Abstract
Background and objectives
Prior studies suggested that women with CKD have higher risk for cardiovascular disease (CVD) and mortality than men, although putative mechanisms for this higher risk have not been identified. We assessed sex differences in (1) CVD risk factors and left ventricular hypertrophy (LVH), and (2) the relationship of left ventricular mass (LVM) with different measures of body size in children with CKD.
Design, setting, participants, and measurements
The study population comprised 681 children with CKD from the Chronic Kidney Disease in Children cohort, contributing 1330 visits. CVD risk factors were compared cross-sectionally by sex. LVH was defined as LVM/height2.7 >95th percentile and LVM relative to estimated lean body mass (eLBM) >95th percentile for age and sex. Differences in LVM by sex were assessed by adjusting for age, weight, height, and eLBM using bivariate and multivariate regression models.
Results
Girls were less likely to have uncontrolled hypertension (26% versus 38%, P=0.001), had lower diastolic BP z-scores (+0.3 versus +0.6, P=0.001), and had lower prevalence of high triglycerides (38% versus 47%, P=0.03) compared with boys. When LVH was defined by LVM indexed to height, girls had higher prevalence of LVH (16% versus 9%, P=0.01); when LVH was defined by LVM relative to eLBM, prevalence of LVH was similar between girls and boys (18% versus 17%, P=0.92). In regression models adjusting for eLBM, no sex differences in LVM were observed.
Conclusions
Despite lack of increased prevalence of CVD risk factors, indexing LVM to height showed a higher proportion of LVH among girls, while estimates of LVH based on eLBM showed no sex differences. Indexing LVM to eLBM may be an alternative to height indexing in children with CKD.
Keywords: chronic kidney disease; left ventricular hypertrophy; pediatric nephrology; cardiovascular disease; Body Size; Body Weight; child; female; Humans; hypertension; Hypertrophy, Left Ventricular; Male; Prevalence; Renal Insufficiency, Chronic; risk factors; Sex Characteristics; Triglycerides
Introduction
CKD increases the risk of cardiovascular disease (CVD) in children and young adults, and cardiac-related death is the leading cause of mortality in this population (1,2). Young women with CKD have been shown to be at even greater risk for mortality than young men, although the reason for the higher risk of death has not been determined (3,4).
A recently published report from the Chronic Kidney Disease in Children (CKiD) study suggested over four-fold higher odds of left ventricular hypertrophy (LVH) among girls compared with boys with CKD, adjusted for known contributors to LVH, including hypertension and anemia (5). LVH is a significant predictor of cardiovascular outcomes and is associated with high rates of morbidity and mortality in adults with CKD (6–8). Previous studies in adults have shown higher risk of LVH among women compared with men at the same level of hypertension, suggesting possible sex differences in the pathogenesis of LVH (9,10). However, sex-specific risk factors for LVH have not previously been studied in the pediatric CKD population.
In addition, there is no agreed best method of defining LVH in children. Different methods of indexing left ventricular mass (LVM) for body size result in significantly different estimates of LVH prevalence (11). While height and weight are related to LVM and sex, lean body mass (LBM) may better account for sex and age differences in LVM (12). However, transformations of height and weight are often used to approximate LBM. Recently published equations for estimated lean body mass (eLBM) may be used to account for sex differences in LVM (13), but it is not known whether these equations apply to children with CKD, who tend to have lower height and higher weight for their age and sex (14). Since LVM is directly related to LBM independent of sex (12), we sought to explore this relationship in children with CKD.
In order to evaluate the association of LVH with sex among children with CKD, we sought to (1) compare CVD risk factors by sex to investigate whether comorbidities not previously examined may explain the higher LVH prevalence among girls, and (2) compare sex differences in LVM normalized relative to different measures of body size.
Materials and Methods
Study Population and Design
The CKiD study is an observational cohort study of CKD in children being conducted at 46 centers in North America (study website available at http://www.statepi.jhsph.edu/ckid). Inclusion criteria include age 1–16 years and eGFR of 30–90 ml/min per 1.73 m2, calculated using the bedside CKiD study formula (15). The full details of the CKiD study protocol, including exclusion criteria, have been described previously (16). The CKiD study protocol has been reviewed and approved by the institutional review boards of each participating center, and the study protocol adhered to the Declaration of Helsinki. All participants and/or guardians provided written informed consent.
