Abstract
How teeth are replaced during normal growth and development has long been an important question for comparative and developmental anatomy. Non‐standard model animals have become increasingly popular in this field due to the fact that the canonical model laboratory mammal, the mouse, develops only one generation of teeth (monophyodonty), whereas the majority of mammals possess two generations of teeth (diphyodonty). Here we used the straw‐coloured fruit bat (Eidolon helvum), an Old World megabat, which has two generations of teeth, in order to observe the development and replacement of tooth germs from initiation up to mineralization stages. Our morphological study uses 3D reconstruction of histological sections to uncover differing arrangements of the first and second‐generation tooth germs during the process of tooth replacement. We show that both tooth germ generations develop as part of the dental lamina, with the first generation detaching from the lamina, leaving the free edge to give rise to a second generation. This separation was particularly marked at the third premolar locus, where the primary and replacement teeth become positioned side by side, unconnected by a lamina. The position of the replacement tooth, with respect to the primary tooth, varied within the mouth, with replacements forming posterior to or directly lingual to the primary tooth. Development of replacement teeth was arrested at some tooth positions and this appeared to be linked to the timing of tooth initiation and the subsequent rate of development. This study adds an additional species to the growing body of non‐model species used in the study of tooth replacement, and offers a new insight into the development of the diphyodont condition.
Keywords: dental lamina, diphyodont, fruit bat, mammal, tooth development, tooth replacement
Introduction
Teeth are mineralized appendages found in all vertebrate classes. Although tooth morphology and distribution vary, the initial steps of development are much the same across clades. Reciprocal and sequential interactions between cranial neural crest‐derived mesenchyme and ectodermal epithelium (or endodermal in the case of pharyngeal teeth) lie at the core of tooth morphogenesis (Chai et al. 2000; Soukup et al. 2008; Rothova et al. 2012). During their development, teeth pass through a series of well‐characterized stages, defined by the morphology of the dental epithelium: dental lamina, bud, cap and bell (Luckett, 1993).
Teeth are covered by enamel, which is resistant to abrasion and helps prolong the life of the tooth; however, teeth can be lost, or ground down by tooth wear and need to be replaced. Tooth replacement also allows for changes in the morphology of the dentition as the animal ages, as observed in many fish (Streelman et al. 2003; Fraser et al. 2006; Huysseune, 2006; Crucke & Huysseune, 2014; Tucker & Fraser, 2014; Vandenplas et al. 2014). The ability to replace lost teeth is evolutionarily ancient. The presumed basal character of jawed vertebrates is polyphyodonty, meaning they can continuously replace their dentition throughout their lifetime (Soukup et al. 2008; Smith et al. 2009; Tucker & Fraser, 2014; Rasch et al. 2016). Tooth replacement capabilities differ widely throughout the terrestrial vertebrates: most reptiles are polyphyodonts, whereas most mammals are diphyodont and possess two sets of generational teeth. A subset of mammals and some specialized reptiles are monophyodont, where the teeth are non‐replacing. An important aspect that the first two groups (poly‐ and diphyodonts) have in common is the dental successional lamina, a structure crucial for tooth replacement (Handrigan & Richman, 2010; Jernvall & Thesleff, 2012; Tucker & Fraser, 2014).
In diphyodont mammals, the deciduous dentition develops from the primary dental lamina, which interconnects the teeth along the jaw. The second and final generation of teeth develops from a successional dental lamina, an epithelial structure connected to the lingual aspect of the deciduous dental organ (Juuri et al. 2012). The reduced number of tooth generations in mammals has occurred in juxtaposition with an increase in tooth size and complexity (Jernvall & Thesleff, 2012). Much of the process of mammalian tooth development has been discovered by examination of the house mouse, Mus musculus (Lesot et al. 2014; Peterkova et al. 2014). Although the mouse has provided a wealth of valuable information on tooth development, its derived rodent monophyodont dentition with only incisors and molars has limited studies on tooth replacement. To overcome this problem, other mammalian models with complete heterodont dentitions have been introduced into this field of study. The common shrew and house shrew (Sorex araneus, Suncus murinus) retain a more basal eutherian dentition and although they do not display full tooth replacement, several reports have shown that they develop a rudimentary milk dentition. This deciduous dentition is suppressed once the second tooth generation initiates development and only the permanent teeth erupt (Sasaki et al. 2001; Yamanaka et al. 2007; Järvinen et al. 2008).
The ferret and minipig are more appropriate models for study of tooth replacement in mammals as they exhibit diphyodonty as well as heterodonty. All teeth, except for the molars, are replaced once, with the aid of the dental successional lamina (Jarvinen et al. 2009; Stembírek et al. 2010; Jussila et al. 2014). Previous studies have suggested that Wnt signaling components (Axin2, beta‐catenin) and transcription factor Sox2 are involved in the process of tooth replacement as well as cessation of replacement (Jarvinen et al. 2009; Juuri et al. 2013).
