Progress in understanding tumor-specific immune responses, genetic engineering and ex vivo manufacturing, have led to improvements in the safety and feasibility of adoptive transfer of genetically modified T cells. However, rational design, application and evaluation of T-cell therapy requires monitoring methods that can detect, locate and serially quantify these cell-mediated immune responses. Currently, such monitoring methods are chiefly limited to invasive techniques to investigate recovered cell populations for in vitro measurements including histology, flow cytometry, Q-PCR or the detection of cytokines. These assays provide episodic glimpses of the bio distribution of T cells and are limited by the number and sites of sampling. In contrast, imaging provides a methodology for quantitative, non-invasive, longitudinal and spatial in vivo information about the dynamic processes of infused T cells.
In earlier studies, in vivo imaging of lymphocyte migration used cells passively labeled with radiotracers ex vivo. An alternative imaging approach involves stable transduction/transfection of cells with a reporter gene (such as herpes simplex virus-1 thymidine kinase; HSV-TK) that can be visualized by active accumulation of a radiolabeled reporter probe. It has been demonstrated that cells genetically modified to express HSV-TK could be imaged by scintigraphy or positron emission tomography (PET) in rodents after infusions of I-labeled 2′-fluoro-2′-deoxy-1-β-D-arabinofuranosyl-5-iodouracil.1 Bioluminescence imaging (BLI) of luciferase reporter gene expression has also been used to monitor lymphocyte trafficking in a mouse model.2,3 Recently Dobrenkov et al.4 have shown the feasibility of PET and BLI in a prostate cancer model using peripheral blood chimeric antigen receptor (CAR)+ cells and Jensen and co-workers have evaluated the biodistribution of CAR+ T cells by magnetic resonance imaging.5
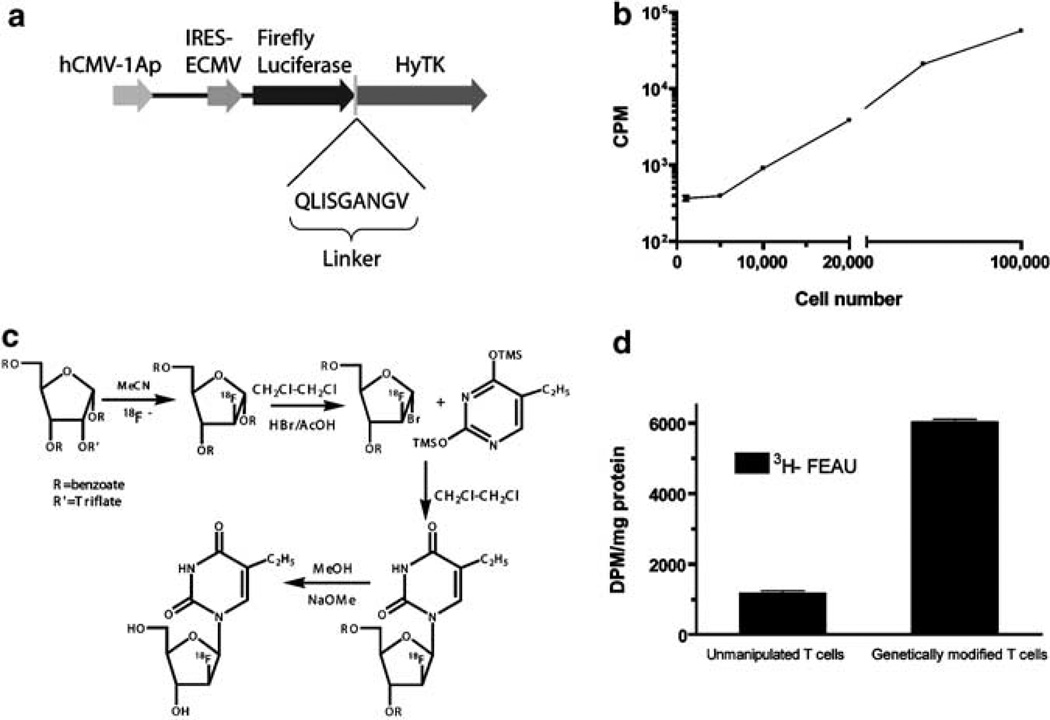
Previously, we have demonstrated that human T cells derived from peripheral blood and umbilical cord blood (UCB) mononuclear cells can be rendered specific for CD19 by the introduction of a CAR.3,6 To track the persistence of infused genetically modified T cells, a multi-function reporter gene (designated ffLucHyTK) was developed which fuses firefly luciferase (ffLuc), hygromycin phosphotransferase (Hy) and HSV-TK (Figure 1).2,3 The four-function reporter transgene can achieve (i) in vitro selection of genetically modified T cells in cytocidal concentrations of hygromycin B, (ii) in vivo ganciclovir-dependent conditional ablation7 and in vivo imaging with (iii) BLI with (iv) PET. A DNA plasmid expressing the fusion gene was expressed in T cells to render them resistant to cytocidal concentrations of hygromycin B and thus co-express the ffLuc and TK imaging genes.
