSEESESPPQENSC corresponding to residues 4–21 of the amino‐terminus of the rat protein; Chemicon (C), raised against a 21‐amino acid peptide corresponding to the C‐terminus of the rat protein; and Immunological Sciences (IS), raised against a synthetic peptide as a part of the human TRPV1 conjugated to an immunogenic carrier protein. These products were used as primary antiserum at dilutions 1 : 100 to 1 : 1000 in TBS‐T containing 5% milk powder and 0.02% sodium azide (NaN3). After TBS‐T rinse, blots were incubated for 60 min, at room temperature, with peroxidase‐conjugated goat anti‐rabbit serum (Sigma Aldrich) diluted 1 : 10 000 in TBS/T. Loading controls were obtained by immunostaining the membranes as above, using a monoclonal mouse antibody against glyceraldehyde 3‐phosphate dehydrogenase (GAPDH; Millipore), diluted 1 : 1000, as primary antiserum, and a peroxidase‐conjugated goat anti‐mouse serum (Chemicon), diluted 1 : 5000, as secondary antiserum. To check for non‐specific staining, blots were stripped and incubated with the relevant secondary antiserum. After TBS‐T rinse, protein bands were visualized on a film (Kodak X‐Omat LS, Kodak, Rochester, NY, USA) using the ECL PLUS method (GE Healthcare). The approximate mw of immunolabelled protein bands was determined by comparing the position of relevant bands on the autoradiography films with that of nearby prestained mw standards.
Immunohistochemistry
Specimens of human TG and caudal brainstem were immediately fixed by immersion in 4% freshly prepared phosphate‐buffered formaldehyde, pH 7.3, for 24 h at 4 °C, and rinsed overnight in 0.1 m phosphate buffer (PB), pH 7.3, containing 10 or 30% sucrose for adult and newborn specimens, respectively. Cryostat consecutive sections (12 μm thick) were collected in series on chrome alum‐gelatin‐coated slides. TRPV1 and CGRP were studied on series of adjacent sections processed by the avidin–biotin–peroxidase complex (ABC) immunohistochemical technique; TRPV1 and SP were studied on single slides processed by double staining indirect immunofluorescence. Endogenous peroxidase activity was blocked with 0.001% phenylhydrazine in phosphate‐buffered saline (PBS) containing 0.2% Triton X‐100 (PBS‐T). The primary antibodies used were: Thermo Scientific anti‐TRPV1, diluted 1 : 100 for immunofluorescence and 1 : 500 for ABC immunohistochemistry; a rabbit polyclonal antibody against CGRP (Chemicon), diluted 1 : 500 for immunofluorescence and 1 : 1000 for ABC immunohistochemistry; a guinea‐pig polyclonal antibody against SP (AbCam), diluted 1 : 500 for immunofluorescence and 1 : 1200 for ABC immunohistochemistry. Biotin‐conjugated goat anti‐rabbit and anti‐guinea‐pig sera (Vector), both diluted 1 : 400, were used as secondary antiserum in the ABC method; Alexa Fluor 488 or 594 goat anti‐rabbit and goat anti‐guinea‐pig sera (Invitrogen), diluted 1 : 500, were used as secondary antiserum in immunofluorescence. The ABC reaction product was revealed with ABC (BioSpa Div.), diluted 1 : 250, followed by incubation with a solution of 0.1 m PB, pH 7.3, containing 0.05% 3‐3′‐diaminobenzidine (Sigma), 0.04% nickel ammonium sulphate and 0.01% hydrogen peroxide. Incubations with primary antiserum were carried out overnight at 4 °C. Incubations with secondary antiserum and ABC lasted 60 and 30 min, respectively, and were performed at room temperature. All antisera and the ABC were diluted in PBS/T. Negative control preparations were obtained by incubating tissue sections in parallel with either PBS‐T alone or with the relevant primary antiserum pre‐absorbed with an excess of the corresponding peptide antigen. Mayer's haematoxylin staining was used as counterstaining on immunostained TG sections or alone, in a dedicated series of brainstem sections for orientation. Slides were observed with an Olympus BX61 microscope and digital images were obtained with a Leica DFC450 C camera by means of las af software. Some immunofluorescence preparations were observed with a Leica TCS SP5X Inverted Supercontinuum Confocal Laser Scanning microscope (Leica Microsystems, Heidelberg, Germany) equipped with a white light laser. Images with a field size of 512 × 512 were generated using a Plan Fluotar 20× lens NA 0.5.
Morphometry
TG sections separated by a minimum of 168 μm were used. Cell size analysis of the immunolabelled and of the whole (labelled and unlabelled) neuronal population was performed on neuronal cell profiles of digital images captured with a 20× lens, from at least four immunostained sections per specimen counterstained with Mayer's haematoxylin. Only neurons where the nuclear profile was evident were considered. Neuronal mean diameters were automatically evaluated by imageproplus software. Statistical parameters (mean, median, SD) and histograms were obtained with statistica 6 software. The percentage of positive perikarya was calculated by using the ratio of the total number of labelled cells found in three to six immunostained sections to the total number of cells found in the same sections after Mayer's haematoxylin counterstaining.
Results
Western blot
Antisera that could reveal the TRPV1 receptor in human tissue were not readily available and Western blot (WB) analysis was used as an initial step in evaluating the immunolabelling capacity of four different commercially available antisera against TRPV1. Protein samples of human pre‐term and adult TG (Table 1, cases 32 and 9, respectively) and caudal medulla oblongata (Table 1, cases 32 and 10, respectively), each probed with four different TRPV1 antibodies, produced different WB outcomes. Two of the antibodies (C and N) did not produce any labelling and one (IS) recognized numerous protein bands at a molecular weight of about 36 kDa and below. Conversely, in all examined specimens, the antibody by TS (Fig. 1) detected a constant, though not very intense, band at about 100 kDa (Fig. 1, arrow), which corresponds to the molecular weight of the receptor monomeric form (Jahnel et al. 2001), as expected in the reducing conditions of the assay. In addition, a strongly immunoreactive band was present at about 180 kDa, which acquired the aspect of a smear in TG samples (Fig. 1, large arrowhead). This is likely to correspond to the dimeric form of the protein, which, as reported by several authors (Jahnel et al. 2001; Rosenbaum et al. 2002; Tóth et al. 2005), is particularly stable and is revealed by Western blot analysis. A further, rather faint band, below 150 kDa (Fig. 1, small arrowhead), which was more evident in medulla oblongata samples, may possibly represent splice variants (Lu et al. 2005; Schumacher & Eilers, 2010) or post‐translational modifications of the receptor (Jahnel et al. 2001; Tóth et al. 2005). This same antibody was also the only one to produce a reliable immunostaining in tissue sections of human TG and brainstem. For this reason, the following data are based exclusively on the TRPV1‐like immunoreactivity obtained with the antibody by TS.
