Abstract
Background and objectives
Trabecular bone score is a gray–level textural measure obtained from dual energy x-ray absorptiometry lumbar spine images that provides information independent of areal bone mineral density. The association between trabecular bone score and incident fractures in adults with reduced kidney function and whether this association differs from that of adults with normal kidney function are unknown.
Design, setting, participants, & measurements
We included 1426 participants ages ≥40 years old (mean age of 67 years) in the community–based Canadian Multicentre Osteoporosis Study. We stratified participants at cohort entry (2005–2008) by eGFR (eGFR<60 ml/min per 1.73 m2 [n=199; 72.4% stage 3a, 25.1% stage 3b, and 2.5% stage 4] versus ≥60 ml/min per 1.73 m2 [n=1227]). Trabecular bone score was obtained from lumbar spine (L1–L4) dual energy x-ray absorptiometry images, with a lower trabecular bone score representing worse bone structure. Over an average of 4.7 years follow-up (maximum follow-up of 5 years), we documented incident fragility (low–trauma) fracture events (excluding craniofacial, foot, and hand sites). We used a modified Kaplan–Meier estimator to determine the 5-year probability of fracture. Cox proportional hazard regression per SD lower trabecular bone score expressed the gradient of fracture risk.
Results
Individuals with an eGFR<60 ml/min per 1.73 m2 who had a trabecular bone score value below the median (<1.277) had a significantly higher 5-year fracture probability than those above the median (18.1% versus 6.2%; P=0.01). The association between trabecular bone score and fracture was independent of bone mineral density and other clinical risk factors in adults with reduced and normal kidney function (adjusted hazard ratio per SD lower trabecular bone score: eGFR<60 ml/min per 1.73 m2: adjusted hazard ratio, 1.62; 95% confidence interval, 1.04 to 2.51; eGFR≥60 ml/min per 1.73 m2: adjusted hazard ratio, 1.44; 95% confidence interval, 1.13 to 1.83).
Conclusions
Lower lumbar spine trabecular bone score is independently associated with a higher fracture risk in adults with reduced kidney function. Additional study is needed to examine the association between trabecular bone score and fractures in individuals with diagnosed CKD-mineral and bone disorder.
Keywords: trabecular bone score; chronic kidney disease; fragility fracture; reduced kidney function; Absorptiometry, Photon; adult; Bone Density; Bone and Bones; Canada; Confidence Intervals; Follow-Up Studies; glomerular filtration rate; humans; Lumbar Vertebrae; minerals; Osteoporosis; risk factors
Introduction
Adults with CKD have an increased fracture risk compared with the general population (1–7); even individuals with mild to moderate declines in kidney function (eGFR=30–59 ml/min per 1.73 m2) have approximately a twofold higher fracture risk compared with adults with normal kidney function (4). Reasons for this increased fracture risk are multifactorial and include CKD-mineral and bone disorder (CKD-MBD; i.e., abnormalities in phosphorous, calcium, parathyroid hormone, or vitamin D metabolism) and muscle wasting (8,9). Unfortunately, in the CKD population, the best method to evaluate bone health and fracture risk has not been identified. Bone mineral density (BMD) is widely used in the general population to risk stratify individuals at an increased fracture risk. However, the utility of BMD in the CKD population is controversial (8). A potential explanation is that BMD only measures one factor potentially contributing to an increased fracture risk, bone mass, providing no information on bone texture, which is also adversely affected in CKD (8). It has been suggested that both bone mass and texture need to be assessed to provide an accurate assessment of fracture risk in individuals with CKD (10).
Trabecular bone score (TBS) is a gray–level textural measure that indirectly assesses bone trabecular microarchitecture, providing information that BMD cannot capture (11,12). TBS measurements can easily be obtained from dual energy x-ray absorptiometry (DXA) images of the lumbar spine (13). Better bone trabecular structure (e.g., dense and well connected trabecular bone) results in a higher TBS value. In contrast to TBS, other non–DXA approaches to BMD assessment used to evaluate fracture risk (e.g., high–resolution peripheral quantitative computed tomography, micromagnetic resonance imaging, and bone biopsy) are limited by high costs, low availability, invasiveness, and their inability to assess spine bone texture (where fractures commonly occur) (13–15). However, these measures have shown some utility in the CKD population (16–18).
In the general population, TBS is considered prognostic, with lower TBS values associated with a higher fracture risk independent of fracture clinical risk factors, and this has been found at multiple fracture sites in addition to the vertebral site (19–23). As a result, TBS is now incorporated into the widely used World Health Organization fracture risk assessment score (FRAX; fracture prediction tool that uses a combination of age, sex, clinical risk factors, and femoral neck BMD [optional] to predict the 10-year probability of major osteoporotic fracture [i.e., forearm, hip, humerus, and clinical vertebral fractures]) (24).
A lower TBS is also associated with fractures in individuals who have undergone kidney transplantation, with diabetes mellitus, or treated with glucocorticoids (25–29). We performed a detailed search of PubMed and other bibliographic databases and found no study assessing TBS in a nontransplant CKD population. Therefore, we examined the association between TBS and fracture risk in adults with reduced kidney function and examined whether this association differs from adults with normal kidney function. In an additional analysis, we examined characteristics associated with a lower TBS. To account for the potential independent contribution of TBS to fracture risk, we also recalculated FRAX fracture probabilities adjusting for TBS.
