Abstract
Background and objectives
Plasma fibroblast growth factor 23 (FGF23) concentrations increase early in the course of CKD in children. High FGF23 levels associate with progression of CKD in adults. Whether FGF23 predicts CKD progression in children is unknown.
Design, setting, participants, & measurements
We tested the hypothesis that high plasma FGF23 is an independent risk factor for CKD progression in 419 children, aged 1–16 years, enrolled in the Chronic Kidney Disease in Children (CKiD) cohort study. We measured plasma FGF23 concentrations at baseline and determined GFR annually using plasma disappearance of iohexol or the CKiD study estimating equation. We analyzed the association of baseline FGF23 with risk of progression to the composite end point, defined as start of dialysis or kidney transplantation or 50% decline from baseline GFR, adjusted for demographics, baseline GFR, proteinuria, other CKD-specific factors, and other mineral metabolites.
Results
At enrollment, median age was 11 years [interquartile range (IQR), 8–15], GFR was 44 ml/min per 1.73 m2 (IQR, 33–57), and FGF23 was 132 RU/ml (IQR, 88–200). During a median follow-up of 5.5 years (IQR, 3.5–6.6), 32.5% of children reached the progression end point. Higher FGF23 concentrations were independently associated with higher risk of the composite outcome (fully adjusted hazard ratio, 2.52 in the highest versus lowest FGF23 tertile; 95% confidence interval, 1.44 to 4.39, P=0.002; fully adjusted hazard ratio, 1.33 per doubling of FGF23; 95% confidence interval, 1.13 to 1.56, P=0.001). The time to progression was 40% shorter for participants in the highest compared with the lowest FGF23 tertile. In contrast, serum phosphorus, vitamin D metabolites, and parathyroid hormone did not consistently associate with progression in adjusted analyses.
Conclusions
High plasma FGF23 is an independent risk factor for CKD progression in children.
Keywords: chronic kidney disease; progression of chronic renal failure; fibroblast growth factor 23; CKiD; parathyroid hormone; mineral metabolism; adult; child; cohort studies; Confidence Intervals; Demography; Fibroblast Growth Factors; Follow-Up Studies; glomerular filtration rate; humans; iohexol; kidney; kidney transplantation; Minerals; Phosphorus; proteinuria; renal dialysis; Renal Insufficiency, Chronic; risk factors; Vitamin D
Introduction
Disordered mineral metabolism develops during the early stages of CKD, leading to skeletal abnormalities, cardiovascular disease, and impaired growth in children. Together, these complications comprise the clinical syndrome of CKD mineral and bone disorder. Critical to the pathogenesis of CKD mineral and bone disorder is an excess of fibroblast growth factor 23 (FGF23), a bone-derived circulating peptide. In children and adults with CKD, plasma FGF23 concentrations increase progressively as GFR declines (1–3). By suppressing the renal reabsorption of phosphate and the synthesis of 1,25-dihydroxyvitamin D [1,25(OH)2D] (4–6), excess FGF23 initiates the development of secondary hyperparathyroidism (2,7). Moreover, in adult patients with CKD, increased plasma FGF23 is associated with adverse outcomes, including premature death (8,9), left ventricular hypertrophy (10,11), cardiovascular disease events (12), kidney disease progression (13–15), and renal allograft dysfunction (16,17).
Development of ESRD in children compromises life expectancy, yielding mortality rates for children on dialysis that are 30- to 150-fold higher than in healthy children of comparable age (18–21). Known risk factors for progression of childhood CKD include proteinuria, hypertension, glomerular disease, and low GFR (22). However, it is not known whether abnormalities in mineral metabolism, in particular excess circulating FGF23, are associated with CKD progression in children. Such an association is suggested by observations that, in children with a glomerular etiology of CKD, plasma FGF23 is higher (3) and the rate of CKD progression can be faster than in children with CKD of nonglomerular etiology. Identification of modifiable factors that affect CKD progression is critically important to the development and early implementation of therapeutic strategies that prevent or delay progression and its complications, including cardiovascular disease, the leading cause of death in CKD.