In the present study, we have analysed the dentition of a series of straw‐coloured fruit bat embryos (Eidolon helvum) (Fig. 1A‐I). These mammals belong to the order Chiroptera, suborder Megachiroptera, also known as Old World fruit bats, and are native to the African continent. Their gestation period lasts 9 months and commences with a long pre‐implantation period, followed by the actual embryonic development of 4 months (Fayenuwo & Halstead, 1974; Mutere, 1967). Although the lengthy preservation of the specimens prevented us from acquiring any molecular data, and given that these animals are not currently available for such studies due to accessibility and near‐threatened conservation status, we provide here a unique and informative analysis of this diphyodont animal with a complete heterodont dentition. Importantly, our histological investigation has uncovered new aspects regarding the timing of tooth initiation and the relationship between the deciduous and permanent teeth, and shows different modalities of replacement at different regions of the jaw.
Figure 1.
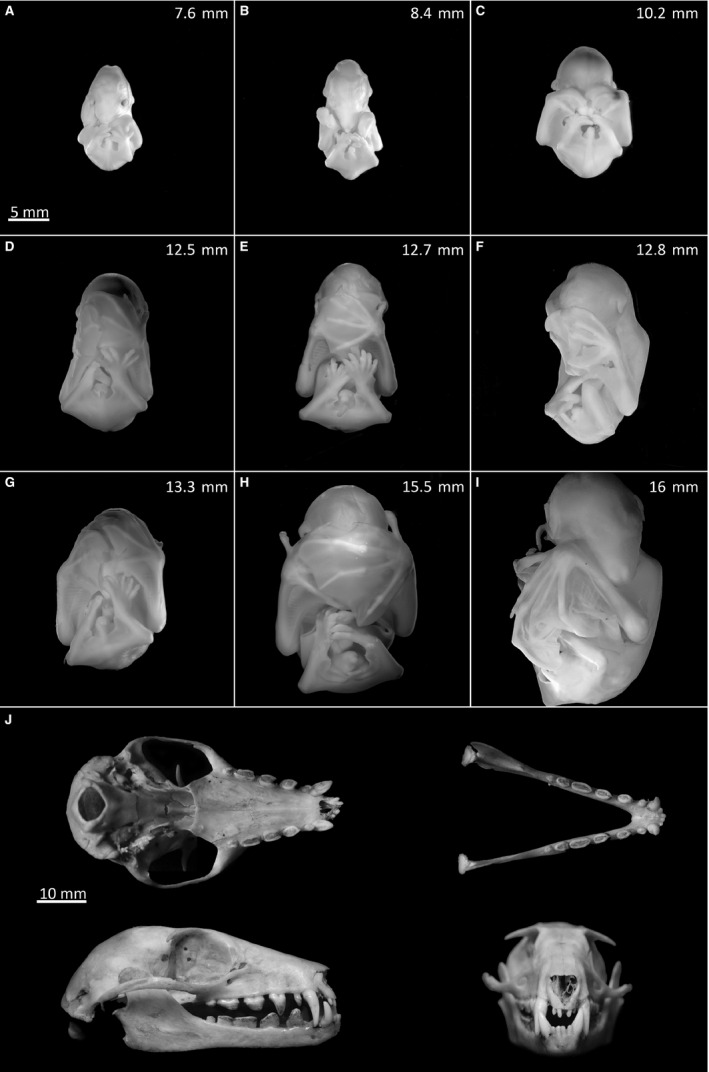
Fruit bat embryonic development (A‐I) and adult fruit bat skull (J). (A‐I) The nine fruit bat embryos used for this study were photographed frontally in ascending order of head length. (J) Top half: bottom and top views of the adult upper and lower jaws, respectively. Bottom half: sagittal and frontal views.
Materials and methods
Pregnant straw‐coloured fruit bat females were previously captured and culled in Nigeria in 1972–73 following an approved cull of bats on the campus of Ife University (Fayenuwo & Halstead, 1974). Uteri were dissected out and a range of stages of 11 fruit bat embryos were fixed in Bouin's fixative solution, dehydrated through a series of industrial methylated spirits (IMS) and stored for the long term in 70% IMS. Upon receipt, the embryos were rehydrated using IMS and decalcified with EDTA. In this study we divided the 11 embryos into nine stages (Fig. 1).
Embryos were photographed under a Leica MZ FLIII stereoscope and subsequently dehydrated using a series of increasing concentrations of methanol (30–100%), followed by isopropanol. Heads were embedded in paraffin wax and sectioned frontally at a thickness of 8 mm. Sections were stained using a trichrome stain (Picro‐Sirius Red, haematoxylin and Alcian Blue) and photographed in an anterior‐to‐posterior direction using a Nikon Eclipse 80i microscope.
Whereas staging is available for a species of New World fruit bat (Cretekos et al. 2005), this is a member of a different suborder (Microchiroptera) to Old World fruit bats (Megachiroptera) (Nowak, 1999). An embryological staging series was not available for the suborder Megachiroptera, therefore we used head length to stage our embryos. The lengths of the heads were measured in imagej as follows: a square was drawn around the head to include all extremities and a horizontal line parallel to the base of the square was drawn from the tip of the nose to the back of the head, the length of which was measured. The average of two different measurements was used as the final head length (referred to in Fig. 1).
Three‐dimensional reconstructions of dental tissues were generated from histology images in fiji – imagej 1.47v, using the trackem2 Plugin (Schindelin et al. 2012, 2015). The 3D objects were processed (smoothening and pseudocolouring) in blender 2.71.
The adult fruit bat skull is part of the skull collection of the Department of Craniofacial Development and Stem Cell Biology at King's College London.