Materials and Methods
Canadian Multicentre Osteoporosis Study
Details on the prospective observational study, the Canadian Multicentre Osteoporosis Study (CaMos), have previously been published (30,31). In brief, individuals were randomly selected to participate in the CaMos using residential phone numbers; approximately 42% of approached individuals participated in the baseline interview, DXA, and spine x-rays (31). A standardized interviewer–administered questionnaire along with weight, height, and BMD measurements were then taken for participants every 5 years beginning in 1995 (31). Blood samples were comprehensively collected in the CaMos year 10, with the Montreal CDL Labs analyzing all serum creatinine samples. To comply with the Helsinki Declaration, all participants provided written informed consent. McGill University along with each participating study center’s ethics review board provided study approval.
Cohort
To ensure a sufficient sample of participants with available blood samples, the study baseline for this analysis was the interview and assessment at year 10 (cohort entry; 2005–2008). Individuals ages ≥40 years old who had both a serum creatinine measurement and TBS were eligible. Creatinine values were missing from individuals at the CaMos center that did not collect blood work (Hamilton) and from individuals who did not consent to have blood taken. We excluded individuals who died between years 10 and 11, could not be reached at year 11, or dropped out of the study at year 11 because of their uncertain fracture status. We also excluded individuals who had a previous organ transplant. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (32). We used the Kidney Disease Improving Global Outcomes (KDIGO) guidelines to define kidney function at cohort entry (reduced kidney function: eGFR<60 ml/min per 1.73 m2 and normal kidney function: eGFR≥60 ml/min per 1.73 m2) (33). For individuals with reduced kidney function, we further defined kidney function according to the following CKD stages: 3a, 3b, and 4 (eGFR=45–59, 30–44, and 15–29 ml/min per 1.73 m2, respectively) (33).
BMD and TBS
All BMD values in the CaMoS were adjusted to a common phantom measured each assessment year on each of the participating DXA machines. To measure areal BMD, we used the Hologic QDR DXA Scanner (Marlborough, MA) at four centers and the Lunar Scanner (Waukesha, WI) at five centers. Standard methods converted Lunar data to Hologic values (34–37). Information on the BMD crosscalibration and quality control program can be found elsewhere (38). We compared each individual’s BMD with the Third National Health and Nutrition Examination Survey reference range for white women ages 20–29 years old to calculate femoral neck T scores (39); lumbar spine (L1–L4) T scores were calculated using the United States manufacturer’s reference values (39). The Bone Disease Center at the Lausanne University Hospital in Switzerland provided TBS values using our cohort’s anonymized lumbar spine DXA images. The TBS iNsight Software (version 2.1; Medimaps, Merignac, France) calculated TBS values using the same anteroposterior spine region as used for the lumbar spine BMD measurement.
Covariates
We defined bisphosphonate therapy as use of one of the following medications at cohort entry: zoledronate, pamidronate, risedronate, alendronate, clodronate, or etidronate. We characterized previous corticosteroid use as evidence of oral or intravenous glucocorticoids for ≥3 months before cohort entry. We considered rheumatoid arthritis as evidence of a self–reported rheumatoid arthritis diagnosis and evidence of treatment (i.e., sulfasalazine, betamethasone, prednisone, hydroxychloroquine, leflunomide, adalimumab, methotrexate, etanercept, or infliximab). We defined prevalent fragility fractures as a self-reported fracture caused by low trauma (i.e., a fall from a standing height or less) before cohort entry (excluding those at craniofacial, foot, hand, and ankle sites); self–reported parental hip fracture was ascertained at the CaMos years 5 and 10. We defined all other covariates by self-report or measurement at or before cohort entry. We divided weight (in kilograms) by height squared (in meters2) to ascertain body mass index. To calculate the 10-year probability of major osteoporotic fracture (i.e., low–trauma hip, forearm, humerus, or clinical vertebral fractures), we used the Canadian FRAX tool (FRAX Desktop Multi-Patient Entry, version 3.7) (Supplemental Table 1).
Incident Fractures
We included all incident fragility (low–trauma) fractures (excluding craniofacial, foot, and hand sites). Fractures were ascertained through self-report within 5 years of cohort entry or in person at the CaMos study year 15 (last follow-up time) (30). The following mechanisms were used to verify self-reported fractures: additional information from a structured interview (e.g., fracture date, location, cause, and treatment) plus hospital or treating physician verification (30). In an additional analysis, we assessed incident major osteoporotic fractures.