Figure 1.
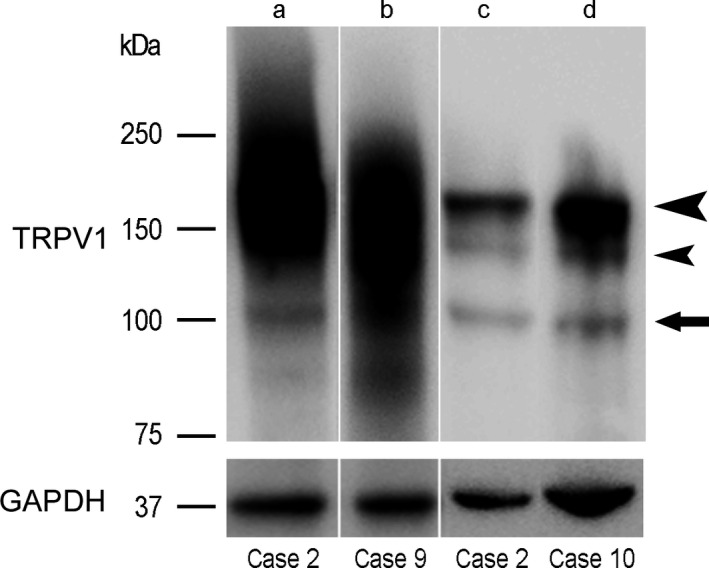
Western blot analysis of TRPV1 and GAPDH in tissue homogenates of human trigeminal ganglion (lanes a, b) and caudal medulla oblongata (lanes c, d) from a pre‐term newborn (case 2; lanes a, c) and two adults (cases 9 and 10, respectively; lanes b, d). Arrow points to the band at the monomer level; large arrowhead points to the band/smears possibly representing the dimeric form; small arrowhead points to a band possibly representing splice variants or post‐translational forms of the protein.
Immunohistochemistry
In the TG, TRPV1‐like immunoreactive (LI) neurons were present at all ages examined and were distributed throughout the whole ganglion (Fig. 2). The immunoreactive perikarya contained a dark‐brown precipitate, typically in the form of very thin granules scattered throughout the cytoplasm, although occasionally a patchy labelling, suggestive of a discrete localization in the Golgi apparatus and in the Nissl substance (data not shown), could be observed. They stood out against the pale background of the unstained tissue structures. Perikaryal lipofuscin deposits were common in adult specimens. They were easily recognizable for their yellow to light brown colour in bright field (arrows in Fig. 2G) and vivid orange autofluorescence under fluorescein filter combination, as well as for their coarse aspect and compartmentalized localization in the cell soma. Occasionally, positive fibres, isolated or in thin bundles, occurred among neuronal cell bodies (Fig. 2B,G). Centrally, TRPV1‐like immunoreactivity labelled extensive filamentous and dot‐like elements, similar to those described in the dorsal horn and spinal trigeminal nucleus of laboratory animals, and suggestive of fibre tracts and terminals. Labelled cell bodies were rare. No such stained elements were detectable in control preparations.
Figure 2.
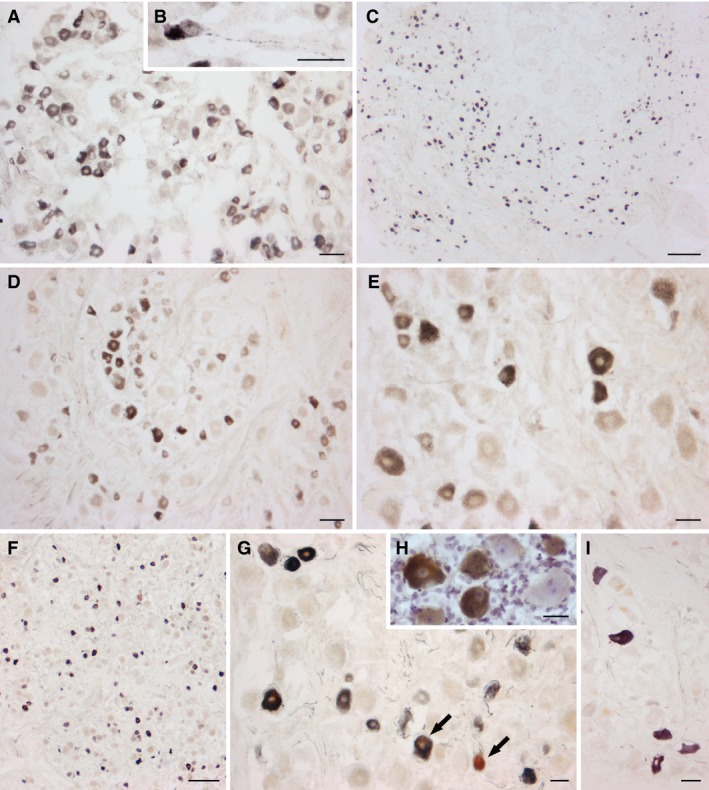
Human trigeminal ganglion. TRPV1‐LI neurons from two pre‐term newborns (A, B, case 1; C, case 3), a full‐term newborn (D, E, case 5) and two adults (F, G, H, case 8; I, case 10). (B) High magnification of a TRPV1‐labelled TG neuron with a varicose proximal process. (H) Immunostained section with Mayer's modified haematoxylin counterstaining. Arrows in (G) point to neurons containing small (right) and large (left) light brown deposits of lipofuscins. Scale bars: (A, D, E, G, I) 50 μm, (C, F) 250 μm, (B, H) 25 μm.
In the TG, TRPV1‐LI neurons were heterogeneous in both density of labelling and cell size, and were detected rather evenly throughout the ganglion, with no apparent preferential localization in distinct areas. Figure 3 shows the frequency histograms of the TRPV1‐LI neurons (Fig. 3, left column) and of the whole neuronal population detected after haematoxylin staining of the same sections (Fig. 3, right column). These pertain to TG specimens from two pre‐term newborns (Table 1, cases 1, 3), a full‐term newborn (Table 1, case 4), and two adults (Table 1, case 8, 10). The neuronal mean cell diameter and size range increased with age. In the adult TG, the haematoxylin stained neuronal population had a mean cell diameter ranging from about 10 to 85 μm, whereas most of the immunoreactive neurons (about 78%) had a mean cell diameter below 40 μm, thus falling into the class of small‐ and medium‐sized cells. The percentage of TRPV1‐LI neurons found in the same subjects is reported in Table 2. It varied from about half of the total population at the earliest age we could examine, to about one‐fifth at late perinatal ages, and one‐third in the adults.