Figure 1.
(a) Schematic of the fusion transgene ffLucHyTK expressed from the DNA plasmid ffLucHyTK-pMG.3 (b) Luciferase activity of genetically modified T cells, incubated with D-Luciferin (1.43 mg/ml in 200 µl) in a 96-well Opti-plate and the luminescence measured (CPM) using TopCount (Perkin-Elmer). (c) 2′-Deoxy-2′-[18F]fluoro-5-ethyl-1-β-d-arabinofuranosyluracil ([18F]-FEAU) was synthesized as described earlier.8 Briefly, 1,3,5-tri-O-benxoyl-2-trifluoromethasulfonyl-ribose was radiolabeled with 18F-fluoride using Bu4N18F to the corresponding 2-18F-fluoro-arabinose. The 2-18F-fluoro-arabinose was then converted to its 1-bromo-derivative, which was condensed with protected 5-ethyluracil. Hydrolysis of the protecting groups in the sugar moiety with NaOMe produced the desired radiolabeled nucleoside, which was purified by HPLC. Solvent was evaporated, and then the product was re-constituted in saline and filtered through a 0.22 µ Millipore filter. The radiochemical yield was 20% (decay corrected, d.c.) from the end of bombardment. Radiochemical purity was > 99% and specific activity was > 74 GBq/µmol. (d) In vitro radiotracer uptake study, genetically modified or unmanipulated T cells (107) were centrifuged (1000 g, 5 min) and the pellet was resuspended in 6ml of RPMI media containing 10% fetal bovine serum and the radiotracers [14C]-thymidine (0.01 µCi/ml) (Moravek, Brea, CA, USA) and [3H]-FEAU (0.1 µCi/ml) (Moravek). The cell suspension was subsequently divided into three 10mm2 dishes (2 ml per dish) and incubated for 120 min. Each suspension was then transferred into a 15ml tube, and centrifuged at 1000 g for 2 min. A 100 µl aliquot of supernatant was transferred to a pre-weighed scintillation tube, and the rest was removed by aspiration before snap-freezing the cell pellet on dry ice. The frozen pellets were transferred to pre-weighed scintillation vials, weighed and thoroughly resuspended in 0.5 ml of Soluene-350 (Perkin-Elmer, Boston, MA, USA) before adding 4ml of Insta-Fluor Plus scintillation fluid (Perkin-Elmer). Radioactive β emissions of the media and the cell pellets were measured within precalibrated energy ranges using a Packard Tri-Carb 3100TR scintillation counter to quantitate [3H]-FEAU and [14C]-thymidine uptakes. Activity accumulation ratios of cell pellet to media ((DPM/g cells)/(DPM/g media)) were then determined and plotted.
This was achieved using UCB-derived T cells that underwent electro-transfer with the plasmid ffLucHyTK-pMG (Figure 1a). We demonstrated that the genetically modified and propagated TK+ T cells could be visualized by selective incorporation of radiolabeled 2′-fluoro-2′deoxy-1-β-D-arabionofuranosyl-5-ethyl-uracil (FEAU) in a xenogenic mouse model. Radiolabeled FEAU is a specific substrate for HSV1-TK enzyme, which accumulates in HSV1-TK-transfected cells through phosphorylation by the HSV1-TK enzyme, and that the magnitude and spatial distribution of accumulation of FEAU-derived radioactivity in the body reflects the location and density of TK+ cells. Initially, in vitro uptake studies were conducted to evaluate the accumulation and retention of [3H]FEAU. The genetically modified T cells exhibited selective accumulation of FEAU as compared with untransfected T cells (1185 and 6031 disintegrations per minute (DPM)/mg protein respectively) (Figure 1d). The proliferation rate of transfected and untransfected cells was equivalent as demonstrated by [14C]-labeled thymidine uptake. The HSV-TK-derived enzymatic activity detected by in vitro accumulation of FEAU was high and adequate for in vivo imaging with microPET.