Statistical Analyses
For baseline characteristics, we used means (SD) or medians (interquartile ranges) to describe continuous variables and proportions to describe categorical variables. We used the t test or Mann–Whitney U test as appropriate to compare continuous baseline characteristics and the chi-squared test or Fisher exact test to compare proportions. We used a modified Kaplan–Meier estimator accounting for the competing risk of death to express the 5-year fracture-free probability (40), stratifying by individuals who were above or below the median TBS; to determine if there was a statistically significant difference between the two groups, we used the log-rank test. We also used the Kaplan–Meier estimator to compare the 5-year observed major osteoporotic fracture risk with the 5-year FRAX predicted major osteoporotic fracture risk (40); we calculated 5-year FRAX major osteoporotic fracture probabilities by dividing the 10-year estimates by two (41). In the general population, TBS has been found to predict incident fracture independent of FRAX (23,42,43); therefore, to account for the independent contribution of TBS, in an additional analysis, we calculated TBS–adjusted FRAX fracture probabilities using a previously defined algorithm described in Supplemental Appendix (42). We used Cox proportional hazards analysis to determine unadjusted and adjusted hazard ratios per SD lower TBS to express the gradient of fragility fracture risk; the proportional hazard assumption was satisfied. We log transformed FRAX scores because of the skewed distribution. We examined the interaction of kidney function and TBS in the prediction of fragility fracture in unadjusted and adjusted Cox models assessing kidney function as both continuous and categorical variables. To determine predictors of TBS, we used multivariable linear regression. We considered a two–tailed P value <0.05 to represent statistical significance. We performed all analyses using SAS, version 9.3 (SAS Institute Inc., Cary, NC).
Results
We included 1426 individuals, 199 with an eGFR<60 ml/min per 1.73 m2 (72.4% CKD stage 3a, 25.1% CKD stage 3b, and 2.5% CKD stage 4) and 1227 with an eGFR≥60 ml/min per 1.73 m2 (Supplemental Figure 1). Comparing individuals with an eGFR<60 ml/min per 1.73 m2 with individuals with an eGFR≥60 ml/min per 1.73 m2, individuals with a lower eGFR were significantly older (75.7 versus 65.7 years old), were more likely to have had a fracture before cohort entry (25.1% versus 16.3%), and had a significantly lower mean lumbar spine TBS (1.275 versus 1.297) (Table 1). There were no significant differences in type 2 diabetes and bisphosphonate use between the two groups. All individuals self-identified as being of white race. Comparing women with an eGFR<60 ml/min per 1.73 m2 with men, they had a significantly higher fracture risk during follow-up (11.1% versus 0.5%) and a significantly higher median FRAX without BMD score (16.7 versus 7.6). Similar differences were found comparing men and women with an eGFR≥60 ml/min per 1.73 m2.
Table 1.
Baseline characteristics by eGFR
| Characteristic | eGFR, ml/min per 1.73 m2 | P Value | |
|---|---|---|---|
| <60, n=199 | ≥60, n=1227 | ||
| Women | 144 (72.4%) | 847 (69.0%) | 0.34 |
| Age, yr | 75.7±7.1 | 65.7±9.8 | <0.001 |
| Body mass index, kg/m2 | 26.7±3.7 | 26.6±4.1 | 0.95 |
| Previous fracture | 50 (25.1%) | 200 (16.3%) | 0.002 |
| Parent fractured hip | 26 (13.1%) | 161 (13.1%) | 0.98 |
| Current smoking | 14 (7.0%) | 122 (9.9%) | 0.20 |
| Corticosteroid use for >3 mo | 4 (2.0%) | 10 (0.81%) | 0.12 |
| Rheumatoid arthritis | 0 | 7 (0.57%) | 0.60 |
| Secondary osteoporosisa | 15 (7.5%) | 53 (4.3%) | 0.05 |
| ≥3 Alcoholic beverages per d | 0 | 14 (1.1%) | 0.24 |
| Lumbar spine TBS (L1–L4) | 1.275±0.103 | 1.297±0.106 | <0.01 |
| Femoral neck T score | −1.31±0.89 | −1.08±0.91 | 0.001 |
| Lumbar spine T score | −0.42±1.71 | −0.66±1.40 | 0.05 |
| FRAX 10-yr major osteoporotic fracture probability without BMD, % | 13.9 (9.0–22.2) | 7.7 (4.9–13.4) | <0.001 |
| FRAX 10-yr major osteoporotic fracture probability with BMD, % | 10.6 (7.4–16.2) | 7.0 (4.8–11.5) | <0.001 |
| eGFRb | 49.8±8.4 | 81.2±11.3 | <0.001 |
| Stage 3a | 144 (72.4%) | ||
| Stage 3b | 50 (25.1%) | ||
| Stage 4 | 5 (2.5%) | ||
| Fall in the past 12 mo | 48 (24.1%) | 321 (26.2%) | 0.54 |
| Bisphosphonate usec | 59 (29.6%) | 300 (24.4%) | 0.12 |
| Type 2 diabetes | 17 (8.5%) | 74 (6.0%) | 0.18 |
| Excellent, very good, or good self–reported current health | 179 (89.9%) | 1153 (94.0%) | 0.03 |
| Albumin, g/dl | 4.4±0.27 | 4.5±0.25 | <0.001 |
| Parathyroid hormone,d pg/ml | 63.1 (48.1–88.1) | 55.0 (43.7–68.9) | <0.001 |
| Missing | 28 (14.1%) | 240 (19.6%) | |
| Serum 25(OH)D, ng/ml | 27.2±9.8 | 28.4±9.2 | 0.50 |
| Missing | 22 (11.1%) | 221 (18.0%) | |
| Serum calcium, mg/dl | 9.6±0.52 | 9.5±0.41 | 0.09 |
| Serum phosphate, mg/dl | 3.4±0.47 | 3.4±0.48 | 0.03 |
Data are means±SD, medians (interquartile ranges), or N (%). Baseline characteristics were taken at year 10 of the study. TBS, trabecular bone score; FRAX, fracture risk assessment score; BMD, bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
Defined as any of the following: hyperthyroidism, premature menopause (<45 years old), chronic liver disease, type 1 diabetes, hypogonadism, chronic malnutrition/malabsorption, or osteogenesis imperfect (24).
eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. eGFR<60 ml/min per 1.73 m2 encompasses CKD stages 3a, 3b, and 4 as defined by the Kidney Disease Improving Global Outcomes guideline.
Defined as a composite of alendronate, clodronate, etidronate, risedronate, pamidronate, and zoledronate at cohort entry.
Reference range for the parathyroid hormone assay was 21.8–104.5 pg/ml and measured by the Liaison (Diasorin Incorporated) assay.
We followed individuals for an average of 4.7 years (6646 person-years). During follow-up, there were 103 incident fragility fracture events (11.6% [n=23] with an eGFR<60 ml/min per 1.73 m2 and 6.5% [n=80] with an eGFR≥60 ml/min per 1.73 m2), 39 individuals died (4.5% [n=9] with an eGFR<60 ml/min per 1.73 m2 and 2.4% [n=30] with an eGFR≥60 ml/min per 1.73 m2 ), and 52 individuals were lost to follow-up (8.5% [n=17] with an eGFR<60 ml/min per 1.73 m2 and 2.9% [n=35] with an eGFR≥60 ml/min per 1.73 m2).
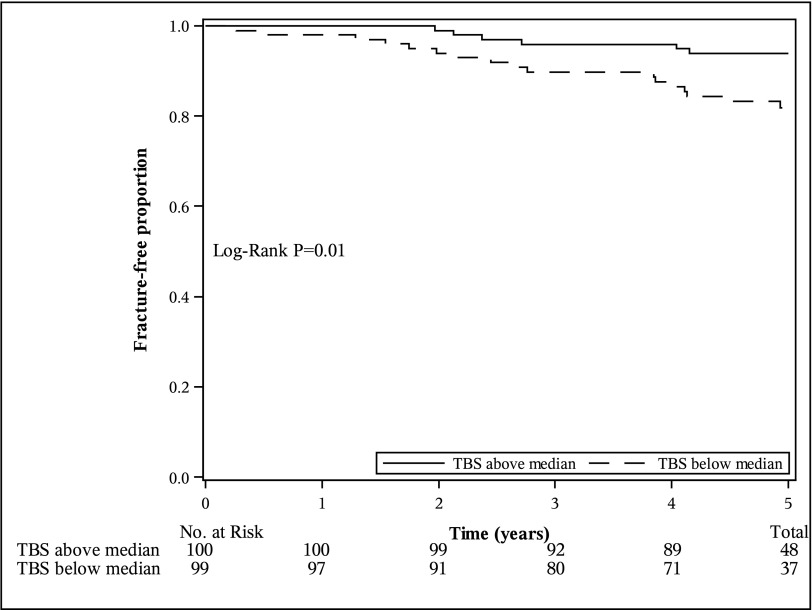
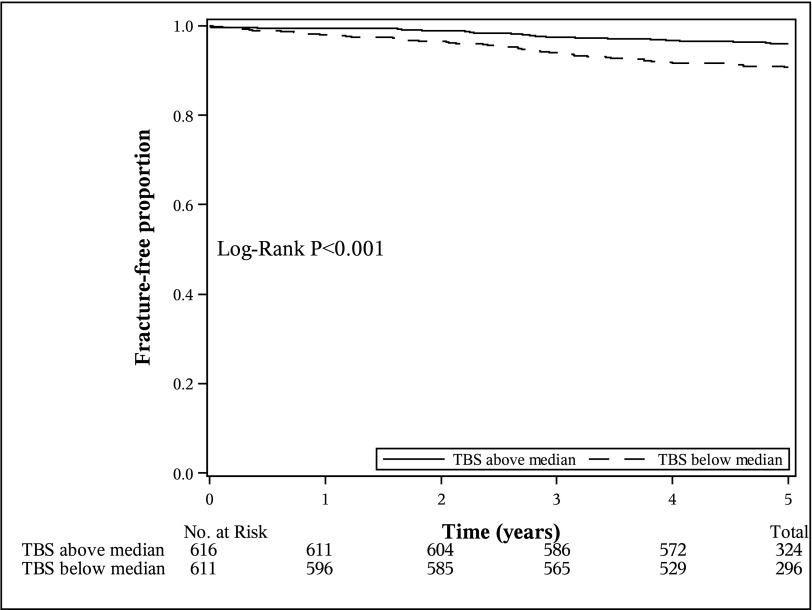
Comparing the 5-year fragility fracture probability in individuals with a TBS value below versus above the median, individuals with an eGFR<60 ml/min per 1.73 m2 who had a TBS value below the median (<1.277) had a significantly higher probability of fragility fracture (P=0.01) than those who had a value above the median (Figure 1). Specifically, individuals with an eGFR<60 ml/min per 1.73 m2 who had a TBS value below the median had a 5-year fragility fracture probability of 18.1% (95% confidence interval [95% CI], 11.7% to 27.6%) versus 6.2% (95% CI, 2.8% to 13.3%) for individuals with a TBS above the median. Similar results were found for individuals with normal kidney function (P<0.001) (Figure 2).