In the present study, we tested the hypothesis that high plasma FGF23 is a risk factor for kidney disease progression, independent of baseline GFR and proteinuria, in children with predialysis CKD who were enrolled in the Chronic Kidney Disease in Children (CKiD) observational cohort study (23).
Materials and Methods
The CKiD study is a prospective observational cohort study of children with CKD (23). Briefly, participants aged 1–16 years old with an eGFR between 30 and 90 ml/min per 1.73 m2 (2,24), were enrolled from 54 pediatric nephrology sites in the United States and Canada. Recipients of solid organ, bone marrow, or stem cell transplants were excluded; additional exclusion criteria are described in Furth et al. (23). The Institutional Review Board at each study site approved the study protocol, and informed consent was obtained from each participant and parent or guardian. The study is registered with Clinicaltrials.gov (registration no.: NCT00327860).
Participants
Participants underwent annual study visits at which BP, GFR, and laboratory values were determined (23). GFR was directly measured by plasma disappearance of iohexol (25) at the enrollment visit, 1 year later, and every other year thereafter. When not directly measured, GFR was estimated using the CKiD study estimating equation that is based on serum creatinine and cystatin C concentrations (26). We use GFR to refer to either the iohexol measured value (when available) or the estimated value, for each individual.
The CKiD study enrolled 586 participants from January, 2005 to July, 2009; for the present analysis, we included 419 participants for whom specimens were available for baseline measurement of FGF23, parathyroid hormone (PTH), and vitamin D metabolites at the first follow-up visit, which occurred within six months of enrollment. We found no differences in baseline characteristics between those participants included in the present study and those not included, with respect to age, gender, glomerular diagnosis, and GFR, respectively.
Assays
Specimens for PTH, FGF23, and vitamin D metabolites were stored at −80°C until measurements were made. We measured plasma C-terminal FGF23 concentrations in duplicate by second generation ELISA (Immutopics Int., San Clemente, CA); inter- and intra-assay coefficients of variation were 11.5% and 5.7%, respectively. We defined 101 RU/ml as the upper limit of the normal range in healthy children (mean age, 12±4 years) (3). Serum concentrations of vitamin D metabolites were measured in duplicate by Heartland Assays (Ames, IA); 25-hydroxyvitamin D (25OHD) by chemiluminescence immunoassay (27,28), and 1,25(OH)2D by radioimmunoassay (29), as described (3). Serum creatinine, intact PTH, calcium, phosphorus, and urine protein and creatinine concentrations were determined in the CKiD Central Biochemistry Laboratory, University of Rochester, as described (3). Cystatin C was determined by nephelometry (Siemens Dade-Behring). Serum calcium concentrations were corrected for serum albumin concentrations using the following formula: corrected calcium=measured calcium+0.8×(4.0−serum albumin).
Covariates
Proteinuria was categorized based on the ratio of urine protein-to-creatinine concentration (Up/c), as <0.5 mg/mg, 0.5 to <2.0 mg/mg, or ≥2.0 mg/mg. Baseline systolic BP was categorized as a z-score relative to gender, age, and height-matched values (30). Hypertension was defined as a systolic or diastolic BP ≥95th percentile value for gender, age, and height (30). The primary diagnoses of CKD were categorized as either nonglomerular (obstruction/reflux, hypoplasia/dysplasia, cystic disease, pyelonephritis/interstitial nephritis, other) or glomerular (FSGS, familial nephritis, hemolytic uremic syndrome, other). Since serum phosphorus concentration varies with age in healthy children (31), we expressed phosphorus values for each participant as a z-score relative to age-matched values in 493 healthy individuals aged 1–20 years old (31).