Tooth abbreviation
Upper deciduous dentition: incisors i1, i2; canines c1; premolars pm1, pm2, pm3. Lower deciduous dentition: incisors i1, i2; canines c1; premolars pm1, pm2, pm3. Upper permanent dentition: incisors I1, I2; canines C1; premolars PM2, PM3; molars M1, M2. Lower permanent dentition: incisors I1, I2; canines C1; premolars PM2, PM3; molars M1, M2, M3. For simplicity the three premolars were here labelled 1–3, although in some text such teeth would be labelled 2–4 to highlight the existence of an ancestral first premolar that does not form in modern Old World fruit bats.
Results and discussion
Bat embryos were staged according to head size and divided into nine stages (Fig. 1A‐I). Over this period the forelimbs developed from hand plates to large wings with elongated digits and the heads extended from 7.6 to 16 mm in length. These stages are approximately equivalent to stages 17–22 of the New World fruit bat species Carollia perspicillata (Cretekos et al. 2005); however, anatomical differences such as lack of leaf nose structures in Eidolon helvum make direct comparison inaccurate. The straw‐coloured fruit bat is heterodont and diphyodont, with the deciduous dentition comprising two incisors, one canine and three premolars in each jaw quadrant. The adult dentition exhibits an additional two molars on the upper jaw and three molars on the lower jaw (Fig. 1J). Here we refer mostly to the upper jaw dentition; however, the same developmental pattern occurs on the lower jaw.
Development of the fruit bat dentition
The fruit bat embryos used in this study presented a wide range of tooth developmental stages, from dental lamina to mineralization stage. The youngest embryo (7.6 mm) had already initiated all antemolar first‐generation teeth, with the exception of pm1, which was still at the dental lamina stage and had no mesenchymal condensation visible at this point. There was no evidence of a dental lamina in the molar region at this stage. The second incisor i2 had developed to the bud stage, showing a sharp inclination toward the lingual side of the oral cavity (Fig. 2A1,B1). The tissue containing i1 for the earliest stage was lost; however, inferring from the later stages this tooth germ likely developed similarly to i2. The incisors only reached the cap stage at 13.3 mm and the late differentiation stage was not observed until the oldest embryo, 16.0 mm (Fig. 3A1‐A3,B1‐B3). No evidence of a second generation of incisors was apparent at any of the available stages. In contrast, c1 had already developed to early cap stage at 7.6 mm, and advanced rapidly through development, with the bud of C1 being clearly visible by 12.5 mm (Fig. C1‐C4). The c1 commenced the secretion of hard tissues by 13.3 mm and by the oldest stage, 16.0 mm, C2 had already surpassed c1 in size and reached the late differentiation stage (Fig. 3C1–C3).
Figure 2.
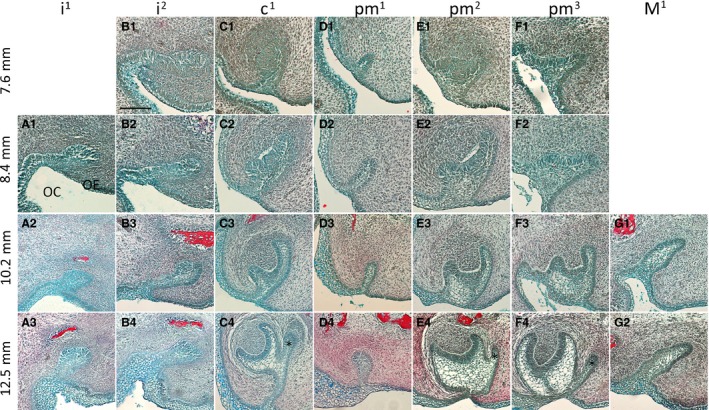
Development of the fruit bat dentition from initiation to late cap stage. Frontal histological sections of upper jaw tooth germs, stained with a Picro‐Sirius Red, Alcian Blue and haematoxylin trichrome stain. (A1‐A3) First incisor. (B1‐B4) Second incisor. (C1‐C4) Canine. (D1‐D4) First premolar. (E1‐E4) Second premolar. (F1‐F4) Third premolar. (G1‐G) First molar. Asterisks mark the initiation of the second tooth generation. OC, oral cavity; OE, oral epithelium. Labial and lingual are to the left and right sides of each image, respectively. Scale bars: 100 μm.
Figure 3.