Figure 1.
The Modified Kaplan–Meier estimator for fragility fracture accounting for the competing risk of death in individuals with an eGFR<60 ml/min per 1.73 m2 stratified by median trabecular bone score differed significantly (TBS; TBS below median <1.277; TBS above median ≥1.277). There were 17 fragility fractures in individuals with a TBS below the median and six fragility fractures in individuals with a TBS above the median.
Figure 2.
The Modified Kaplan–Meier estimator for fragility fracture accounting for the competing risk of death in individuals with an eGFR≥60 ml/min per 1.73 m2 stratified by median trabecular bone score differed significantly (TBS; TBS below median <1.304; TBS above median ≥1.304). There were 56 fragility fractures in individuals with a TBS below the median and 24 fragility fractures in individuals with a TBS above the median.
When assessing the gradient of risk for fragility fracture, we found a significant association between fracture and TBS in both the unadjusted and adjusted analyses (Table 2). For example, the fragility fracture hazard ratio per SD lower TBS for individuals with an eGFR<60 ml/min per 1.73 m2 was 1.62 (95% CI, 1.04 to 2.51) after adjustment for FRAX with femoral neck BMD plus spine BMD. The hazard ratios per SD lower TBS were comparable in the normal and reduced kidney function groups. When including an interaction term in the Cox model, we found that kidney function (measured as continuous and categorical variables) did not alter the association between TBS and fracture (kidney function [reduced versus normal] × TBS interaction; P=0.74).
Table 2.
Hazard ratios for lumbar spine trabecular bone score to predict fragility fractures in individuals with reduced and normal kidney function
| Adjustment(s) | eGFR, ml/min per 1.73 m2 | |
|---|---|---|
| <60 | ≥60 | |
| Unadjusted | 1.83 (1.22 to 2.75) | 1.79 (1.44 to 2.23) |
| Age and sex | 1.75 (1.14 to 2.68) | 1.60 (1.28 to 2.01) |
| FRAX major fracture probability without femoral neck BMDa | 1.74 (1.13 to 2.68) | 1.57 (1.25 to 1.97) |
| FRAX major fracture probability with femoral neck BMDa | 1.57 (1.02 to 2.42) | 1.50 (1.19 to 1.90) |
| FRAX major fracture probability with femoral neck BMDa plus spine BMD | 1.62 (1.04 to 2.51) | 1.44 (1.13 to 1.83) |
Data are presented as hazard ratio per SD lower lumbar spine trabecular bone score (95% confidence interval). FRAX, fracture risk assessment score; BMD, bone mineral density.
FRAX major fracture probability (log transformed).
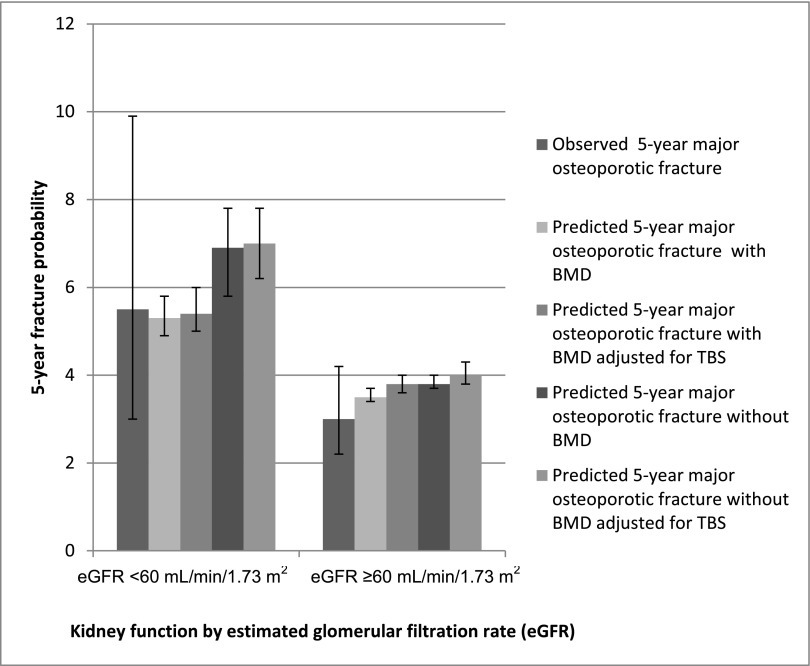
After adjusting fracture probabilities to account for TBS’s independent contribution to fracture risk, we found that the FRAX 5-year predicted probability of major osteoporotic fracture changed minimally in individuals with reduced and normal kidney function (Figure 3). For example, in individuals with an eGFR<60 ml/min per 1.73 m2, the observed 5-year major osteoporotic fracture probability was 5.5% (95% CI, 3.0% to 9.9%), and the FRAX predicted probability with BMD was 5.3% (95% CI, 4.9% to 5.8%), whereas the FRAX predicted probability with BMD accounting for the independent contribution of TBS was 5.4% (95% CI, 5.0% to 6.0%).