Statistical Analyses
We summarized continuous variables as mean±SD or median and interquartile range (IQR) for skewed data, in the overall cohort and according to tertiles of FGF23: tertile 1, <100 RU/ml (within the normal range); tertile 2, 100–169 RU/ml; and tertile 3, ≥170 RU/ml. We expressed categorical variables as frequencies and proportions. We compared mean or median values of biochemical parameters between FGF23 tertiles using one-way ANOVA or the Kruskal–Wallis test, as appropriate. We used simple linear and multivariable regression analyses to examine associations between GFR and FGF23, 1,25(OH)2D, and other parameters of mineral metabolism. Values of FGF23 and PTH were natural log transformed to satisfy normality assumptions. Analyses were performed using STATA 12 (StataCorp., College Station, TX) and SAS 9.12 (SAS Institute Inc., Cary, NC) software. A P value of <0.05 was considered statistically significant.
The primary outcome was progression of CKD, defined as a composite event of either initiation of dialysis or kidney transplantation or 50% reduction in GFR from the level at enrollment (baseline). Participants were censored for loss to follow-up or at the close of the observation period in August, 2013. The primary exposure variable was the tertile of plasma FGF23 concentration, the lowest tertile (normal range) serving as the reference category; we also analyzed risk of CKD progression according to continuous FGF23 concentrations, reported per doubling of FGF23. Secondary exposure variables were phosphorus z-score, 25OHD, and 1,25(OH)2D concentrations. We used multivariable Cox proportional hazards regression to examine the risk of progression to the composite event according to tertiles of baseline plasma FGF23, after verifying proportionality assumptions. In model 1, we adjusted for age, gender, and race. In model 2, we additionally adjusted for baseline GFR and proteinuria. In model 3, we additionally adjusted for systolic BP, medication use (angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, vitamin D analogs, and phosphate binders), and glomerular diagnosis of CKD. Model 3 is the primary model of interest in the present analysis. In model 4, we additionally adjusted for mineral metabolites (phosphorus z-score, tertiles of 25OHD and 1,25(OH)2D concentration). We performed similar risk analyses for each of the secondary exposures (phosphorus z-score, 25OHD, and 1,25(OH)2D concentrations); in model 4, we additionally adjusted for FGF23 and mineral metabolites other than the exposure variable. Serum PTH was measured in only 219 participants based on availability of suitable serum specimens; thus, we performed separate regression analyses that included PTH as a covariate, within that subset of participants.
In separate complementary analyses, we examined the association between tertiles of baseline plasma FGF23 concentration and time to the composite event using parametric time-failure regression, assuming log normal distribution of failure times with adjustment for the covariates described above, as done previously in the CKiD study (22). With this approach, the interpretation for the association was expressed in terms of relative time to the composite event according to tertiles of FGF23 (e.g., a relative time of 0.6 would indicate a 40% shorter time to the event compared with the reference exposure).
Results
Study Population
Characteristics of the 419 participants are summarized in Table 1. In the overall cohort, the median baseline GFR was 44 ml/min per 1.73 m2 (IQR, 33–57), and range was 16–109 ml/min per 1.73 m2. Three percent of participants had CKD stage 1, 20% had stage 2, 59% had stage 3, and 18% had stage 4. The median plasma FGF23 level was 132 RU/ml (IQR, 88–200), a value 2.3-times higher than that in healthy children of comparable age (31); FGF23 excess (>101 RU/ml) was observed in 65% of participants. Participants in the highest FGF23 tertile had significantly lower GFR and serum 1,25(OH)2D and albumin concentrations, higher serum phosphorus, phosphorus z-score, and PTH values, and more severe proteinuria, compared with those in the lowest FGF23 tertile (Table 1).
Table 1.