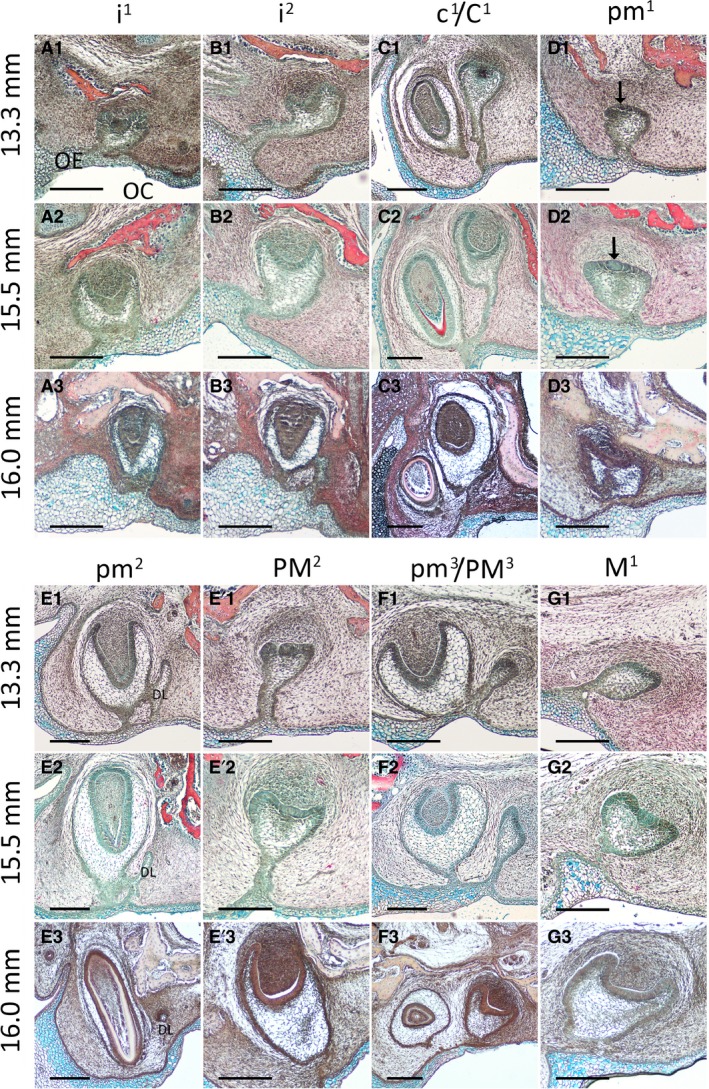
Development of the fruit bat dentition from late cap to mineralization stage. Frontal histological sections of upper jaw tooth germs, stained with a Picro‐Sirius Red, Alcian Blue and haematoxylin trichrome stain. (A1‐A3) First incisor. (B1‐B3) Second incisor. (C1‐C3) Canine. (D1‐D3) First premolar; arrows point to enamel knot. (E1‐E3) Deciduous second premolar. DL: dental lamina (E'1‐E'3) permanent second premolar. (F1‐F3) Third premolar. (G1‐G3) First molar. OC, oral cavity; OE, oral epithelium. Labial and lingual are to the left and right sides of each image, respectively. Scale bars: 200 μm.
The pm1 had only reached the dental lamina stage at 7.6 mm (Fig. 2D1). Following dental lamina elongation into the underlying mesenchyme, the early bud stage was apparent at 10.2 mm, with mesenchyme starting to condense immediately underneath (Fig. 2D1‐D4). A cap stage tooth germ was clearly identified at 13.5 mm by the presence of the enamel knot appearing as a very well‐defined ball of cells in the middle of the inner enamel epithelium (Fig. 3D1‐D2). The late cap stage was reached in the oldest embryo, but no sign of a dental lamina elongation for a second generation was observed at any point (Fig. 3D3).
The pm2 reached the cap stage at 7.6 mm, developed further and started to separate from the dental lamina at 12.5 mm, when PM2 began to develop from the dental lamina (Fig. 2E1‐E4). Interestingly, although the dental lamina could be observed next to pm2 as it developed, PM2 itself formed immediately posterior to the deciduous tooth, and could be observed in sections after pm2 (Fig. 3E,E’). Mineralization of pm2 was apparent at 15.5 mm, when PM2 was advancing through the cap stage (Fig. 3E2, E'2). PM3 lagged slightly behind in development compared with PM2, with mineralization not yet present in the oldest specimen. Unlike PM2, PM3 developed from the dental lamina directly on the lingual side of the first‐generation tooth (Figs 2F1‐F4 and 3F1‐F3).
Our histological analysis revealed that overall, the incisors and particularly the first premolar exhibited a slower pace of development compared with the canine, second and third premolars. The incisors and first premolars found in the adult fruit bat are remarkably small in size compared with the rest of the dentition (Fig. 1J). Taking into consideration the lack of visible evidence of a successional lamina next to the incisors and first premolars, it could be argued that the fruit bat does not replace its dentition at these three loci.
In the specimens we had available, only the first molar (of two for the upper jaw and three for the lower jaw) was observed, the other molars presumably forming by successional development at more mature stages; for example, in the mouse the third molar does not initiate until after birth (Chlastakova et al. 2011). The M1 formed as an elongation of the posterior free end of the dental lamina. The bud stage was apparent at 10.2 mm and by 16 mm it had advanced through to the early bell stage, when its connection to the oral epithelium began to break down. No extension of the dental lamina was apparent posterior to M1 at this stage (Figs 2G1‐2 and 3G1‐G3).
Generally in the case of replacing teeth, as the deciduous tooth germ grew in size, it began to detach from the dental lamina, which then became spatially free to generate another tooth germ (for example see Fig. 2C1‐C4 followed by Fig. 3C1‐C3). The lingual cervical loops of all antemolar tooth germs developed as part of the dental lamina before the two structures began to separate and detach. This aspect was more apparent when viewed in three‐dimensional space (Fig. 5 – upper panel).
Figure 5.