Figure 3.
Concordance identified between observed and fracture risk assessment score (FRAX) mean predicted 5-year major osteoporotic fracture risk according to eGFR. Observed major osteoporotic fracture risk was calculated using the modified Kaplan–Meier estimator accounting for the competing risk of death. There were 10 major osteoporotic fracture events in individuals with an eGFR<60 ml/min per 1.73 m2 and 37 major osteoporotic events in individuals with an eGFR≥60 ml/min per 1.73 m2. Error bars are 95% confidence intervals.
The multivariable linear regression analysis revealed that the following predictors were associated with a significantly lower TBS: older age (per 10 years), higher body mass index (per 5 kg/m2), previous fracture, parental hip fracture, current smoking, and bisphosphonate use (Table 3). There was no significant association between kidney function (eGFR<60 ml/min per 1.73 m2 compared with ≥60 ml/min per 1.73 m2) and TBS (0.011; 95% CI, −<0.01 to 0.03).
Table 3.
Multivariable linear regression analysis of correlates of trabecular bone score
| Variables | TBS | 95% CI |
|---|---|---|
| Age, per 10 yr | −0.030a | −0.04 to −0.02a |
| Sex, women versus men | −0.006 | −0.02 to 0.01 |
| BMI, per 5 kg/m2 | −0.033a | −0.04 to −0.03a |
| Prior fracture | −0.025a | −0.04 to −0.01a |
| Parental hip fracture | −0.016a | −0.03 to −<0.01a |
| Current smoking | −0.065a | −0.08 to −0.05a |
| Glucocorticoid use | 0.012 | −0.04 to 0.06 |
| Osteoporosis therapy | −0.039a | −0.05 to −0.03a |
| Reduced kidney function versus normalb | 0.011 | −<0.01 to 0.03 |
TBS, trabecular bone score; 95% CI, 95% confidence interval; BMI, body mass index.
Statistically significant (P<0.05).
Reduced kidney function was defined as an eGFR<60 ml/min per 1.73 m2, and normal kidney function was defined as an eGFR≥60 ml/min per 1.73 m2.
Discussion
In this study, we found that lower lumbar spine TBS was associated with a higher risk of fragility fracture in individuals with an eGFR<60 ml/min per 1.73 m2, and this association was similar to that found in individuals with an eGFR≥60 ml/min per 1.73 m2. Moreover, the association between TBS and fracture was independent of age, sex, FRAX score, and BMD in both groups. These results suggest that TBS may be a useful additional measure for clinicians to predict fracture risk in those with reduced kidney function.
Similar to what has been found in the kidney transplant population (28), we found that a lower TBS was associated with a higher risk of fracture in individuals with reduced kidney function who have not received a transplant. For example, each SD lower TBS was associated with a 62% higher risk of fragility fracture independent of FRAX with BMD and lumbar spine BMD. Moreover, individuals with reduced kidney function who had a TBS value below the median had a significantly higher fragility fracture probability compared with those who had a TBS value above the median.
Previous studies have found that FRAX may accurately predict fracture in individuals with CKD (41,44). However, accounting for other factors, such as TBS, in addition to the FRAX score may further improve its predictive ability. We found that, similar to in the general population, after adjusting for TBS, the 5-year FRAX fracture probabilities changed minimally (23,42). However, as seen in the general population, adjusting for TBS may have stronger effects on fracture probabilities when restricting to different ages and fracture risk categories (e.g., intermediate risk) (42); because of our limited sample size, this was not able to be assessed and therefore, should be considered in future studies.
The ability of BMD to predict fracture in individuals with CKD has been controversial, with the current KDIGO guidelines suggesting that BMD should not be performed in individuals with CKD stages 3–5 who have evidence of CKD-MBD (8); it is important to note that this guideline may be revised in light of recent studies (45–47). Nevertheless, BMD only provides information on one aspect contributing to an increased fracture risk: bone mass. As a result, individuals with a normal BMD may fracture because of altered bone texture. Other non–DXA approaches to BMD assessment are limited by costs, availability, and invasiveness, whereas TBS can be simply obtained from already available DXA images. Therefore, TBS may not only be an accurate but also, a practical method to obtain a more complete assessment of fracture risk in individuals with reduced kidney function. It is important to note that TBS measurements are minimally affected by osteoarthritis; however, measurements are less affected than BMD (48).