Baseline characteristics of the study population by tertile of plasma FGF23 concentration
| Characteristic | Overall Cohort | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|---|
| FGF23, RU/ml | <100 | 100–169 | ≥170 | |
| n | 419 | 141 | 139 | 139 |
| Age, yr | 11.1 [7.8, 14.5] | 10.7 [7.4, 14.2] | 11.1 [7.7, 14.3] | 12.2 [8.2, 14.9] |
| Female, n (%) | 160 (38) | 52 (37) | 50 (36) | 58 (42) |
| Race, n (%) | ||||
| White | 292 (70) | 98 (70) | 95 (68) | 99 (71) |
| Black | 83 (20) | 31 (22) | 29 (21) | 23 (17) |
| Multiracial or other | 44 (11) | 12 (9) | 15 (11) | 17 (12) |
| Hispanic | 56 (14) | 27 (19) | 12 (9) | 17 (13) |
| Primary CKD diagnosis, n (%) | ||||
| Glomerular | 89 (21) | 27 (19) | 20 (14) | 42 (30) |
| Obstruction/reflux | 159 (38) | 55 (39) | 60 (43) | 44 (32) |
| Hypoplasia/dysplasia | 69 (16) | 20 (14) | 25 (18) | 24 (17) |
| Other nonglomerular | 83 (20) | 36 (26) | 26 (19) | 21 (15) |
| Cystic diseasea | 19 (5) | 3 (2) | 8 (6) | 8 (6) |
| Hypertensive, n (%) | 86 (21) | 22 (16) | 26 (19) | 38 (28) |
| GFR, ml/min per 1.73 m2 | 44 [33, 57] | 52 [41, 71] | 45 [35, 56]b | 35 [28, 47]b,c |
| Mineral metabolism | ||||
| Serum calcium, mg/dl | 9.4±0.4 | 9.4±0.4 | 9.4±0.4 | 9.4±0.5 |
| Serum phosphorus, mg/dl | 4.6±0.8 | 4.4±0.6 | 4.6±0.8 | 4.7±0.9b |
| Serum phosphorus z-score | −0.1±1.5 | −0.4±1.2 | −0.1±1.4 | 0.3±1.7b,c |
| Serum iPTH, pg/mld | 54 [32, 97] | 39 [24, 55] | 62 [38, 108]b | 82 [44, 1454]b |
| Plasma FGF23, RU/ml | 132 [88, 200] | 75 [60, 88] | 133 [115, 149]b | 250 [200, 405]b,c |
| Serum 25OHD, ng/ml | 27±12 | 27±10 | 29±12 | 26±13 |
| Serum 1,25(OH)2D, pg/ml | 31±11 | 33±11 | 32±12 | 22±11b,c |
| Serum albumin, g/dl | 4.3±0.4 | 4.4±0.3 | 4.3±0.3 | 4.1±0.6b,c |
| Urine protein-to-creatinine ratio, mg/mg | 0.4 [0.2, 1.0] | 0.2 [0.1, 0.6] | 0.3 [0.1, 0.8] | 0.7 [0.3, 1.8]b,c |
| Medication use, n (%) | ||||
| Antihypertensive (ACE/ARB) | 227 (54) | 79 (56) | 72 (52) | 76 (55) |
| Phosphate binders | 86 (21) | 23 (16) | 24 (17) | 39 (28) |
| Active vitamin D | 155 (37) | 32 (23) | 46 (33) | 77 (55) |
| Nutritional vitamin D | 21 (5) | 8 (6) | 6 (4) | 7 (5) |
Data are means±SD, medians [25th, 75th percentile] or n (%). FGF23, fibroblast growth factor; iPTH, immunoreactive parathyroid hormone; 25OHD, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Recessive polycystic kidney disease (n=13), juvenile nephronophthisis (n=4), dominant polycystic kidney disease (n=2).
P<0.05 versus tertile 1.
P<0.05 versus tertile 2.
n=219.
Across the cohort, log plasma FGF23 concentrations varied inversely with GFR (R=−0.41; P<0.001), inversely with serum 1,25(OH)2D concentrations (R=−0.26; P<0.001), directly with phosphorus z-score (R=0.22; P<0.001), and directly with log of the Up/c (R=0.32; P<0.001); each of these associations remained significant after adjustment for GFR. Plasma FGF23 did not correlate with serum calcium or 25OHD concentrations.