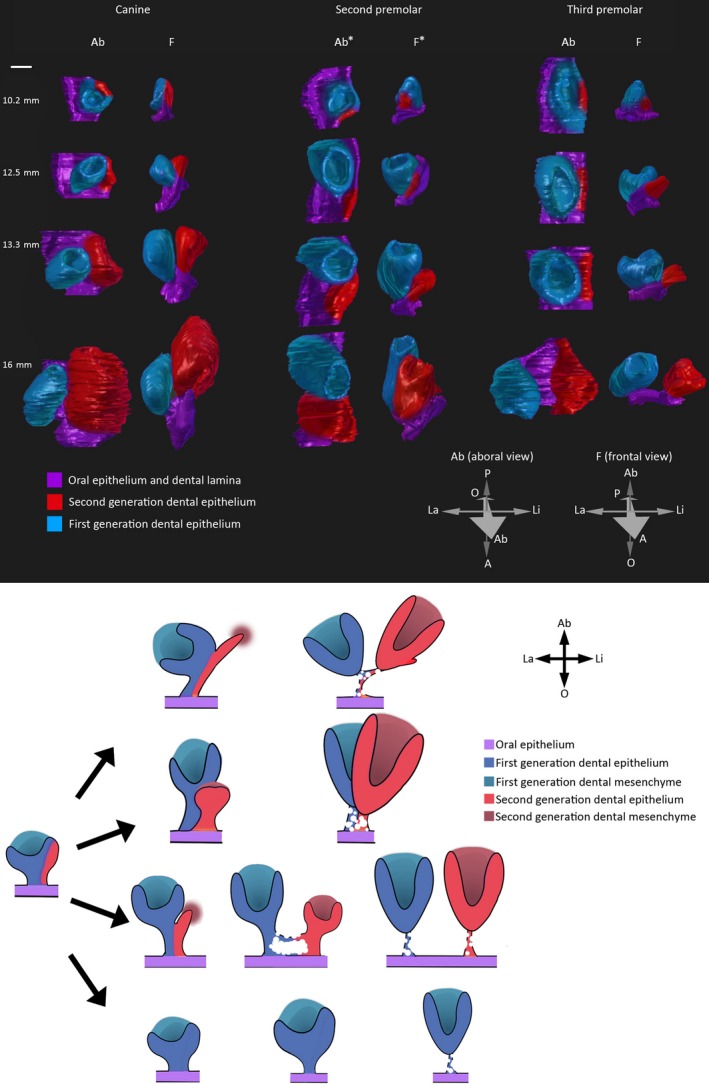
Different modalities of tooth replacement throughout the jaw. Upper panel: three‐dimensional reconstructions of replacing tooth germs during four stages of embryonic fruit bat development. Orientation arrows: La, labial; Li, lingual; A: anterior; P, posterior; O, oral; Ab, Aboral. Ab* and F*: anterior and posterior directions are reversed for better visualisation of both tooth germs. Scale bar: 100 μm. Lower panel: Schematic diagram summarizing the different modalities of replacement tooth germ development in the fruit bat. The second‐generation tooth germ develops from the dental lamina growing on the lingual side of the first‐generation tooth germ; depending on the location in the jaw, this may develop directly on the lingual side (canine), directly posterior (second premolar) or may become completely separated from the first‐generation tooth germ (third premolar). In the case of the first premolar, no second‐generation tooth germ develops.
At the early cap stage, the lingual cervical loops of c1, pm2 and pm3 were more elongated than their labial cervical loops, likely representing the extension of the dental lamina to give rise to the second generation of teeth (Fig. 2C2,E2,F2).
The pm2 and pm3 exhibited a very thick and short dental stalk (dental lamina connecting the tooth to the oral epithelium) when compared with the other teeth, especially the canine, which has a thin and very long dental stalk (Fig. 2C,E,F). These differences appear to correlate with the tooth length in adult animals: longer canines and larger, shorter premolars (Fig. 1J).
Interestingly, the cervical loops of c1 pointed towards the labial side, whereas the first‐generation premolars did not exhibit this bias and developed in a parallel direction to the oral–aboral plane (Figs 2C,E,F and 3C,E,F). This aspect may be related to the degree of separation of the two generations of tooth germs at the same dental locus on the oral epithelium later in development. The two generations of canine teeth developed closer together, perhaps owing to the early tilting of the cervical loops having allowed for sufficient space for the second generation to develop. On the other hand, the two successional third premolars became progressively more separated from each other on the oral epithelium, possibly to make more space for their further development. At the oldest stage, the two premolar tooth germs appeared almost as if they belonged to two different tooth rows (Fig. 3F2‐3). Lastly, the first premolar did not show tilting or different lengths of the cervical loops – both aspects could be correlated to the lack of replacement at this locus.
In contrast with C1 and PM3, which commenced their development on the dental lamina located directly on the lingual side of their deciduous counterparts, this process occurred slightly differently at the second premolar locus. The replacement tooth germ developed immediately posterior to the first‐generation tooth germ (Figs 3E,E’ and 5), and therefore was not observed in the same plane on frontal sections (Fig. 3E1‐E3). This could represent another adaptation to accommodate the large tooth germ size combined with restricted spatial availability. This offset positioning of two generations of tooth germs belonging to the same family has also been encountered in other species, such as the short‐tailed opossum and to some extent in the guinea pig (van Nievelt & Smith, 2005, E. M. Popa, A. S. Tucker pers. obs.).