Clinical Variables
Casual BP was measured at study entry, then at annual intervals after enrollment (17). Casual BPs were classified according to the National High Blood Pressure Education Program Fourth Report on the Diagnosis, Evaluation, and Treatment of High BP in Children and Adolescents (18): normotensive (<90th percentile), prehypertensive (≥90th and <95th percentiles), and hypertensive (≥95th percentile). Ambulatory BP was measured as previously described (19). Elevated systolic and diastolic BP was based on mean wake or sleep states greater than the expected 95th percentile (20) or 25% of readings within each state greater than the same threshold. Abnormally high ambulatory BP was defined as elevated systolic or diastolic BP.
Weight, height, GFR, and blood and urine samples were obtained on the day of the echocardiographic evaluation. GFR was determined by plasma iohexol disappearance curves (iGFR) and using the bedside CKiD study creatinine-based equation (eGFR) (15,21); details of GFR measurement have been previously published (22). Plasma vitamin D and fibroblast growth factor 23 (FGF23) were measured as previously described (23,24). Blood and urine specimens were analyzed at the study’s central biochemistry laboratory (University of Rochester, Rochester, NY).
Echocardiography
Echocardiography measurements were obtained every other year, starting 1 year after study entry. M-mode and Doppler echocardiography were performed at each center; analyses of echocardiographic data were performed by the Cardiovascular Core Imaging Research Laboratory at Cincinnati Children’s Hospital Medical Center. For achievement of standardization of echocardiographic images across centers, qualifying recordings were sent to each center and certified at the core laboratory.
LVM was measured by two-dimensional directed M-mode echocardiography at rest according to the American Society of Echocardiography criteria (25). This method applies measurements of LV end-diastolic cavity (LVED), interventricular septal thickness, and posterior wall thickness and accurately predicts LVM through the following equation:
 |
LVM index (g/m2.7) was calculated by dividing LVM by height in meters to the power of 2.7 (12).
Statistical Analyses
The first component of the analyses described CVD risk factors by sex at visit 2 when the first echocardiography data were collected. The second component described differences in LVM by sex, adjusting for different metrics of body size, including age, weight, height, and eLBM, using bivariate and multivariate regression models. Subjects who had repeat echocardiograms could contribute multiple LVM measurements, with corresponding age, weight, height, and eLBM updated for that visit.
Characterizing CVD and CKD Risk Profiles by Sex.
Univariate descriptive statistics were used to characterize CVD and CKD risk profiles. Wilcoxon rank sum and Fisher exact tests compared continuous and categorical variables. We calculated the prevalence of LVH among girls and boys as defined by Khoury et al. as left ventricular mass index (LVMI) =(LVM/height2.7) >95th percentile for sex and age (26). We performed repeated linear regression models of LVMI (in the log scale) and logistic regression models of LVH, with the primary independent variable being sex. These models used generalized estimating equations to account for repeated observations within individuals. The base model included the same covariates as those reported by Kupferman et al. (5), including age, race, visit, systolic BP z-score, height, eGFR, anemia, antihypertensive medications, CKD duration, percent life with CKD, and glomerular diagnosis. To investigate additional variables that may explain sex differences, we included Tanner stage, cholesterol, vitamin D levels, and FGF23 levels in separate models and compared the adjusted effect of sex to the base model.
We also calculated the prevalence of LVH using recently published reference centiles expressing LVM relative to eLBM, with LVH defined as LVMI-for-eLBM >95th percentile (27), and compared with LVH estimates based on the Khoury equation (26). eLBM was calculated for each subject visit according to sex-specific equations described by Foster et al. (13):
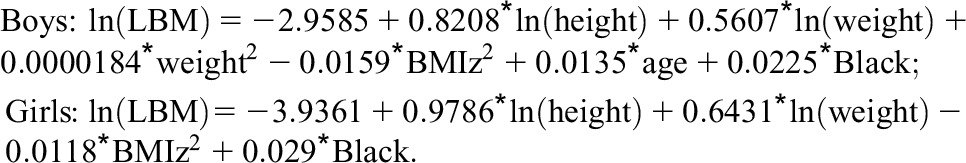 |
Association of LVM with Different Metrics of Body Size, by Sex.