CKD Progression
Over a median of 5.5 years (IQR, 3.5–6.6) of observation, 136 participants (32.5%) progressed to the composite end point; 121 participants underwent dialysis or received a kidney transplant, and 15 experienced a 50% decline in GFR from baseline values. Fifteen participants were lost to follow-up after their baseline visits. Seventy-four percent (n=308) of participants underwent four or more longitudinal measurements of GFR.
FGF23 and Risk of Progression
Kaplan–Meier curves illustrate that higher tertiles of FGF23 were associated with higher probability of reaching the composite end point (Figure 1). In Cox proportional hazards models, the risk of progression was 2.3-fold and 5.0-fold higher in participants with FGF23 levels in the middle and highest tertiles, respectively, compared with those in the lowest FGF23 tertile (normal range) after adjustment for age, gender, and race (model 1, Figure 2, Table 2). With full multivariable adjustment for baseline GFR, proteinuria, BP, glomerular disease, and medication use, higher FGF23 concentrations remained significantly associated with progression, the risk being 2.5-fold higher in the highest versus the lowest FGF23 tertile (model 3, Figure 2, Table 2). The association between FGF23 and progression was also significant when plasma FGF23 was modeled as a continuous variable; each doubling of FGF23 was associated with a 33% higher risk of progression (model 3, Table 2). The associations were not attenuated when further adjusted for concentrations of other mineral metabolites (model 4).
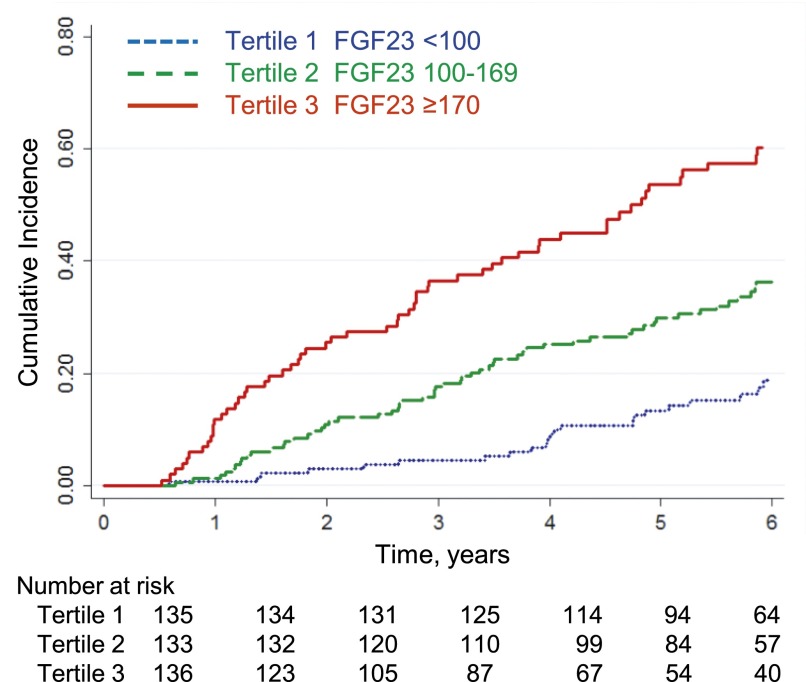
Figure 1.
Higher tertiles of baseline plasma FGF23 concentration associate with higher cumulative incidence of progression to dialysis, transplant, or 50% decline in GFR from baseline. Log-rank test for trend, P<0.001. FGF23, fibroblast growth factor 23.