Appearance of the dental lamina
Classically, the initiation of tooth replacement in diphyodont mammals takes place as the dental lamina extends on the lingual side of the cap stage predecessor tooth germ; the lamina appears as an epithelial offshoot elongating at an acute angle and buds at the apical end to give rise to a second‐generation tooth germ (Leche, 1895; Jarvinen et al. 2009). However, this phenotype varied to some extent in the fruit bat embryo dentition. The most overtly different appearance of the dental lamina of replacing teeth was observed in the lower jaw third premolar; the outer enamel epithelium/dental lamina of the first‐generation tooth became separated from the oral epithelium by a small island of mesenchyme (Fig. 4A). More lingually, an inpocketing of the oral epithelium re‐converged with the dental lamina epithelium, the free end of which extended further into the underlying condensing mesenchyme (Fig. 4A,A1). The marked separation of the two successional premolars on the oral epithelium has not been previously described and constitutes a clear divergence from the classical description of successive tooth formation in diphyodonts (Jarvinen et al. 2009; Stembírek et al. 2010; Buchtová et al. 2012; Jussila et al. 2014). Variation is also present in the morphology of the reptile dental lamina. In the corn snake, ball python, leopard gecko, and Madagascan ground gecko the dental lamina is permanently attached to the oral epithelium (Buchtová et al. 2008; Handrigan & Richman, 2010; Handrigan et al. 2010; Zahradnicek et al. 2012; Gaete & Tucker, 2013), whereas in the alligator the dental lamina giving rise to successional teeth loses its connection to the oral epithelium, yet still continues to generate successive teeth for the whole lifespan of the animal (Wu et al. 2013). In some species of fish, successional teeth have been shown to develop directly from the outer epithelium of the predecessor tooth rather than from a dental lamina (Huysseune & Thesleff, 2004; Fraser et al. 2006; Huysseune & Witten, 2008; Vandenplas et al. 2014).
Figure 4.

Interesting morphological aspects of the dental lamina. (A, A1) Frontal histological sections of lower jaw third premolar at 12.5 mm. (B, B1) Upper jaw second premolar at 16 mm; magnification of image in Fig. 3 (E3). Black arrows point to fragmenting outer enamel epithelium. Red arrows point to fragmenting dental lamina. (C, C1) Upper jaw canine at 16 mm, magnification of image in Fig. 3 (C3). Arrowheads point to dental lamina rudiment. OC, oral cavity; OE, oral epithelium. Labial and lingual are to the left and right sides of each image, respectively. All sections were stained with a Picro‐Sirius Red, Alcian Blue and haematoxylin trichrome stain. Scale bars: 50 μm.
Regression of the dental lamina is an important process for limiting the tooth generation to two in diphyodont animals. In the minipig, the basement membrane supporting the dental lamina epithelium breaks down, and the cells undergo a combination of epithelial to mesenchymal transition, migration and apoptosis (Buchtová et al. 2012). At 16 mm, the bat dental lamina was fragmented at several points along the antero‐posterior axis of the jaw, with many epithelial clusters of cells present in the associated mesenchyme (Fig. 4B), mirroring the situation previously reported in the minipig (Buchtová et al. 2012). A similar epithelial breakdown pattern was also observed in the outer enamel epithelium of first‐generation teeth (Fig. 4B,B1), which might indicate loss of the connection with the oral epithelium and from the replacement tooth germ, in preparation for eruption.
The canine was the first second‐generation tooth germ to initiate development and it advanced through development ahead of the other second‐generation teeth. Interestingly, at 16 mm, the second‐generation canine exhibited an epithelial elongation on its lingual side (Fig. 4C,C1). This was reminiscent of the dental lamina appearance during tooth replacement initiation observed in the bat at 12.5 mm as well as other diphyodont species (Jarvinen et al. 2009; Buchtová et al. 2012; Olley et al. 2014;). However, the canine locus is only replaced once, not twice; the dental lamina epithelium is likely to have lost its potential for tooth replacement by this later stage and probably regresses shortly thereafter. A similar occurrence has been observed in the mouse, where a rudiment of the dental lamina was observed to protrude on the lingual side of the molars during late prenatal stages, after which time it began to reduce in size (Dosedělová et al. 2015). In humans, a primordium of the third dentition has been described as an epithelial projection appearing lingual to the permanent tooth germ (Ooë, 1981). This indicates that the dental lamina could remain competent to generate further teeth if the correct signals were operating.
The mammalian dental lamina appears to have a limited lifespan and limited competence to generate successional teeth, as indicated by its regression after the initiation of the second tooth generation in diphyodonts. We propose a scenario whereby the earlier the development of the first tooth is initiated, the higher the likelihood the dental lamina will produce more successional teeth. This hypothesis could explain the visible attempt to produce a third generation for the canine locus, which is one of the first deciduous teeth (and the first permanent tooth) to start developing. It also explains the lack of replacement of the late developing first premolar. Slowly developing tooth germs also appeared to be less likely to develop a replacement, as in the case of the incisors which remained at the bud stage for long periods of time, while elsewhere in the jaw, tooth germs had advanced from early bud to late cap. This timing must be carefully balanced at a molecular level, as crossing this threshold and initiating secondary teeth too early could lead to inhibition of the development of the deciduous dentition, as has been proposed to occur in the shrew (Järvinen et al. 2008). It remains to be investigated what the molecular controls behind the exact timing of tooth initiation are and how this timing may affect the competence of the diphyodont dental lamina to produce successional teeth.