We analyzed LVM in relation to different metrics of body size including age (years), weight (kg), height (cm), and eLBM (kg), using echocardiographic data from all available visits. Four bivariate models were of the form:
where Z represents included covariates: age centered at 14 years, weight in the log scale centered at 46 kg, height in the log scale centered at 150 cm [as well as the quadratic of log(height)], and eLBM in the log scale centered at 30 kg. These values were chosen as the mean levels for the population, and centering provides an interpretable intercept. Z×Girls represents covariate interactions with sex to evaluate sex differences in the effect of covariates on LVM. Generalized estimating equations assuming an independent correlation structure were used to account for repeated measurements within individuals. The differences by sex were determined by the statistical significance of the parameters α1 and δ1.
Results
Cardiovascular, Metabolic, and Kidney Disease Risk Factors, by Sex
The analysis included 681 children (411 boys and 270 girls). Table 1 presents demographic characteristics by sex at first visit with an echocardiography study. The age range for the total cohort was 2.0–19.2 years and was similar by sex. Boys and girls had similar distributions of black race; girls were more likely to be of Hispanic ethnicity. Girls tended to have more advanced Tanner stage, consistent with earlier pubertal development among normal girls compared with boys (28). CKD severity, as measured by GFR and proteinuria, did not differ by sex. Compared with boys, girls had a lower height-for-age z-score and a lower eLBM. There was no difference in the prevalence of obesity by sex.
Table 1.
Baseline demographic features comparing boys and girls
| Variable | Boys (n=411) | Girls (n=270) | P Value |
|---|---|---|---|
| Age, yr | 11.0 [7.4, 14.4] | 11.3 [7.8, 14.8] | 0.29 |
| Black race | 22 (92) | 19 (52) | 0.34 |
| Hispanic ethnicity | 12 (49) | 18 (49) | 0.03 |
| Tanner stage | 0.01 | ||
| 1 | 62 (242) | 51 (129) | |
| 2 | 8 (33) | 9 (23) | |
| 3 | 8 (32) | 11 (28) | |
| 4 | 15 (59) | 15 (39) | |
| 5 | 7 (27) | 14 (36) | |
| Glomerular diagnosis | 23 (94) | 33 (90) | 0.004 |
| CKD duration, yr | 8.5 [4.4, 13.1] | 7.4 [3.3, 11.5] | 0.04 |
| iGFR, ml/min per 1.73 m2 | 53.6 [38.2, 72.4] | 49.6 [38.9, 68.0] | 0.18 |
| eGFR, ml/min per 1.73 m2 | 52.6 [37.9, 66.9] | 53.2 [41.0, 67.7] | 0.41 |
| Urine protein-to-creatinine, mg/mg | 0.33 [0.11, 1.06] | 0.39 [0.13, 0.94] | 0.33 |
| Height, cm | 140.8 [118.0, 163.3] | 139.4 [120.5, 157.5] | 0.24 |
| Height z-score | −0.47 [−1.26, 0.28] | −0.66 [−1.59, 0.15] | 0.05 |
| Weight, kg | 37.9 [22.6, 56.1] | 37.6 [24.4, 54.2] | 0.98 |
| Weight z-score | 0.09 [−0.77, 0.89] | −0.10 [−1.03, 0.98] | 0.31 |
| Body mass index, kg/m2 | 18.5 [16.3, 21.8] | 18.7 [16.3, 23.3] | 0.37 |
| BMI z-score | 0.47 [−0.31, 1.29] | 0.43 [−0.31, 1.38] | 0.95 |
| Obese | 17 (67) | 17 (45) | 0.92 |
| Lean body mass, kg | 27.4 [17.3, 42.5] | 25.9 [17.1, 36.0] | 0.03 |
Continuous variables expressed as median [interquartile range]. Categorical variables expressed as percentage (number of subjects). eGFR calculated using bedside CKiD equation. iGFR, iohexol GFR; BMI, body mass index.