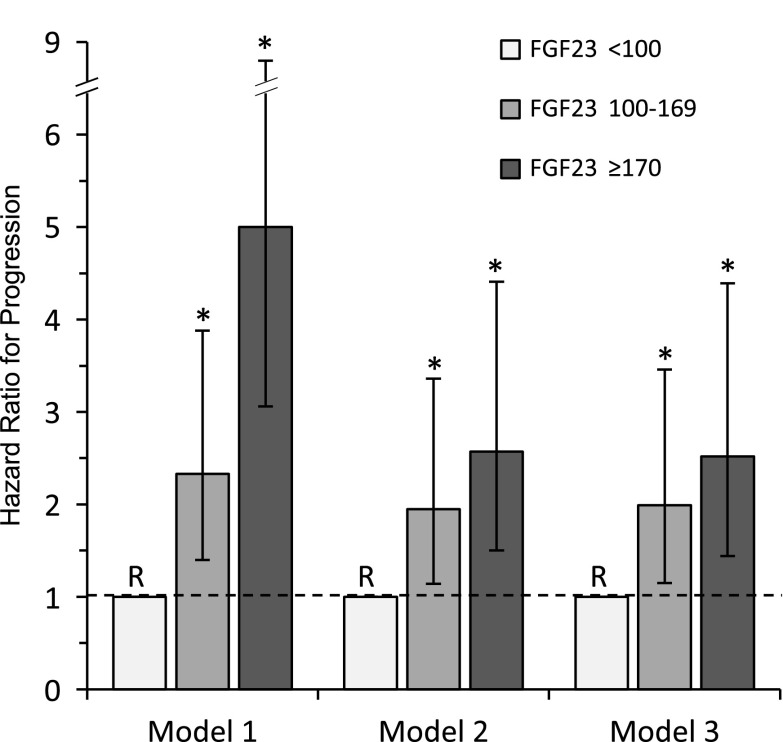
Figure 2.
Hazard ratio (and 95% confidence interval) for the composite end point of dialysis, transplant, or 50% decline in GFR from baseline, according to tertiles of baseline plasma FGF23 concentrations. Model 1: adjusted for gender, age, and race. Model 2: adjusted for covariates in model 1 plus GFR and Up/c. Model 3: adjusted for covariates in model 2 plus systolic BP z-score, glomerular diagnosis, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, active vitamin D, and phosphate binder use. Tertile 1 (FGF23<100 RU/ml) is the reference group (R) in all models. Bars represent 95% confidence intervals. Asterisks indicate P<0.02 relative to the reference group. FGF23, fibroblast growth factor 23; Up/c, urine protein-to-creatinine concentration.
Table 2.
Risk of progression to the composite end point according to indices of mineral metabolism
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimally Adjusteda | +Adjusted for GFR, Up/cb | Multivariable Adjustedc | +Mineral Metabolitesd | |||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Plasma FGF23 tertiles, RU/ml | ||||||||||||
| <100 | Reference | Reference | Reference | Reference | ||||||||
| 100–169 | 2.33 | 1.40 to 3.88 | 0.001 | 1.95 | 1.14 to 3.36 | 0.02 | 1.99 | 1.15 to 3.46 | 0.01 | 1.96 | 1.09 to 3.52 | 0.02 |
| ≥170 | 4.99 | 3.06 to 8.12 | <0.001 | 2.57 | 1.50 to 4.41 | 0.001 | 2.52 | 1.44 to 4.39 | 0.002 | 2.58 | 1.42 to 4.67 | 0.01 |
| P for linear trend | <0.001 | 0.003 | 0.01 | |||||||||
| Plasma FGF23, per doubling | 1.70 | 1.49 to 1.94 | <0.001 | 1.31 | 1.12 to 1.53 | 0.001 | 1.33 | 1.13 to 1.56 | 0.001 | 1.36 | 1.14 to 1.64 | 0.001 |
| Serum 25OHD tertiles, ng/ml | ||||||||||||
| >33 | Reference | Reference | Reference | Reference | ||||||||
| 23–33 | 1.41 | 0.88 to 2.27 | 0.46 | 1.24 | 0.75 to 2.10 | 0.40 | 1.23 | 0.74 to 2.06 | 0.43 | 1.26 | 0.75 to 2.11 | 0.39 |
| <23 | 1.66 | 1.01 to 2.72 | 0.04 | 1.22 | 0.71 to 2.12 | 0.47 | 0.97 | 0.54 to 1.74 | 0.91 | 0.94 | 0.51 to 1.74 | 0.84 |
| P for linear trend | 0.13 | 0.68 | 0.58 | 0.49 | ||||||||
| Serum 25OHD, per 5 ng/ml lower | 1.11 | 1.02 to 1.21 | 0.02 | 1.04 | 0.95 to 1.42 | 0.40 | 1.00 | 0.91 to 1.10 | 0.94 | 1.00 | 0.91 to 1.10 | 0.95 |
| Serum 1,25(OH)2D tertiles, pg/ml | ||||||||||||
| >34 | Reference | Reference | Reference | Reference | ||||||||