Successional teeth develop in different arrangements
We performed three‐dimensional reconstructions at the three replacing tooth loci we have described above – canine, second premolar and third premolar – to understand the developing relationship between the deciduous and replacement tooth at these three loci (Fig. 5, upper panel). Following the development of these tooth germs in 3D space we suggest that the first‐generation tooth germs develop with their lingual cervical loops embedded in the dental lamina. As the tooth germs advance in development, they begin to cleave from the dental lamina and slowly detach from it, with the epithelial connection between the tooth germ and dental lamina becoming visibly thinner. The lamina continued its growth at an angle towards the lingual side and gave rise to the second‐generation tooth germ; however, this process unfolded slightly differently for the three different tooth families: C1 developed on the lingual side of c1, whereas PM2 developed directly posterior to pm2. After initiation of PM3, the two successional third premolars detached from each other laterally, and exhibited separate epithelial connections to the oral epithelium.
Given the morphology of the dental epithelial tissues throughout development, it is likely that the location from which the second tooth generation develops on the dental lamina is specified very early on. It has been suggested that signals from the first‐generation tooth germ are required for the development of a second generation; however, in dogs with X‐linked hypohidrotic ectodermal dysplasia, which lack the deciduous dentition, it was possible to rescue the permanent set by administration of recombinant Eda protein, suggesting that the development of the deciduous and permanent dentitions can be independent processes (Casal et al. 2007).
Conclusions
In this study we have given an account of the morphological aspects of tooth development and replacement in a diphyodont animal with a complete dentition. We conclude that there are subtle variations to the tooth replacement phenotype when comparing the process at different tooth loci (summarized in Fig. 5, lower panel). These findings also reiterate the process of tooth replacement in diphyodont animals whereby the second‐generation tooth forms by growth of the dental lamina and not by budding of the deciduous tooth epithelium on the lingual side (Leche, 1895; Jarvinen et al. 2009). Related to this, an interesting question to answer would be how the process of separation of first‐generation tooth germs from the dental lamina occurs in diphyodonts and what cellular events are involved in this process. Furthermore, we have shown the first instance of a successional lamina rudiment attached to a second‐generation tooth in a diphyodont animal. Although this particular species is no longer available for experimental purposes, other more common species of Chiroptera could be screened for this dental lamina rudiment. Obtaining gene expression data on this structure could lead to a better understanding of why the development of a further generation of teeth is suppressed in diphyodonts.
Author contributions
E.M.P. obtained the data, performed data analysis and prepared the figures. E.M.P. and A.S.T. designed the study and wrote the manuscript. N.A. helped obtain part of the data and critically reviewed the manuscript.
Acknowledgements
We would like to thank Professor Moya Meredith Smith for kindly providing us with the fruit bat specimens. The authors declare no conflict of interest.
References
- Buchtová M, Handrigan GR, Tucker AS, et al. (2008) Initiation and patterning of the snake dentition are dependent on Sonic Hedgehog signaling. Dev Biol 319, 132–145. [DOI] [PubMed] [Google Scholar]
- Buchtová M, Stembirek J, Glocova K, et al. (2012) Early regression of the dental lamina underlies the development of diphyodont dentitions. J Dent Res, 91, 491–498. [DOI] [PubMed] [Google Scholar]
- Casal ML, Lewis JR, Mauldin EA, et al. (2007) Significant correction of disease after postnatal administration of recombinant ectodysplasin A in canine X‐linked ectodermal dysplasia. Am J Hum Genet 81, 1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, et al. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Chlastakova I, Lungova V, Wells K, et al. (2011) Morphogenesis and bone integration of the mouse mandibular third molar. Eur J Oral Sci 119, 265–274. [DOI] [PubMed] [Google Scholar]
- Cretekos CJ, Weatherbee SD, Chen C, et al. (2005) Embryonic staging system for the short‐tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive‐bred animals. Dev Dyn 233, 721–738. [DOI] [PubMed] [Google Scholar]
- Crucke J, Huysseune A (2014) Blocking VEGF signaling delays development of replacement teeth in Zebrafish. J Dent Res 94, 157–165. [DOI] [PubMed] [Google Scholar]
- Dosedělová H, Dumková J, Lesot H, et al. (2015) Fate of the molar dental lamina in the monophyodont mouse. PLoS One 10, e0127543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayenuwo JO, Halstead LB (1974) Breeding cycle of straw‐colored fruit bat, Eidolon helvum, at Ile‐Ife, Nigeria. J Mammal 55, 453–454. [PubMed] [Google Scholar]
- Fraser GJ, Berkovitz BK, Graham A, et al. (2006) Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of the osteichthyan dentition. Evol Dev 8, 446–457. [DOI] [PubMed] [Google Scholar]
- Gaete M, Tucker AS (2013) Organized emergence of multiple‐generations of teeth in snakes is dysregulated by activation of Wnt/Beta‐catenin signalling. PLoS One 8, e74484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handrigan GR, Richman JM (2010) A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol 348, 130–141. [DOI] [PubMed] [Google Scholar]