Table 2 describes CVD risk factors by sex. Girls were less likely to have uncontrolled hypertension (26% versus 38%, P=0.001), had lower diastolic BP z-scores (+0.3 versus +0.6, P=0.001), and had lower prevalence of high triglycerides (38% versus 47%, P=0.03) compared with boys. Girls were more likely to receive antihypertensive therapy and angiotensin-converting enzyme inhibitors. There was no sex difference in prevalence of ambulatory hypertension. Girls had lower vitamin D-1,25 levels compared with boys (30.4 versus 33.6, P=0.01), although there was a similar distribution of vitamin D-25 and FGF23 levels between sexes. Girls also had slightly lower median hemoglobin compared with boys (12.4 versus 12.8 g/dl, P=0.01). We performed a subgroup analysis restricted to postpubertal boys and girls (i.e., Tanner stage 4 or 5). This analysis showed similar results, indicating that girls did not have a higher prevalence of CVD risk factors. A similar pattern of lower median hemoglobin levels and lower vitamin D-1,25 levels, with similar vitamin D-25 and FGF23 levels among postpubertal girls compared with boys was observed.
Table 2.
Cardiovascular disease risk factors comparing boys and girls
| Variable | Boys (n=411) | Girls (n=270) | P Value |
|---|---|---|---|
| Systolic BP z-score | 0.39 [−0.3, 1.03] | 0.17 [−0.62, 0.92] | 0.05 |
| Diastolic BP z-score | 0.59 [−0.05, 1.16] | 0.30 [−0.26, 1.0] | 0.001 |
| Uncontrolled hypertension | 38 (155) | 26 (69) | 0.001 |
| Elevated ambulatory systolic BP | 45 (121) | 39 (72) | 0.25 |
| Elevated ambulatory diastolic BP | 42 (113) | 40 (73) | 0.70 |
| Ambulatory hypertension | 54 (146) | 48 (88) | 0.21 |
| Antihypertensive therapy | 59 (243) | 69 (185) | 0.02 |
| Angiotensin-converting enzyme inhibitor therapy | 44 (182) | 57 (154) | 0.001 |
| Angiotensin receptor blocker therapy | 9 (38) | 10 (27) | 0.79 |
| Calcium channel blocker | 13 (54) | 13 (35) | 1.00 |
| >1 antihypertensive medication | 14 (59) | 18 (48) | 0.24 |
| High phosphorous | 9 (39) | 7 (18) | 0.21 |
| Hemoglobin, g/dl | 12.8 [11.8, 13.8] | 12.4 [11.6, 13.4] | 0.01 |
| Total cholesterol, mg/dl | 169 [147, 191] | 173 [150, 197] | 0.25 |
| High total cholesterol | 25 (98) | 21 (57) | 0.35 |
| HDL cholesterol, mg/dl | 49 [40, 58] | 50 [42, 59] | 0.23 |
| Low HDL cholesterol | 12 (47) | 9 (25) | 0.37 |
| LDL cholesterol, mg/dl | 96 [74, 114] | 98 [81, 117] | 0.14 |
| High LDL cholesterol | 12 (47) | 11 (30) | 0.90 |
| Triglycerides, mg/dl | 101 [70, 150] | 98 [73, 141] | 0.96 |
| High triglycerides | 47 (187) | 38 (101) | 0.03 |
| Vitamin D-1,25 DOH | 33.6 [25.4, 42.4] | 30.4 [23.5, 38.6] | 0.01 |
| Vitamin D-25 OH | 28.3 [21.1, 35.5] | 28.3 [19.7, 35.7] | 0.73 |
| Plasma FGF23, RU/ml | 127.4 [81.2, 217.9] | 144.4 [91.4, 186.8] | 0.38 |
| Number of echocardiographic studies | 0.44 | ||
| 1 study | 52 (213) | 47 (127) | |
| 2 studies | 26 (105) | 27 (73) | |
| ≥3 studies | 23 (93) | 26 (70) | |
| LVMI at visit 2, g/m2.7 | 31.6 [26.1, 37.8] | 29.9 [25.0, 37.1] | 0.23 |
| LVMI at visit 4, g/m2.7 | 31.2 [26.7, 36.2] | 30.3 [24.2, 35.1] | 0.05 |
| LVMI at visit 6, g/m2.7 | 26.1 [21.5, 32.5] | 26.9 [22.3, 32.5] | 0.60 |
Continuous variables expressed as median [interquartile range]. Categorical variables expressed as percentage (number of subjects). Vitamin D-1,25 DOH, 1,25-dihydroxyvitamin D; Vitamin D-25 OH, 25-hydroxyvitamin D; FGF23, fibroblast growth factor 23; LVMI, left ventricular mass index.