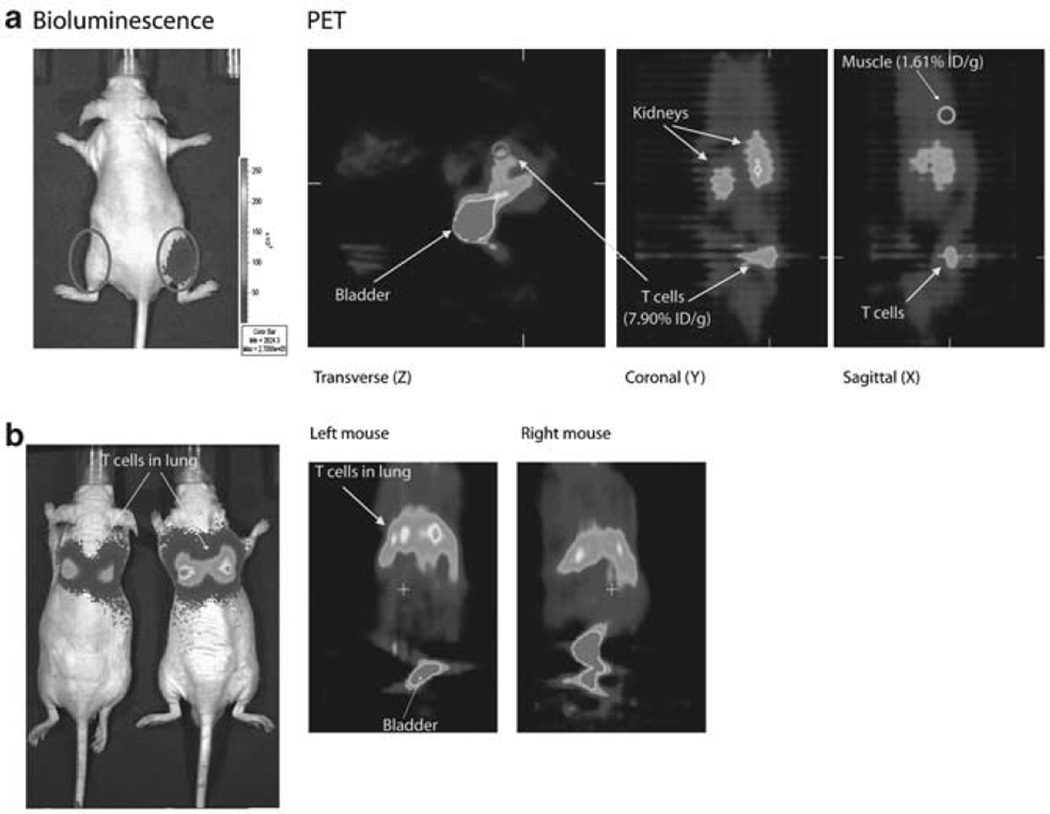
To visualize genetically modified UCB-derived T cells by BLI and µPET, nude mice were injected subcutaneously (right hind leg) or intravenously through tail vein and imaged using an ultrasensitive CCD camera followed by a µPET scan. Bioluminescent images after intraperitoneal injection of d-Luciferin revealed that genetically modified T cells express high bioluminescence (2.22 × 105 p/sec/cm2/sr) in comparison to the control (left uninjected leg) (0.29 × 105 p/sec/cm2/sr). These mice were then imaged by µPET using intravenous infusion of [18F]FEAU and static images were obtained. The signal from the images was used to determine % injected dose (ID)/g of FEAU which is a measure of the amount of tracer accumulated in a given tissue site normalized to the injected amount and to the mass of tissue examined. The %ID/g value for genetically modified T cells (7.9%) was higher than the control (muscle, 1.61%) (Figure 2a). After intravenous infusion of genetically modified UCB-derived T cells, the cells could be visualized in the lung both by BLI and µPET. Ratio of bioluminescent signals from lungs and control tissue (abdomen) was 36. PET images reveal the accumulation of radiotracer in the lungs along with the urinary bladder (Figure 2b).