- Handrigan G.R., Leung K.J., Richman J.M. (2010) Identification of putative dental epithelial stem cells in a lizard with life‐long tooth replacement. Development, 137, 3545–3549. [DOI] [PubMed] [Google Scholar]
- Huysseune A (2006) Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol 50, 637–643. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Thesleff I (2004) Continuous tooth replacement: the possible involvement of epithelial stem cells. BioEssays 26, 665–671. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Witten PE (2008) An evolutionary view on tooth development and replacement in wild Atlantic salmon (Salmo salar L.). Evol Dev 10, 6–14. [DOI] [PubMed] [Google Scholar]
- Jarvinen E, Tummers M, Thesleff I (2009) The role of the dental lamina in mammalian tooth replacement. J Exp Zool B Mol Dev Evol 312, 281–291. [DOI] [PubMed] [Google Scholar]
- Järvinen E, Välimäki K, Pummila M, et al. (2008) The taming of the shrew milk teeth. Evol Dev 10, 477–486. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I (2012) Tooth shape formation and tooth renewal: evolving with the same signals. Development 139, 3487–3497. [DOI] [PubMed] [Google Scholar]
- Jussila M, Crespo Yanez X, Thesleff I (2014) Initiation of teeth from the dental lamina in the ferret. Differentiation 87, 32–43. [DOI] [PubMed] [Google Scholar]
- Juuri E, Saito K, Ahtiainen L, et al. (2012) Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev Cell 23, 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juuri E, Jussila M, Seidel K, et al. (2013) Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development, 140, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leche W (1895) Zür Entwicklungsgeschichte des Zahnsystems des Säugethiere, zugleich ein Beitrag zur Stammesgeschichte dieser Thiergruppe. I. Ontogenie. Zoologica 6, 1–160. [Google Scholar]
- Lesot H, Hovorakova M, Peterka M, et al. (2014) Three‐dimensional analysis of molar development in the mouse from the cap to bell stage. Aust Dent J 59(Suppl. 1), 81–100. [DOI] [PubMed] [Google Scholar]
- Luckett WP (1993) Ontogenetic staging of the mammalian dentition, and its value for assessment of homology and heterochrony. J Mamm Evol 1, 269–282. [Google Scholar]
- Mutere FA (1967) The breeding biology of equatorial vertebrates: reproduction in the fruit bat, Eidolon helvum, at latitude 0°20′N. J Zool 153(2), 153–161. [Google Scholar]
- van Nievelt AFH, Smith KK (2005) To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31, 324–346. [Google Scholar]
- Nowak RM (1999) Walker's Mammals of the World,. 6th edn, vol. 1 Baltimore: Johns Hopkins University Press. [Google Scholar]
- Olley RC, Olley R, Xavier GM, et al. (2014) Expression analysis of candidate genes regulating successional tooth formation in the human embryo. Front Physiol 5, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooë T. (1981) Human Tooth and Dental Arch Development. Tokyo: Ishiyaku Publishers, 217. [Google Scholar]
- Peterkova R, Hovorakova M, Peterka M, et al. (2014) Three‐dimensional analysis of the early development of the dentition. Aust Dent J 59(Suppl. 1), 55–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch LJ, Martin KJ, Cooper RL (2016) An ancient dental gene set governs development and continuous regeneration of teeth in sharks. Dev Biol, 415, 347–370. [DOI] [PubMed] [Google Scholar]
- Rothova M, Thompson H, Lickert H, et al. (2012) Lineage tracing of the endoderm during oral development. Dev Dyn 241, 1183–1191. [DOI] [PubMed] [Google Scholar]
- Sasaki C, Sato T, Kozawa Y (2001) Apoptosis in regressive deciduous tooth germs of Suncus murinus evaluated by the the TUNEL method and electron microscopy. Arch Oral Biol 46, 649–660. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, et al. (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Rueden C.T, Hiner M.C, et al. (2015) The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82, 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MM, Fraser GJ, Mitsiadis TA (2009) Dental lamina as source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zool B Mol Dev Evol 312, 260–280. [DOI] [PubMed] [Google Scholar]
- Soukup V, Epperlein HH, Horacek I, et al. (2008) Dual epithelial origin of vertebrate oral teeth. Nature 455, 795–798. [DOI] [PubMed] [Google Scholar]
- Stembírek J, Buchtová M, Král T, et al. (2010) Early morphogenesis of heterodont dentition in minipigs. Eur J Oral Sci 118, 547–558. [DOI] [PubMed] [Google Scholar]
- Streelman JT, Webb JF, Albertson RC, et al. (2003) The cusp of evolution and development: a model of cichlid tooth shape diversity. Evol Dev 5, 600–608. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Fraser GJ (2014) Evolution and developmental diversity of tooth regeneration. Semin Cell Dev Biol 25–26, 71–80. [DOI] [PubMed] [Google Scholar]
- Vandenplas S, De Clercq A, Huysseune A (2014) Tooth replacement without a dental lamina: the search for epithelial stem cells in Polypterus senegalus . J Exp Zool B Mol Dev Evol 322, 281–293. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu X, Jiang T‐X, et al. (2013) Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc Natl Acad Sci U S A 110, E2009–E2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Yasui K, Sonomura T, et al. (2007) Development of heterodont dentition in house shrew (Suncus murinus). Eur J Oral Sci 115, 433–440. [DOI] [PubMed] [Google Scholar]
- Zahradnicek O, Horacek I, Tucker AS (2012) Tooth development in a model reptile: functional and null generation teeth in the gecko Paroedura picta . J Anat 221, 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]