Association of LVM with Different Metrics of Body Size, by Sex
Median LVMI was similar between sexes at all visits (Table 2). Using the definition of LVH described by Khoury et al. (26), the prevalence of LVH was higher among girls compared with boys at visit 2 (16% versus 9%, P=0.01). However, when defining LVH using LVM relative to eLBM centile curves (27), there was no longer a difference in the prevalence of LVH between girls and boys (18% versus 17%, P=0.92).
Table 3 shows the results of the multivariable linear regression model of LVMI and logistic regression model of LVH with the primary independent variable of sex, adjusted for CVD risk factors (including 145 cases of LVH out of 1398 person visits). These models indicate no differences in LVMI between boys and girls in the base linear model, and increased odds of LVH among girls in the base logistic model. The increased odds of LVH among girls persisted after adjustment for CVD risk factors. Of note, the current CKiD study dataset used in this analysis includes more echocardiography studies since the previous report (5), explaining differences with the previous results but consistent inferences.
Table 3.
Multivariable regression models of LVMI and LVH
| Model | LVMI (in the Log Scale) Girls versus Boys, % (95% CI) | Odds of LVH among Girls versus Boys (95% CI) |
|---|---|---|
| Base model | −3.6 (−7.3 to +0.2) | 2.30 (1.44 to 3.67) |
| Base model+Tanner stage | −4.9 (−8.5 to −1.1) | 2.25 (1.40 to 3.63) |
| Base model+high triglycerides | −3.5 (−9.2 to +0.5) | 2.17 (1.33 to 3.52) |
| Base model+FGF23 | −1.9 (−6.7 to +3.1) | 3.08 (1.74 to 5.44) |
| Base model+Vitamin D-1,25 DOH | −4.1 (−8.2 to +0.3) | 1.97 (1.12 to 3.48) |
| Base model+Vitamin D-25 OH | −4.1 (−8.3 to +0.3) | 1.99 (1.12 to 3.51) |
| Based model+Tanner stage+high triglycerides+FGF23+Vitamin D-1,25 DOH+Vitamin D-25 OH | −3.6 (−9.1 to +2.2) | 2.11 (1.04 to 4.31) |
Base models all adjusted for visit, systolic BP z-score (by visit), height (by visit), eGFR, race (black versus other), anemia, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, other antihypertensive medications, age, CKD duration, percent life with CKD, and glomerular diagnosis. LVMI, left ventricular mass index; LVH, left ventricular hypertrophy; 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; Vitamin D-1,25 DOH, 1,25-dihydroxyvitamin D; Vitamin D-25 OH, 25-hydroxyvitamin D.