Limitations of this study are noted. First, all individuals included were of white race; therefore, results may not be generalizable to other races. Second, individuals with an eGFR<60 ml/min per 1.73 m2 may have not been diagnosed with CKD and were identified through community-based sampling (rather than nephrology clinics). Furthermore, we did not have albumin-to-creatinine ratio values and were not able to classify CKD according to the categories used in the 2012 KDIGO guidelines (33). Third, the majority of individuals in our study had moderate declines in kidney function (i.e., CKD stages 3a and 3b); therefore, these results may not generalize to individuals with more severe declines in kidney function. Future studies should assess the association between TBS and fractures in individuals with more severe CKD and diagnosed CKD-MBD. Fourth, loss to follow-up is a concern, because it can result in attrition bias; however, during our study period, <4% of individuals were lost to follow-up. Fifth, the low number of fracture events limited our statistical power; as a result, we were unable to perform further stratified analyses (e.g., stratification by age, sex, fracture location, and CKD stage).
In conclusion, in individuals with reduced kidney function, there is an association between fragility fracture and TBS, with TBS providing information independent of BMD and FRAX. TBS may be a useful additional tool to predict fracture risk in this unique population. Additional study on individuals with diagnosed CKD-MBD and larger sample sizes are required before TBS should be routinely used.
Disclosures
A.X.G. received an investigator-initiated grant from Astellas (Markham, ON, Canada) and Roche (Basel, Switzerland) to support a Canadian Institutes of Health Research study in living kidney donors, and his institution received unrestricted research funding from Pfizer (Kirkland, QC, Canada). J.D.A has been on the speaker bureaus for Amgen (Thousand Oaks, CA) and Eli Lilly (Toronto, ON, Canada) and has received research grants from Amgen, Eli Lilly, Merck (Kirkland, QC, Canada), and Actavis (Markham, ON, Canada). W.D.L. has been on the speaker bureaus for Amgen, Inc. (Thousand Oaks, CA), Eli Lilly (Indianapolis, IN), and Novartis (Basel, Switzerland) and has had research grants for Novartis, Amgen, Inc., and Genzyme (Mississauga, ON, Canada). The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research, Merck Frosst Canada Ltd. (Kirkland, QC, Canada), Eli Lilly Canada Inc. (Toronto, ON, Canada), Novartis (Dorval, QC, Canada), The Alliance: Sanofi-Aventis & Procter and Gamble Pharmaceuticals Canada Inc. (Toronto, ON, Canada), Servier Canada Inc. (Laval, QC, Canada), Amgen Canada Inc. (Mississauga, ON, Canada), The Dairy Farmers of Canada (Ottawa, ON, Canada), and The Arthritis Society (Toronto, ON, Canada).
Supplementary Material
Acknowledgments
We thank all of the participants in the Canadian Multicentre Osteoporosis Study (CaMos) who made this study possible and members of the CaMos research group who were instrumental in the ongoing success of the CaMos cohort.
K.L.N. is supported by the Canadian Institutes of Health Research Fellowship and the Canadian National Transplant Research Program Astellas Training Award. A.X.G. was supported by the Dr. Adam Linton Chair in Kidney Health Analytics. This research was made possible by infrastructure support from the Lilibeth Caberto Kidney Clinical Research Unit.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Fractures in Patients with CKD: Time for Action,” on pages 1929–1931.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00720116/-/DCSupplemental.
References
- 1.Lindberg JS, Moe SM: Osteoporosis in end-state renal disease. Semin Nephrol 19: 115–122, 1999 [PubMed] [Google Scholar]
- 2.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR; Osteoporotic Fractures Research Group : Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167: 133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, Pouget JG, Lok CE, Hodsman AB, Adachi JD, Garg AX: The three-year incidence of fracture in chronic kidney disease. Kidney Int 86: 810–818, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Dukas L, Schacht E, Stähelin HB: In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int 16: 1683–1690, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, Sarnak M, Siscovick D, Harris T, Cauley J, Newman AB, Robbins J: Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease Improving Global Outcomes Work Group : KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 9.West SL, Jamal SA, Lok CE: Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol Dial Transplant 27: 2384–2388, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Malluche HH, Porter DS, Pienkowski D: Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol 9: 671–680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D: Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: A preliminary spine BMD-matched, case-control study. J Clin Densitom 12: 170–176, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA: Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: An experimental study on human cadaver vertebrae. J Clin Densitom 14: 302–312, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP: Trabecular bone score: A noninvasive analytical method based upon the DXA image. J Bone Miner Res 29: 518–530, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Boutroy S, Bouxsein ML, Munoz F, Delmas PD: In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90: 6508–6515, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cheung AM, Adachi JD, Hanley DA, Kendler DL, Davison KS, Josse R, Brown JP, Ste-Marie LG, Kremer R, Erlandson MC, Dian L, Burghardt AJ, Boyd SK: High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: A review by the Canadian Bone Strength Working Group. Curr Osteoporos Rep 11: 136–146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wehrli FW, Leonard MB, Saha PK, Gomberg BR: Quantitative high-resolution magnetic resonance imaging reveals structural implications of renal osteodystrophy on trabecular and cortical bone. J Magn Reson Imaging 20: 83–89, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Cejka D, Patsch JM, Weber M, Diarra D, Riegersperger M, Kikic Z, Krestan C, Schueller-Weidekamm C, Kainberger F, Haas