Figure 2.
Bioluminescent and µPET imaging. To non-invasively track the UCB-derived T cells in vivo, a DNA plasmid expressing the reporter gene ffLucHyTK was introduced by non-viral gene transfer. Nude mice were injected with ffLucHyTK+ UCB-derived T cells (a) intramuscularly (106) and (b) intravenously (107). Bioluminescent imaging (Xenogen-IVIS model 200 Imaging System) of the genetically modified T cells was performed 5 min following an intraperitoneal injection of d-luciferin (Xenogen Corp; 2 mg/Kg in PBS). MicroPET imaging (Concorde Microsystem Roden R4 microPET) was performed after intravenous injection of radiotracer [18F]-FEAU (3.7MBq in 100 µl of saline) and following a 2-h incubation, static PET images were acquired. PET images were then reconstructed using ordered subset expectation maximization algorithms with subsets 16 and iteration 4. Regional radioactivity concentrations of organs for [18F]-FEAU were estimated by regions of interest (ROI) drawn around the organ on the reconstructed image. The radiotracer uptake levels in the blood (aorta), and muscle were determined and expressed as percent injected dose per gram (%ID/g)±s.d.
These data indicate that the genetically modified UCB-derived T cells expressing the ffLucHyTK fusion transgene can uptake PET-tracer in vitro and can be visualized spatiotemporally in vivo by both BLI and µPET. As the ffLucHyTK is similar to the HyTK transgene used in clinical trials9 these data can be used to provide the proof-of-principle for instituting PET imaging after infusion of donor-derived T cells following UCB transplantation.
This is the first report demonstrating that HyTK fusion gene can be used not only for selection and suicide,3 but also for PET using T cells from UCB. This has translational importance as the TK and HyTK transgenes are currently used in clinical trials,7,9 where co-expression of the TK suicide gene may be necessary to ensure safety of initial protocols infusing genetically modified T cells. As investigators proceed with infusing genetically modified T cells, including from umbilical cord blood, the incorporation of PET imaging is expected to be a useful biologic end point.
Acknowledgments
Support from Cancer Center Core Grant (CA16672); RO1 (CA124782, CA120956); R21 (CA129390, CA116127); DOD (PR064229); The Alliance for Cancer Gene Therapy; The Alex Lemonade Stand Foundation; The Carl C Anderson, Sr and Marie Jo Anderson Charitable Foundation; The Gillson Longenbaugh Foundation; The JP McCarthy Fund Developmental Grant Program The Leukemia and Lymphoma Society; The Lymphoma Research Foundation; The Miller Foundation, The National Foundation for Cancer Research; The National Marrow Donor Program; The Pediatric Cancer Research Foundation.
References
- 1.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 2.Singh H, Serrano LM, Pfeiffer T, Olivares S, McNamara G, Smith DD, et al. Combining adoptive cellular and immunocytokine therapies to improve treatment of B-lineage malignancy. Cancer Res. 2007;67:2872–2880. doi: 10.1158/0008-5472.CAN-06-2283. [DOI] [PubMed] [Google Scholar]
- 3.Serrano LM, Pfeiffer T, Olivares S, Numbenjapon T, Bennitt J, Kim D, et al. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrenkov K, Olszewska M, Likar Y, Shenker L, Gunset G, Cai S, et al. Monitoring the efficacy of adoptively transferred prostate cancer-targeted human T lymphocytes with PET and bioluminescence imaging. J Nucl Med. 2008;49:1162–1170. doi: 10.2967/jnumed.107.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazovic J, Jensen MC, Ferkassian E, Aguilar B, Raubitschek A, Jacobs RE. Imaging immune response in vivo: cytolytic action of genetically altered T cells directed to glioblastoma multiforme. Clin Cancer Res. 2008;14:3832–3839. doi: 10.1158/1078-0432.CCR-07-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 7.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 8.Soghomonyan S, Hajitou A, Rangel R, Trepel M, Pasqualini R, Arap W, et al. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat Protoc. 2007;2:416–423. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MC, Popplewell L, DiGiusto DL, Kalos M, Cooper LJN, Raubitschek A, et al. A first-in-human clinical trial of adoptive therapy using CD19-specific chimeric antigen receptor re-directed T cells for recurrent/refractory follicular lymphoma. Mol Ther. 2007;15:S142. [Google Scholar]