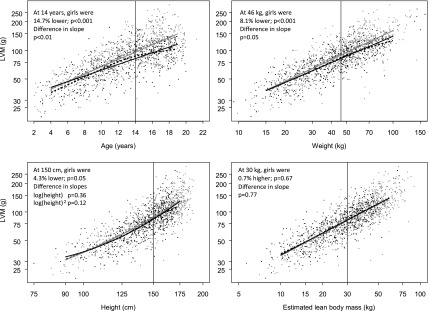
Figure 1 provides the bivariate associations of different metrics of body size. The dashed lines represent nonparametric splines (black for girls, gray for boys), and the solid lines are based on the bivariate linear regressions: the consistency of the two indicate good fit between the model and the data. When adjusting for age, girls had a significantly lower LVM at age 14 [−14.7%; 95% confidence interval (95% CI), −18.5% to −10.6%], and the effect of age on LVM differed by sex: LVM in boys increased by 9.2% per year while LVM in girls increased by 7.1% per year (P for difference <0.01). Similarly, for weight, girls had an 8% lower LVM at the median weight of 46 kg (−8.1%; 95% CI, −11.2% to −4.7%), and the effect of weight on LVM was borderline significant (P=0.05). At the median height of 150 cm, girls were 4.3% lower than boys, and this was also borderline significant (95% CI, −8.3% to +0.02%; P=0.05), although the slopes of height were nonsignificant for the linear term (P=0.36) and the quadratic term (P=0.12). Lastly, there were no differences in LVM between girls and boys at the median eLBM of 30 kg (girls were 0.7% higher; 95% CI, −2.6% to +4.2%) and for the change associated with eLBM (P=0.77). In multivariate analyses, incorporating the predictors from the previous four regressions, there were no differences by sex. Furthermore, eLBM was the strongest predictor of LVM.
Figure 1.
Associations between left ventricular mass (LVM; in grams) and different metrics of body size show no difference in LVM by sex when adjusted for estimated lean body mass. Metrics include age, weight, height, and estimated lean body mass among 411 boys contributing 815 echocardiography studies and 270 girls contributing 515 echocardiography studies.
Discussion
Based on a prior report from the CKiD study showing a four-fold higher odds of LVH among girls compared with boys (5), we further explored sex differences in CVD risk factors and LVH in children with CKD. Estimates of LVH based on traditional height indexing showed a persistently higher proportion of LVH among girls, despite a lack of increased prevalence of CVD risk factors. When defining LVH using LVM relative to eLBM centile curves, there was no longer a difference in LVH prevalence by sex. In a regression model adjusting for eLBM, there were no sex differences in LVM.
If one accepts the current height-based indexing of LVM to define LVH in children, our study did show a higher prevalence of LVH among girls with CKD. One potential explanation for the excess burden of LVH among girls is a sex-specific end-organ response to hypertension. In a study of young adults with hypertension, women were more likely to develop LVH compared with men, adjusted for ambulatory BP and other CVD risk factors (9). In adults with metabolic syndrome, hypertension was associated with LVH in both men and women, but waist circumference, hyperinsulinemia, and hyperglycemia were independently associated with LVH only in women, suggesting possible sex differences in the pathogenesis of LVH (10). Sex differences in cardiac response to hypertension among children with CKD will need to be explored further in future studies.
Another potential explanation for the higher proportion of LVH among girls is that there are other CVD risk factors that may disproportionately affect girls. We did find that girls had slightly lower vitamin D-1,25 levels than boys. Vitamin D deficiency has been associated with CVD and mortality in the CKD population (29–31). However, the significance of slightly lower vitamin D-1,25 levels among girls in the context of similar vitamin D-25 and FGF23 levels is unclear. We also found that girls had slightly lower hemoglobin levels compared with boys, but the difference was small and likely not of clinical significance. Finally, there may be other, currently unmeasured variables that may account for the higher proportion of LVH among girls with CKD. For example, both boys and girls with CKD are known to have hormonal variations with pubertal development (32); future studies are needed to determine if these hormonal abnormalities may explain a sex-specific risk for LVH.