Figure 3.
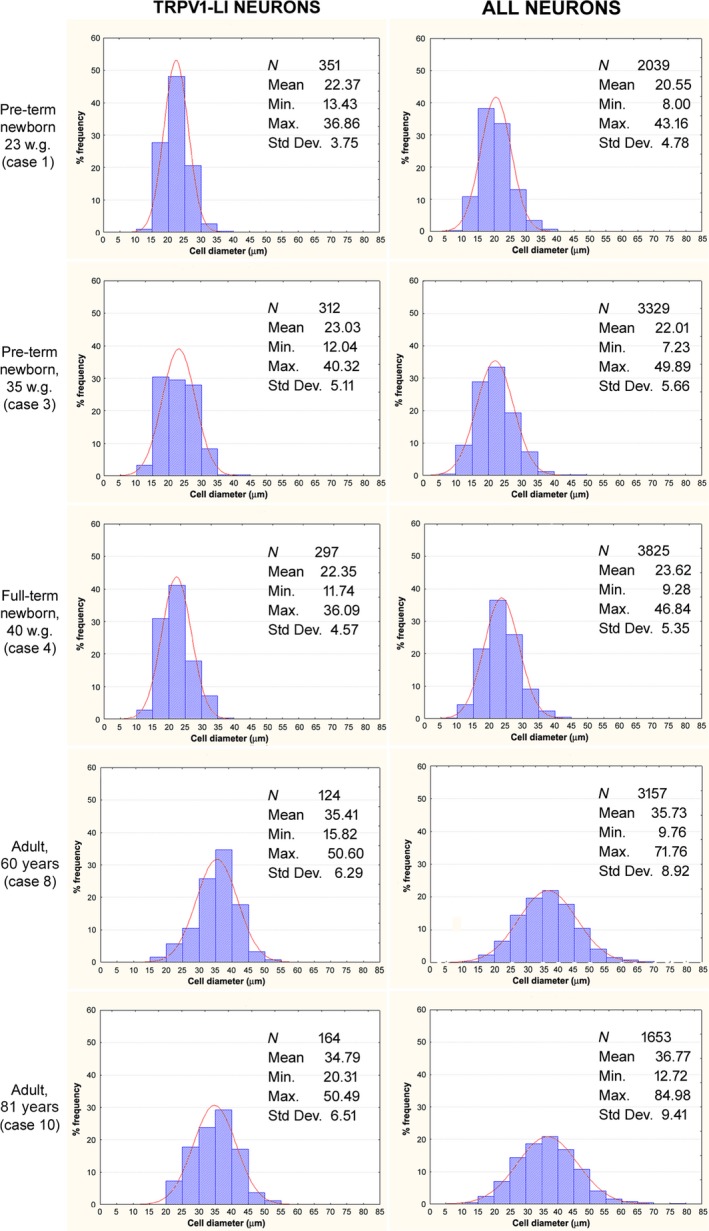
Human trigeminal ganglion. Size frequency histograms of TRPV1‐LI neurons (left column) and all neurons (right column) detected in the same TG sections of specimens from two pre‐term newborns (cases 1 and 3), one full‐term newborn (case 4), and two adults (cases 8 and 10). X‐axis values represent the mean cell diameters expressed in μm; Y‐axis reports values of relative percent frequency. Curves superimposed on the histograms represent the theoretical normal distribution.
Table 2.
Percentage of TRPV1‐positive trigeminal ganglion neurons
| Specimen | % |
|---|---|
| 23 w.g. (case 1) | 51.77 ± 0.02% (1053+/2034) |
| 35 w.g. (case 3) | 21.23 ± 0.01% (706+/3326) |
| 40 w.g. (case 4) | 17.31 ± 0.01% (663+/3831) |
| 60 y (case 8) | 32.92 ± 0.02% (1038+/3153) |
| 81 y (case 10) | 32.17 ± 0.02% (542+/1685) |
In the spinal trigeminal nucleus, the immunoreactivity was localized mostly to its caudal subnucleus (Sp5C; Fig. 4), whereas the interpolar and oral sections contained rare elements. Immunoreactive fibre tracts and terminals were abundant in the spinal tract and deep substantia gelatinosa (inner lamina II) of Sp5C, less profusely distributed in lamina I and scarce in the superficial substantia gelatinosa (outer lamina II) of Sp5C (Fig. 4A,C). Occasional TRPV1‐LI cell bodies were observed in lamina I (Fig. 4D). The density of immunoreactive structures was generally higher in specimens of pre‐ and full‐term newborns than at later ages.
Figure 4.