M: Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol 6: 2264–2271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trombetti A, Stoermann C, Chevalley T, Van Rietbergen B, Herrmann FR, Martin PY, Rizzoli R: Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos Int 24: 1721–1732, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R: Trabecular bone score improves fracture risk prediction in non-osteoporotic women: The OFELY study. Osteoporos Int 24: 77–85, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Rabier B, Héraud A, Grand-Lenoir C, Winzenrieth R, Hans D: A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone 46: 176–181, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Hans D, Goertzen AL, Krieg MA, Leslie WD: Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The Manitoba study. J Bone Miner Res 26: 2762–2769, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Popp AW, Meer S, Krieg MA, Perrelet R, Hans D, Lippuner K: Bone mineral density (BMD) and vertebral trabecular bone score (TBS) for the identification of elderly women at high risk for fracture: The SEMOF cohort study [published online ahead of print May 27, 2015]. Eur Spine J [DOI] [PubMed]
- 23.McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJ, Fujita Y, Glüer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren Ö, Lorentzon M, Mellström D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA: A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31: 940–948, 2016 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization: FRAX World Health Organization Fracture Risk Assessment Tool, 2011. Available at: http://www.shef.ac.uk/FRAX/index.aspx. Accessed December 4, 2015
- 25.Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program : TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 98: 602–609, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Ulivieri FM, Silva BC, Sardanelli F, Hans D, Bilezikian JP, Caudarella R: Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 47: 435–448, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Paggiosi MA, Peel NF, Eastell R: The impact of glucocorticoid therapy on trabecular bone score in older women. Osteoporos Int 26: 1773–1780, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Naylor KL, Lix LM, Hans D, Garg AX, Rush DN, Hodsman AB, Leslie WD: Trabecular bone score in kidney transplant recipients. Osteoporos Int 27: 1115–1121, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Cho SK, Kim JY, Choi YY, Sung YK: Association between trabecular bone score and risk factors for fractures in Korean female patients with rheumatoid arthritis. Mod Rheumatol 26: 540–545, 2016 [DOI] [PubMed]
- 30.Tenenhouse A, Joseph L, Kreiger N, Poliquin S, Murray TM, Blondeau L, Berger C, Hanley DA, Prior JC; CaMos Research Group.Canadian Multicentre Osteoporosis Study : Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: The Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 11: 897–904, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster RS: Research notes: The Canadian Multicentre Osteoporosis Study (CaMos): Background, rationale, methods. Can J Aging 18: 376–387, 1999 [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Hui SL, Gao S, Zhou XH, Johnston CC Jr., Lu Y, Glüer CC, Grampp S, Genant H: Universal standardization of bone density measurements: A method with optimal properties for calibration among several instruments. J Bone Miner Res 12: 1463–1470, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Genant HK, Grampp S, Glüer CC, Faulkner KG, Jergas M, Engelke K, Hagiwara S, Van Kuijk C: Universal standardization for dual x-ray absorptiometry: Patient and phantom cross-calibration results. J Bone Miner Res 9: 1503–1514, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Fan B, Lu Y, Genant H, Fuerst T, Shepherd J: Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int 21: 1227–1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Fuerst T, Hui S, Genant HK: Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 12: 438–444, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Berger C, Goltzman D, Langsetmo L, Joseph L, Jackson S, Kreiger N, Tenenhouse A, Davison KS, Josse RG, Prior JC, Hanley DA; CaMos Research Group : Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 25: 1948–1957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr., Lindsay R: Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8: 468–489, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Leslie WD, Lix LM, Wu X; Manitoba Bone Density Program : Competing mortality and fracture risk assessment. Osteoporos Int 24: 681–688, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, Adachi JD, Morin S, Goltzman D, Lentle B, Jackson SA, Josse RG, Jamal SA: Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol 10: 646–653, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D, Johansson H, Kanis JA: Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96: 500–509, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Leslie WD, Johansson H, Kanis JA, Lamy O, Oden A, McCloskey EV, Hans D: Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25: 2271–2277, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Jamal SA, West SL, Nickolas TL: The clinical utility of FRAX to discriminate fracture status in men and women with chronic kidney disease. Osteoporos Int 25: 71–76, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Ketteler M, Elder GJ, Evenepoel P, Ix JH, Jamal SA, Lafage-Proust MH, Shroff R, Thadhani RI, Tonelli MA, Kasiske BL, Wheeler DC, Leonard MB: Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: A commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int 87: 502–528, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, Kuwahara M, Sasaki S, Tsukamoto Y: Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients--a single-center cohort study. Nephrol Dial Transplant 27: 345–351, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF; Health, Aging, and Body Composition Study : Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 7: 1130–1136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolta S, Briot K, Fechtenbaum J, Paternotte S, Armbrecht G, Felsenberg D, Glüer CC, Eastell R, Roux C: TBS result is not affected by lumbar spine osteoarthritis. Osteoporos Int 25: 1759–1764, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.