Alternatively, the excess proportion of LVH among girls without a corresponding higher prevalence of known CVD risk factors may indicate that the current definition of LVH may not accurately categorize cardiac hypertrophy in this population. There is no consensus on how best to define LVH in children. LVM is known to be strongly associated with sex, height, body surface area, and LBM (33,34). It is important that LVM be scaled to the most appropriate measure of body size to allow meaningful comparisons across a range of body sizes. LVM scales best to LBM in the normal population (12,34–37). Since it is not always clinically feasible to measure LBM, height has often been used as a surrogate for LBM, and LVM has traditionally been indexed to height2.7 (38,39). In adults, an absolute LVMI>51 g/m2.7 has been associated with higher cardiovascular morbidity and mortality (39). However, the relationship between LVM and height differs across ages (11,26,40), and a single cut-off point for LVM/height2.7 has been shown to be inappropriate for defining LVH across pediatric age groups (40). To address this, Khoury et al. developed quantile curves for LVM/height2.7, and LVH has traditionally been defined as LVMI >95th percentile for sex and age (26).
Prior CKiD study publications have used these 95th percentile curves to define LVH. However, this definition of LVH may be limited by the relatively small sample size of 2273 boys and girls to represent the normal population aged 0–18 years. Additionally, the assumption that there is a constant difference in LVMI by sex across all ages at the 95th percentile (i.e., LVH threshold for boys is about 2.7 g/m2.7 higher than girls, regardless of age) may not be the case. Previous studies in children with CKD have shown that indexing LVM to different measures of body size leads to different estimates of LVH prevalence (11). Additionally, LVM indexed to height leads to underestimation of relative LVM in thin individuals and overestimation of LVM in overweight individuals (34). Since individuals in the CKiD study are, on the whole, relatively overweight, this may have led to an overestimation of LVH prevalence.
Since LVM is expected to depend primarily on LBM, our analysis showed that an estimating equation for LBM explained differences in LVM between boys and girls, and this relationship was not different by sex. Indeed, Figure 1 shows that, while height was a strong predictor of LVM, eLBM more fully explained sex differences in LVM, as evidenced by complete overlap of the nonparametric splines and model lines between boys and girls. Foster et al. also recently reexamined the data used to generate the LVMI for age curves presented by Khoury et al. to define new LVM reference centiles expressing LVM relative to LBM (27). This study yielded an overall lower estimate of the prevalence of LVH. In fact, when these new centile curves were applied to the current CKiD study population, a substantial number of subjects were reclassified compared with the Khoury method, and girls and boys had a similar percentage of LVH. Since a simple equation using age, height, weight, and BMI z-scores has been developed, further analysis of LVM indexed to LBM in other samples of typically developing youth and in the CKD population would further our understanding of appropriate cut-offs to define LVH.
Limitations of this study include not having data on all potential CVD risk factors that may explain sex differences in LVH. In addition, we used cut-offs for LVH which were developed in cohorts of healthy children and have not been validated in the CKD population, although these cut-offs are typically used clinically for children with CKD. Finally, we defined LVH based on echocardiographic criteria. Future studies using cardiac magnetic resonance imaging may provide more accurate measures of cardiac hypertrophy.
In conclusion, this study shows similar CVD risk profiles in girls and boys with CKD, and highlights the current limitations in defining LVH in children with CKD. As clinicians frequently use LVH as a marker of end-organ damage, an accurate definition is essential in order to classify CVD risk and prevent future morbidity and mortality. Indexing LVM to eLBM may be an alternative to height indexing, and further research in a normal population should investigate this relationship more closely.
Disclosures
None.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in Children (CKiD) study with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri, Kansas City (B.W.) and Children’s Hospital of Philadelphia (S.L.F.); central biochemistry laboratory (George Schwartz) at the University of Rochester Medical Center; and data coordinating center (Alvaro Muñoz) at the Johns Hopkins Bloomberg School of Public Health.