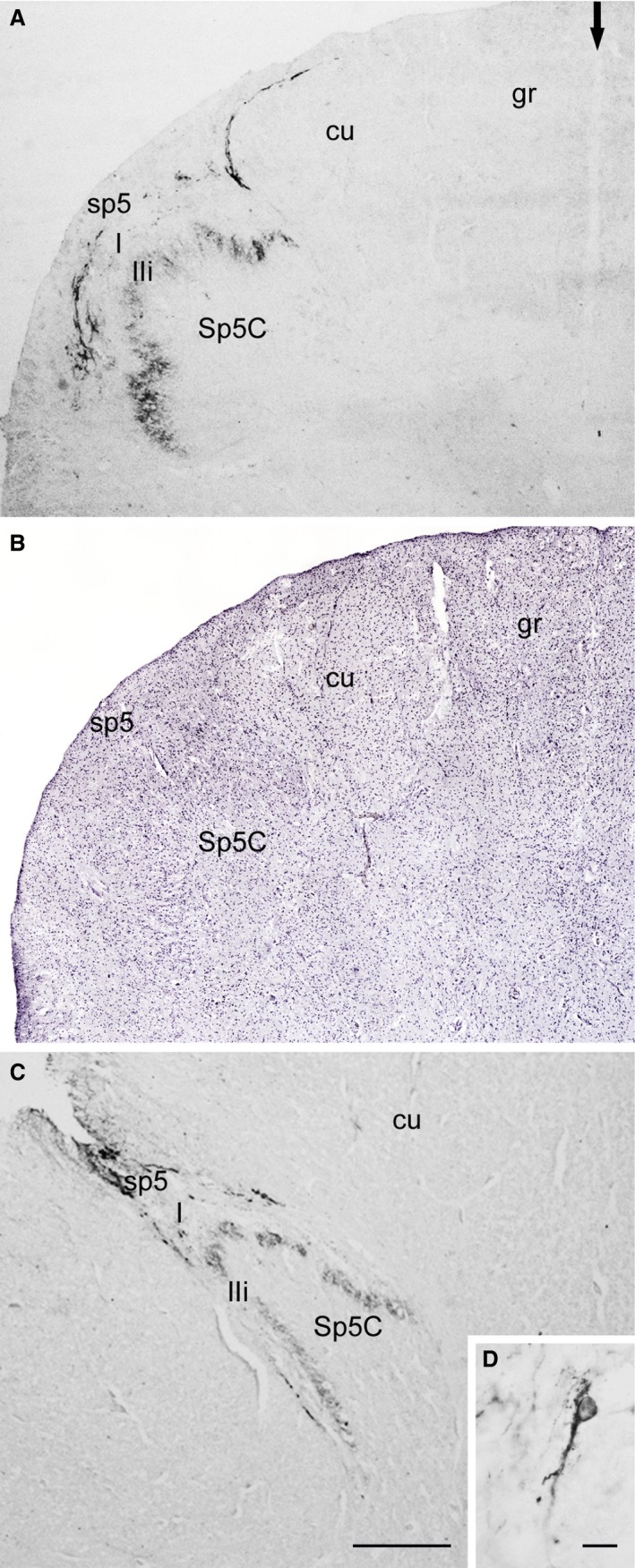
Human spinal trigeminal nucleus, caudal part (Sp5C). Pre‐term newborn (A, B, case 3), adult (C, D, case 10). (A,C) TRPV1‐LI fibre‐ and terminal‐like elements are localized to the spinal trigeminal tract (sp5) and to lamina I (I) and inner lamina II (IIi) of the Sp5C. Arrow in (A) indicates the posterior median septum. (B) Same field as in (A) (left dorsal quadrant) as seen in a consecutive section stained with Mayer's modified haematoxylin. (D) A positive cell in lamina I. cu, cuneate fascicle; gr, gracile fascicle. Scale bars: (A, B, C) 500 μm, (D) 20 μm.
Analysis of codistribution and colocalization of TRPV1 with CGRP or SP was performed in consecutive sections immunostained with ABC (TRPV1 and CGRP) and in double‐immunofluorescence stained sections (TRPV1 and SP) of TG and Sp5C. In the TG, the TRPV1‐LI neuronal population overlapped to some extent with that immunoreactive to either CGRP or SP (Fig. 5). The extent of coexistence of the markers was different in samples at different ages (Table 3). In the newborn, about a quarter and a third of the TRPV1‐LI neurons contained CGRP and SP, respectively; alternatively, about 60% of the CGRP‐LI neurons and about 40% of those positive for SP were also immunostained for the receptor (Table 3). In the adult TG, almost half of the TRPV1‐LI neurons were double‐labelled for CGRP and about a quarter of them were double‐labelled for SP; conversely, about a third of the neuronal population that was immunoreactive to either neuropeptide was also labelled for the receptor (Table 3). Centrally, the three markers were present in the spinal tract and in the Sp5C superficial laminae. However, comparison of immunostaining for TRPV1 and CGRP (consecutive sections, Fig. 6), and for TRPV1 and SP (double‐labelling, Fig. 7) showed that their density and laminar distribution does not entirely correspond. In particular, a good codistribution of immunoreactivity to TRPV1 and either peptide occurs in lamina I and inner lamina II, whereas TRPV1‐LI elements are more abundant than the SP‐ and CGRP‐LI ones in the spinal tract, and the opposite occurs in outer lamina II. Analysis of double‐labelled sections revealed that co‐localization of TRPV1 with SP appeared infrequently when using conventional fluorescence observation (Fig. 7A–C). This was confirmed by confocal microscopy (Fig. 7D).
Figure 5.
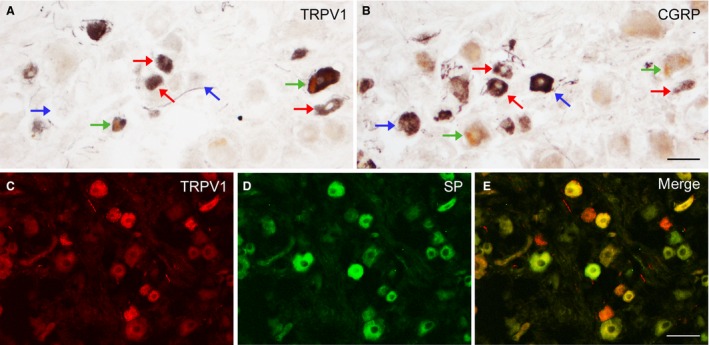
Human trigeminal ganglion. Adult (A, B, case 8); full‐term newborn (C–E, case 5). (A, B) Adjacent sections immunostained for TRPV1 (A) and CGRP (B) showing neurons double‐stained for TRPV1 and CGRP (red arrows) and neurons labelled solely for either TRPV1 (green arrows) or CGRP (blue arrows). (C–E) Double‐labelling for TRPV1 (C) and SP (D). (E) An overlay of panels (C) and (D) showing TRPV1+/SP + neurons (yellow), TRPV1+/SP − neurons (red) and TRPV1−/SP + neurons (green). Scale bars: (A, B) 50 μm, (C, D, E) 50 μm.
Table 3.
Percentage of TRPV1‐LI neurons of the human TG showing also immunoreactivity for neuropeptides (indicated as TRPV1+CGRP+ and TRPV1+SP+), and of either CGRP‐ or SP‐LI neurons showing also immunoreactivity for TRPV1 (indicated as CGRP+TRPV1+ and SP+TRPV1+)
| % TRPV1+ neurons containing neuropeptides | % CGRP+ neurons containing TRPV1 | % SP+ neurons containing TRPV1 | |
|---|---|---|---|
| Full‐term newborn 42 w.g. (case 5) | |||
| TRPV1 |
61.11 ± 0.05% 11 (CGRP+TRPV1+)/18 CGRP+ |
41.1 ± 0.06% 7 (SP+TRPV1+)/17 SP+ |
|
| CGRP |
26.19 ± 0.02% 11 (TRPV1+CGRP+)/42 TRPV1+ |
||
| SP |
33.3 ± 0.04% 7 (TRPV1+SP+)/21 TRPV1+ |
||
| Adult 60 y (case 8) | |||
| TRPV1 |
34.88 ± 0.01% 30 (CGRP+TRPV1+)/86 CGRP+ |
37.5 ± 0.04% 9 (SP+TRPV1+)/24 SP+ |
|
| CGRP |
47.61 ± 0.02% 30 (TRPV1+CGRP+)/63 TRPV1+ |
||
| SP |
25 ± 0.02% 9 (TRPV1+SP+)/36 TRPV1+ |
||
Figure 6.