The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (grant nos.: U01-DK-66143, U01-DK-66174, U01-DK-082194, and U01-DK-66116). Funding for this study was also provided by the FOCUS on Health and Leadership for Women Junior Faculty Award for Research in Women’s Cardiovascular Health, funded by the Edna G. Kynett Memorial Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Foster BJ, Dahhou M, Zhang X, Platt RW, Hanley JA: Change in mortality risk over time in young kidney transplant recipients. Am J Transplant 11: 2432–2442, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ: Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA 309: 1921–1929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M; CKiD Study Group : BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 25: 167–174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP: Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS; CREED Investigators : Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 12: 2768–2774, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Palatini P, Mos L, Santonastaso M, Saladini F, Benetti E, Mormino P, Bortolazzi A, Cozzio S: Premenopausal women have increased risk of hypertensive target organ damage compared with men of similar age. J Womens Health (Larchmt) 20: 1175–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Halldin M, Fahlstadius P, de Faire U, Vikström M, Hellénius ML: The metabolic syndrome and left ventricular hypertrophy--the influence of gender and physical activity. Blood Press 21: 153–160, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Borzych D, Bakkaloglu SA, Zaritsky J, Suarez A, Wong W, Ranchin B, Qi C, Szabo AJ, Coccia PA, Harambat J, Mitu F, Warady BA, Schaefer F; International Pediatric Peritoneal Dialysis Network : Defining left ventricular hypertrophy in children on peritoneal dialysis. Clin J Am Soc Nephrol 6: 1934–1943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA: Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol 76: 699–701, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Foster BJ, Platt RW, Zemel BS: Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol 39: 171–182, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: a review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA; Chronic Kidney Disease in Children Study Group : Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 19.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S; Chronic Kidney Disease in Children Study Group : Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 60: 43–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W: Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr 130: 178–184, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Ng DK, Schwartz GJ, Jacobson LP, Palella FJ, Margolick JB, Warady BA, Furth SL, Muñoz A: Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int 80: 423–430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kumar J, McDermott K, Abraham AG, Friedman LA, Johnson VL, Kaskel FJ, Furth SL, Warady BA, Portale AA, Melamed ML: Prevalence and correlates of 25-hydroxyvitamin D deficiency in the Chronic Kidney Disease in Children (CKiD) cohort. Pediatr Nephrol 31: 121–129, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB: Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devereux RB, Reichek N: Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55: 613–618, 1977 [DOI] [PubMed] [Google Scholar]
- 26.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR: Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22: 709–714, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Foster BJ, Khoury PR, Kimball TR, Mackie AS, Mitsnefes M: New Reference Centiles for Left Ventricular Mass Relative to Lean Body Mass in Children. J Am Soc Echocardiogr 29: 441–447.e2, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Bordini B, Rosenfield RL: Normal pubertal development: part II: clinical aspects of puberty. Pediatr Rev 32: 281–292, 2011 [DOI] [PubMed] [Google Scholar]
- 29.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Mëtivier F: Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18: 613–620, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75: 88–95, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Meuwese CL, Carrero JJ: Chronic kidney disease and hypothalamic-pituitary axis dysfunction: the chicken or the egg? Arch Med Res 44: 591–600, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Daniels SR, Meyer RA, Liang YC, Bove KE: Echocardiographically determined left ventricular mass index in normal children, adolescents and young adults. J Am Coll Cardiol 12: 703–708, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Foster BJ, Gao T, Mackie AS, Zemel BS, Ali H, Platt RW, Colan SD: Limitations of expressing left ventricular mass relative to height and to body surface area in children. J Am Soc Echocardiogr 26: 410–418, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Dewey FE, Rosenthal D, Murphy DJ Jr, Froelicher VF, Ashley EA: Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 117: 2279–2287, 2008 [DOI] [PubMed] [Google Scholar]
- 36.George KP, Birch KM, Pennell DJ, Myerson SG: Magnetic-resonance-imaging-derived indices for the normalization of left ventricular morphology by body size. Magn Reson Imaging 27: 207–213, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Hense HW, Gneiting B, Muscholl M, Broeckel U, Kuch B, Doering A, Riegger GA, Schunkert H: The associations of body size and body composition with left ventricular mass: impacts for indexation in adults. J Am Coll Cardiol 32: 451–457, 1998 [DOI] [PubMed] [Google Scholar]
- 38.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH: Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20: 1251–1260, 1992 [DOI] [PubMed] [Google Scholar]
- 39.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH: Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25: 1056–1062, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Foster BJ, Mackie AS, Mitsnefes M, Ali H, Mamber S, Colan SD: A novel method of expressing left ventricular mass relative to body size in children. Circulation 117: 2769–2775, 2008 [DOI] [PubMed] [Google Scholar]