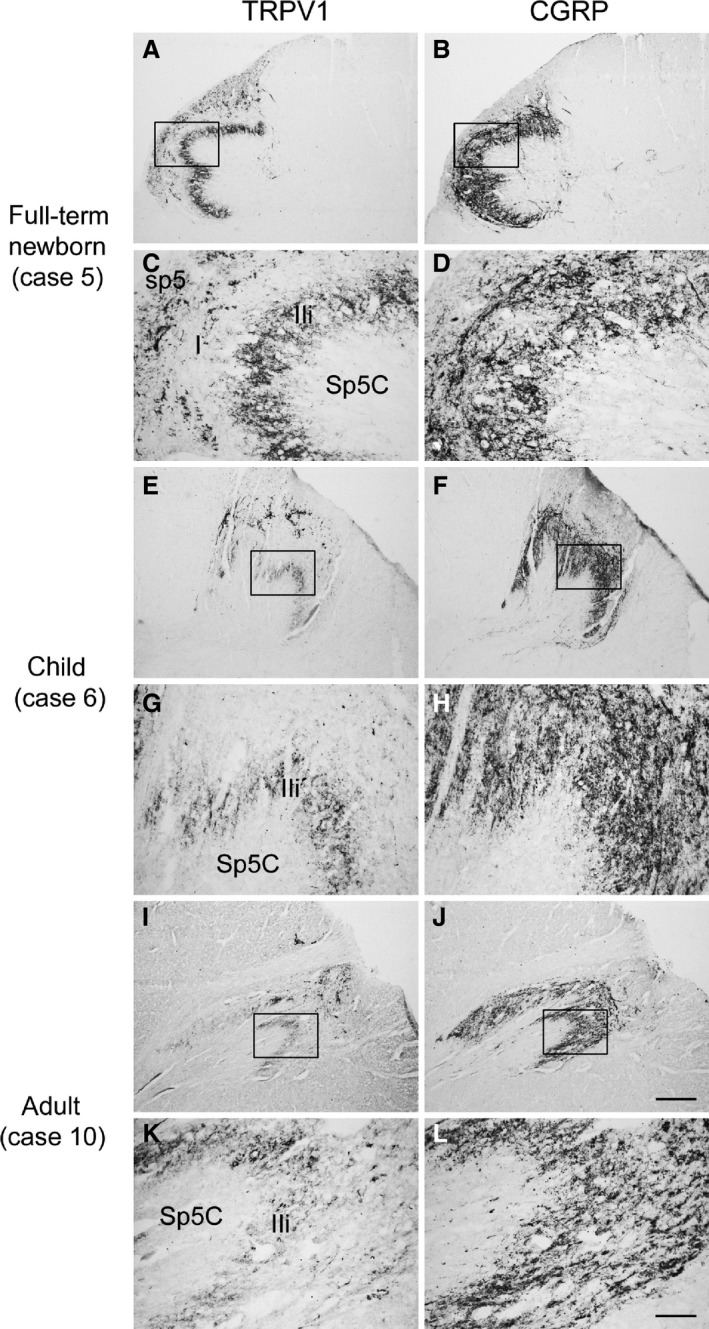
Human spinal trigeminal nucleus, caudal part (Sp5C). Pairs of adjacent sections of medulla oblongata from full‐term newborn (A–D, case 5), child (E–H, case 6), and adult (I–L, case 10) specimens showing the partial codistribution of TRPV1‐ (A, E, I) and CGRP‐like immunoreactivity (B, F, J). (C, D, G, H, K, L) Higher magnifications of microscopic fields in box in (A, B, E, F, I, J), respectively. I, lamina I; IIi, inner lamina II; sp5, spinal trigeminal tract. Scale bars: (A, B, E, F, I, J) 500 μm, (C, D, G, H, K, L) 100 μm.
Figure 7.
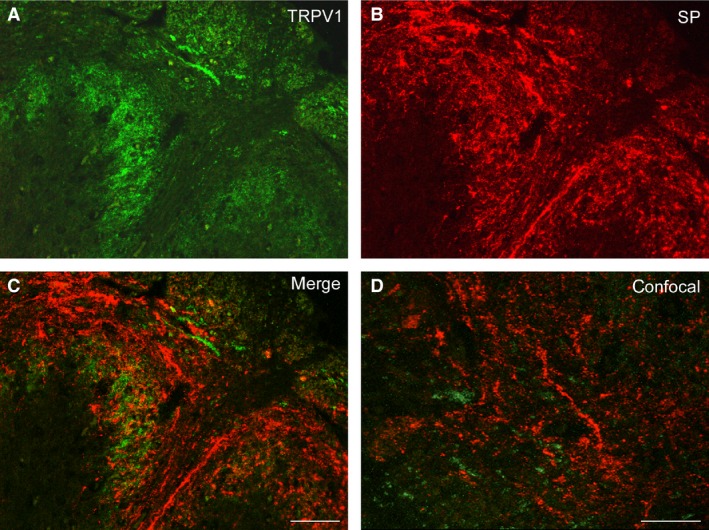
Human spinal trigeminal nucleus, caudal part (Sp5C). Full‐term newborn (case 5). Double immunostaining for TRPV1 (A) and SP (B). (C) Overlay of (A) and (B). (D) Field of the Sp5C superficial laminae as viewed by laser scanning confocal microscopy. Scale bars: (A, B, C) 100 μm, (D) 50 μm.
Discussion
This study provides the first description of the localization of TRPV1‐LI structures in the human trigeminal primary sensory neurons and spinal nucleus at ontogenetic stages spanning from prenatal life to old age. TRPV1‐like immunoreactivity is mostly restricted to TG neurons with a mean diameter below 40 μm and to the protopathic (nociceptive) caudal subdivision of the spinal nucleus. A percentage of the TRPV1‐LI TG perikarya is also immunoreactive to CGRP and SP, and the receptor is partially codistributed with both neuropeptides in the spinal tract and in the superficial laminae of the Sp5C.
Many studies in the literature, which correlate morphological features with neurochemical ones and with functional/pathological involvement in different sensory ganglia and species, classify the neurons in subpopulations of small‐, medium‐ and large‐sized cells. Unfortunately, among different reports (Rambourg et al. 1983; Harper & Lawson, 1985; Lee et al. 1986; Lazarov, 2002; Priestley et al. 2002; Jimenez‐Andrade et al. 2006; Dilkash et al. 2010; Chung et al. 2011b), the size boundaries that define those subpopulations are far from being consistent. Any strict demarcation may appear arbitrary. Yet, given that, in our study, the mean cell diameter of the adult TG perikarya ranges from 10 to 85 μm (and that some of the largest neurons are located outside the TG), we can reasonably say that most of the TRPV1‐LI neurons belong to the small‐ and medium‐sized cell class. This is in keeping with previous studies on rat TG (Caterina et al. 1997; Helliwell et al. 1998; Bae et al. 2004; Damann et al. 2006; Cavanaugh et al. 2011) and human (Lauria et al. 2006) and rat DRG (Caterina et al. 1997; Helliwell et al. 1998; Guo et al. 1999; Michael & Priestley, 1999; Aoki et al. 2005; Cavanaugh et al. 2011; Quartu et al. 2014). The small‐ and medium‐sized TRPV1‐positive primary afferent neurons are related to unmyelinated (C) or thinly myelinated (Adelta) afferents (Guo et al. 1999; Michael & Priestley, 1999; Caterina & Julius, 2001; Bae et al. 2004; Holzer, 2008) and are considered to be nociceptive. In accordance with this, clinical findings report that TRPV1 receptor plays a role in pain and hyperalgesia associated with inflammation, injury, acidosis and cancer (Holzer, 2008). As regards the trigeminal system, a positive correlation between TRPV1‐LI sensory afferents and pain score has been shown in dental pain (Morgan et al. 2005) and chronic burning mouth syndrome (Yilmaz et al. 2007), and we detected an increased density of the TRPV1‐LI vascular innervation of scalp arteries in chronic migraine (Del Fiacco et al. 2015). The percentage of the TRPV1‐positive neurons we detected in the adult TG is double the percentage that was previously reported by Hou et al. (2002). We do not have a clear‐cut explanation for this divergence. Among the antibodies we probed, the one that belonged to the same brand as that used in the aforementioned study did not yield any immunochemical labelling, either in WB or in histochemistry. On this issue, it is pertinent to remark that extremely different values, ranging from 20 to 54%, have been reported in the adult rat TG by different groups (Ichikawa & Sugimoto, 2001; Bae et al. 2004; Cavanaugh et al. 2011). Such a disparity in the number of TRPV1‐LI neurons also occurs in studies of rodent DRG, where scored TRPV1‐positive neurons vary from about 23% to more than 50% (Guo et al. 1999; Aoki et al. 2005; Cavanaugh et al. 2011; Quartu et al. 2014). Although, with respect to the data on DRG, the longitudinal variations existing at different spinal levels (Špicarová & Paleček, 2008) must also be considered, the use of different fixative solutions, antibodies (even different lots of the same brand) and methodological procedures may possibly account for the discrepancies observed.
Our observations suggest the occurrence of age‐related changes. In the TG, the number of TRPV1‐labelled neurons amounted to half of the total ganglion neurons at the earliest age we could examine (23 weeks of gestation). This proportion declined to about one‐fifth at late perinatal ages (35 and 40 weeks of gestation) and we counted up to one‐third in the adults. Although the number of examined specimens is small, the observed differences might represent an aspect of the reported shaping and functional maturation of the nociceptive circuitry (Fitzgerald, 2005; Fitzgerald & Walker, 2009). Centrally, the immunoreactive elements in the Sp5C were more dense at perinatal life stages than in childhood and adult life, and the described laminar distribution was already obvious at the earliest pre‐term age we could examine. These findings differ substantially from the delayed pattern of expression and laminar organization detected in the rat spinal cord during development (Guo et al. 2001).
The distribution of TRPV1‐positive elements in the Sp5C has a close similarity with that reported in the adult rat spinal dorsal horn by Guo et al. (1999, 2001) and Valtschanoff et al. (2001). As well as describing the occurrence of TRPV1‐LI nerve fibres and terminals in laminae I and II inner, they stress the paucity of immunoreactivity in lamina II outer, they report the presence of the receptor in local neurons, and they show that most immunoreactive terminals are distinct from those immunoreactive to substance P in both laminae. Their findings and ours differ substantially from the localization of the receptor in the rat Sp5C shown by Bae et al. (2004). The latter detect the TRPV1‐positive innervation in lamina I and II outer and show the colocalization of the receptor with CGRP and SP. This appears to represent a remarkable species difference. In the rat, in both lamina II inner of the spinal dorsal horn (Guo et al. 1999) and lamina II outer of the Sp5C (Bae et al. 2004), the immunoreactivity to the receptor occurs in nerve fibres and terminals that bind the isolectin Griffonia simplicifolia B4 (IB4). IB4 is considered to be the marker of the non‐peptidergic nociceptive primary sensory neurons, and it has been suggested that IB4 binding neurons expressing the TRPV1 receptor are important in neuropathic pain (Snider & McMahon, 1998) and thermal nociception (Guo et al. 1999). It will therefore be interesting further to characterize the human TG and Sp5C structures in terms of their IB4 binding ability and compare it with their TRPV1 immunoreactivity.
The likelihood of a primary afferent origin for the TRPV1‐LI material in the human spinal trigeminal nucleus rests upon experimental data showing such an origin in the rodent Sp5C (Mezey et al. 2000; Cavanaugh et al. 2011) and spinal dorsal horn (Guo et al. 1999; Špicarová & Paleček, 2008). Yet, the occurrence of TRPV1‐LI cells in the human trigeminal nucleus suggests that the possibility of a central origin for the TRPV1‐LI elements should not be dismissed, as can also be inferred from the evidence of TRPV1 mRNA in homogenates of rat spinal cord (Mezey et al. 2000; Sanchez et al. 2001; Quartu et al. 2014) and of TRPV1 protein in the rat dorsal horn GABAergic interneurons (Kim et al. 2012) and glial cells (Doly et al. 2004; Chen et al. 2009).
Earlier studies have provided evidence for the colocalization of the TRPV1 receptor with the neuropeptides CGRP and SP in TG (Ichikawa & Sugimoto, 2000; Hou et al. 2002; Bae et al. 2004) and DRG neurons (Guo et al. 1999; Michael & Priestley, 1999; Aoki et al. 2005; Hwang et al. 2005; Cavanaugh et al. 2011; Quartu et al. 2014). Although the coexistence values we have obtained tend to match those reported for rat TG (Bae et al. 2004), they differ substantially from the previous data on human TG. The latter show that only about 10 and 8% of the TRPV1‐positive neurons also contain CGRP and SP, respectively (Hou et al. 2002). As mentioned above, different sampling conditions and methodological approaches may account for such a discrepancy. A further point to be considered is that, owing to the intrinsic limitations of studies on human tissues, our results are based on rather small numbers of cells and this also applies to the report by Hou et al. (2002). The analysis of a larger number of samples might perhaps reduce the now apparent difference. On the other hand, even in the rat DRG the percentage of coexpression of TRPV1 and either neuropeptide appears to diverge significantly in different studies (Guo et al. 1999; Michael & Priestley, 1999; Aoki et al. 2005; Hwang et al. 2005; Cavanaugh et al. 2011; Quartu et al. 2014). Unfortunately, we could not obtain a satisfactory double immunostaining for TRPV1 and CGRP. However, the colocalization detected in the TG neurons, together with the codistribution observed in the superficial laminae of the Sp5C, implies that at least a certain degree of colocalization in the central afferents cannot be excluded. The colocalization of TRPV1 and SP in the Sp5C does not appear to exist to a degree that is comparable to that observed in the TG. It may be reasoned that the immunofluorescence method and/or milieu are not appropriate for completely revealing the TRPV1 content in primary afferent central endings. Nevertheless, a differential degree of colocalization of TRPV1 with SP and CGRP at primary sensory neuron peripheral and central endings has been reported in the rat lumbar DRG neurons (Guo et al. 1999; Hwang & Valtschanoff, 2003). It may therefore be hypothesized that the TRPV1 and SP (and possibly CGRP) are not transported at the central nerve endings in the same way and to the same extent. Indeed, the western blot analysis shows that the amount of TRPV1‐LI protein detectable in the medulla oblongata is much lower than that observed in the TG.
To summarize, our results demonstrate that in the human trigeminal sensory system:immunoreactivity to the TRPV1 receptor occurs in a subpopulation of mostly small‐ and medium‐sized primary sensory neurons and in the spinal nucleus, where it is concentrated in the caudal subnucleus; these elements are detectable from early pre‐term life to postnatal old age; the TRPV1 receptor partially colocalizes with the neuropeptides CGRP and SP in ganglion neurons; comparison with the literature points to differences with experimental animals in the central distribution of the immunoreactive structures. These data provide supporting evidence for the concept of the involvement of the TRPV1 receptor in the neurotransmission of the protopathic sensory stimuli from the trigeminal territory.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
We thank Dr Geoffrey M. Gray (Department of Philology, Literature and Linguistics, University of Cagliari, Italy) for assistance in language editing.
References
- Aoki Y, Ohtori S, Takahashi K, et al. (2005) Expression and co‐expression of VR1, CGRP, and IB4‐binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine (Phila Pa 1976), 30, 1496–1500. [DOI] [PubMed] [Google Scholar]
- Bae YC, Oh JM, Hwang SJ, et al. (2004) Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol 478, 62–71. [DOI] [PubMed] [Google Scholar]
- Balaban CD, Zhou J, Li HS (2003) Type 1 vanilloid receptor expression by mammalian inner ear ganglion cells. Hear Res 175, 165–170. [DOI] [PubMed] [Google Scholar]
- Benemei S, De Cesaris F, Fusi C, et al. (2013) TRPA1 and other TRP channels in migraine. J Headache Pain 14, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölcskei K, Helyes Z, Szabó A, et al. (2005) Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene‐deficient mice. Pain 117, 368–376. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, et al. (1997) The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Bráz JM, et al. (2011) Restriction of transient receptor potential vanilloid‐1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 31, 10119–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Willcockson HH, Valtschanoff JG (2009) Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol 220, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Jung SJ, Oh SB (2011a) Role of TRP channels in pain sensation. Adv Exp Med Biol 704, 615–636. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee J, Duraes G, et al. (2011b) Lipopolysaccharide‐induced pulpitis up‐regulates TRPV1 in trigeminal ganglia. J Dent Res 90, 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchetti C (2010) Capsaicin jelly against migraine pain. Int J Clin Pract 64, 457–459. [DOI] [PubMed] [Google Scholar]
- Damann N, Rothermel M, Klupp BG, et al. (2006) Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fiacco M, Quartu M, Boi M, et al. (2015) TRPV1, CGRP and SP in scalp arteries of patients suffering from chronic migraine. J Neurol Neurosurg Psychiatry 86, 393–397. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Uzzell V, Dubin AE, et al. (2009) TRPV1 is activated by both acidic and basic pH. J Neurosci 29, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilkash MNA, Ahmed SS, Khan AA (2010) Comparative light microscopic study of trigeminal ganglion neurons in mammals. Curr Neurobiol 1, 25–29. [Google Scholar]
- Dinh QT, Groneberg DA, Peiser C, et al. (2004) Nerve growth factor‐induced substance P in capsaicin‐insensitive vagal neurons innervating the lower mouse airway. Clin Exp Allergy 34, 1474–1479. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Salio C, et al. (2004) The vanilloid receptor‐1 is expressed in rat spinal dorsal horn astrocytes. Neurosci Lett 357, 123–126. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Sun XX, Locke EE, et al. (2009) Capsaicin‐evoked CGRP release from human dental pulp: a model system for the study of peripheral neuropeptide secretion in normal healthy tissue. Pain 144, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (2005) The development of nociceptive circuits. Nat Rev Neurosci 6, 507–520. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Walker SM (2009) Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol 5, 35–50. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Kaeseler Andersen O, Arendt‐Nielsen L (2005) A human experimental capsaicin model for trigeminal sensitization. Gender‐specific differences. Pain 118, 155–163. [DOI] [PubMed] [Google Scholar]
- Gazerani P, Pedersen NS, Staahl C, et al. (2009) Subcutaneous Botulinum toxin type A reduces capsaicin‐induced trigeminal pain and vasomotor reactions in human skin. Pain 141, 60–69. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Del Bianco E, Cecconi R, et al. (1992) Capsaicin releases calcitonin gene‐related peptide from the human iris and ciliary body in vitro . Regul Pept 41, 83–92. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Charbit AR, Andreou AP, et al. (2009) Neurobiology of migraine. Neuroscience 161, 327–341. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, et al. (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11, 946–958. [DOI] [PubMed] [Google Scholar]
- Guo A, Simone DA, Stone LS, et al. (2001) Developmental shift of vanilloid receptor 1 (VR1) terminals into deeper regions of the superficial dorsal horn: correlation with a shift from TrkA to Ret expression by dorsal root ganglion neurons. Eur J Neurosci 14, 293–304. [DOI] [PubMed] [Google Scholar]
- Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell RJ, McLatchie LM, Clarke M, et al. (1998) Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett 250, 177–180. [DOI] [PubMed] [Google Scholar]
- Holzer P (2008) The pharmacological challenge to tame the transient receptor potential vanilloid‐1 (TRPV1) nocisensor. Br J Pharmacol 155, 1145–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Uddman R, Tajti J, et al. (2002) Capsaicin receptor immunoreactivity in the human trigeminal ganglion. Neurosci Lett 330, 233–236. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Valtschanoff JG (2003) Vanilloid receptor VR1‐positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett 349, 41–44. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, et al. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin‐like substances. Proc Natl Acad Sci USA 97, 6155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG (2005) Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res 1047, 261–266. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T (2000) Vanilloid receptor 1‐like receptor‐immunoreactive primary sensory neurons in the rat trigeminal nervous system. Neuroscience 101, 719–725. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T (2001) VR1‐immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res 890, 184–188. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T (2003) The co‐expression of VR1 and VRL‐1 in the rat vagal sensory ganglia. Brain Res 980, 293–296. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, et al. (2004) VR1‐, VRL‐1‐ and P2X3 receptor‐immunoreactive innervation of the rat temporomandibular joint. Brain Res 1008, 131–136. [DOI] [PubMed] [Google Scholar]
- Immke DC, Gavva NR (2006) The TRPV1 receptor and nociception. Semin Cell Dev Biol 17, 582–591. [DOI] [PubMed] [Google Scholar]
- Jahnel R, Dreger M, Gillen C, et al. (2001) Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem 268, 5489–5496. [DOI] [PubMed] [Google Scholar]
- Jimenez‐Andrade JM, Peters CM, Mejia NA, et al. (2006) Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett 405, 62–67. [DOI] [PubMed] [Google Scholar]
- Julius D (2013) TRP channels and pain. Annu Rev Cell Dev Biol 29, 355–384. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim K, Li HY, et al. (2010) Selectively targeting pain in the trigeminal system. Pain 150, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, et al. (2012) TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 74, 640–647. [DOI] [PubMed] [Google Scholar]
- Kim YS, Chu Y, Han L, et al. (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Morbin M, Lombardi R, et al. (2006) Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst 11, 262–271. [DOI] [PubMed] [Google Scholar]
- Lazarov NE (2002) Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol 66, 19–59. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chung K, Chung JM, et al. (1986) Correlation of cell body size, axon size, and signal conduction velocity for individually labeled dorsal root ganglion cells in the cat. J Comp. Neurol 243, 335–346. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275. [PubMed] [Google Scholar]
- Lu G, Henderson D, Liu L, et al. (2005) TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol 67, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Emori Y, Ninomiya Y, et al. (2001) A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Res Mol Brain Res 93, 105–112. [DOI] [PubMed] [Google Scholar]
- Meng J, Ovsepian SV, Wang J, et al. (2009) Activation of TRPV1 mediates Calcitonin Gene‐Related Peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti‐nociceptive potential. J Neurosci 29, 4981–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, et al. (2000) Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1‐like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 97, 3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV (1999) Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 19, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D, Chugh D (2010) Burning mouth syndrome. Int J Oral Sci 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CR, Rodd HD, Clayton N, et al. (2005) Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain 19, 248–260. [PubMed] [Google Scholar]
- Nicoletti P, Trevisani M, Manconi M, et al. (2008) Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia 28, 9–17. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim MS, Fang Z, et al. (2006) Functional expression of thermo‐transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem 281, 17304–17311. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Mai JK (2004) The Human Nervous System. San Diego: Elsevier Academic Press. [Google Scholar]
- Price TJ, Flores CM (2007) Critical evaluation of the colocalization between calcitonin gene‐related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 8, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, et al. (2002) Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol 80, 495–505. [DOI] [PubMed] [Google Scholar]
- Quartu M, Carozzi VA, Dorsey SG, et al. (2014) Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed Res Int 2014, 180428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A, Clemont Y, Beaudet A (1983) Ultrastructural features of six types of neurons in rat dorsal root ganglia. J Neurocytol 12, 47–66. [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Awaya M, Gordon SE (2002) Subunit modification and association in VR1 ion channels. BMC Neurosci 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, Krause JE, Cortright DN (2001) The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience 107, 373–381. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Eilers H (2010) TRPV1 splice variants: structure and function. Front Biosci (Landmark Ed) 15, 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB (1998) Tackling pain at the source: new ideas about nociceptors. Neuron 20, 629–632. [DOI] [PubMed] [Google Scholar]
- Sohn MK, Graven‐Nielsen T, Arendt‐Nielsen L, et al. (2000) Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve 23, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Špicarová D, Paleček J (2008) The role of spinal cord vanilloid (TRPV1) receptors in pain modulation. Physiol Res 57(Suppl. 3), S69–S77. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51, 159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, et al. (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof‐of‐concept. Nat Rev Drug Discov 6, 357–372. Erratum in: Nat Rev Drug Discov 2007, 6, 442. [DOI] [PubMed] [Google Scholar]
- Terrence CF, Jensen TS (2000) Trigeminal neuralgia and other facial neuralgias In: The Headaches. 2nd edn (eds Olesen J, Tfelt‐Hansen P, Welch KMA.), pp. 929–938, Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, et al. (1998) The cloned capsaicin receptor integrates multiple pain‐producing stimuli. Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, et al. (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135, 162–168. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Szallasi A (2010) Targeting TRPV1: challenges and issues in pain management. Open Drug Discov J 2, 37–49. [Google Scholar]
- Urano H, Ara T, Fujinami Y, et al. (2012) Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int J Med Sci 9, 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usunoff KG, Marani E, Schoen JH (1997) The trigeminal system in man. Adv Anat Embryol Cell Biol 136, 1–126. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Rustioni A, Guo A, et al. (2001) Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol 436, 225–235. [PubMed] [Google Scholar]
- Vanderah T, Gould D (2015) Nolte's The Human Brain: An Introduction to its Functional Anatomy, 7th edn Philadelphia, PA 9103‐2899: Elsevier. [Google Scholar]
- Wong GY, Gavva NR (2009) Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev 60, 267–277. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Renton T, Yiangou Y, et al. (2007) Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci 14, 864–871. [DOI] [PubMed] [Google Scholar]
- Zakir HM, Mostafeezur RM, Suzuki A, et al. (2012) Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One 7, e44023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska JM (2002) Trigeminal neuralgia In: Assessment and Management of Orofacial Pain. Pain Research and Clinical Management. (eds Zakrzewska JM, Harrison SD.), pp. 263–366, Amsterdam: Elsevier. [Google Scholar]