| 25–34 | 1.65 | 1.00 to 2.71 | <0.05 | 1.50 | 0.90 to 2.51 | 0.12 | 1.47 | 0.87 to 2.48 | 0.15 | 1.35 | 0.79 to 2.29 | 0.27 |
| <25 | 2.57 | 1.61 to 4.11 | <0.001 | 1.74 | 1.07 to 2.87 | 0.03 | 1.41 | 0.83 to 2.40 | 0.20 | 1.29 | 0.75 to 2.23 | 0.35 |
| P for linear trend | <0.001 | 0.08 | 0.32 | 0.52 | ||||||||
| Serum 1,25(OH)2D, per 10 pg/ml lower | 1.71 | 1.40 to 2.09 | <0.001 | 1.32 | 1.09 to 1.59 | 0.004 | 1.19 | 0.98 to 1.45 | 0.08 | 1.16 | 0.95 to 1.42 | 0.15 |
| Phosphorus z-score tertiles, SD | ||||||||||||
| <-0.75 | 0.83 | 0.53 to 1.29 | 0.41 | 1.18 | 0.74 to 1.88 | 0.48 | 1.39 | 0.85 to 2.28 | 0.18 | 1.31 | 0.77 to 2.22 | 0.31 |
| −0.75–0.48 | Reference | Reference | Reference | Reference | ||||||||
| >0.48 | 1.64 | 1.10 to 2.45 | 0.02 | 1.45 | 0.95 to 2.20 | 0.08 | 1.40 | 0.90 to 2.18 | 0.13 | 1.29 | 0.81 to 2.06 | 0.29 |
| P for linear trend | 0.003 | 0.22 | 0.25 | 0.47 | ||||||||
| Phosphorus z-score, per SD higher | 1.28 | 1.15 to 1.42 | <0.001 | 1.09 | 0.98 to 1.22 | 0.12 | 1.05 | 0.94 to 1.18 | 0.38 | 1.04 | 0.92 to 1.17 | 0.54 |
, additionally adjusted; Up/c, urine protein-to-creatinine ratio; HR, hazard ratio; 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; 25OHD, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Adjusted for gender, age, and race.
Additionally adjusted for GFR, Up/c.
Additionally adjusted for systolic BP z-score, glomerular diagnosis, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, active vitamin D, and phosphate binder use. This is the primary model of interest.
Additionally adjusted for mineral metabolites [phosphorus z-score, tertiles of FGF23, 25OHD, and 1,25(OH)2D] other than the primary exposure variable.
The associations between FGF23 and progression remained significant and quantitatively unchanged when the analysis was restricted to the progression end points of onset of dialysis or transplantation alone: multivariable adjusted hazard ratio (HR), 2.36 in the highest versus lowest FGF23 tertile; 95% confidence interval, 1.31 to 4.27, P=0.004; HR, 1.29 per doubling of FGF23; 95% confidence interval, 1.09 to 1.53, P=0.003.
In separate parametric time-to-failure regression models, higher tertiles of plasma FGF23 and high log FGF23 were independently associated with shorter time to progression to the composite end point. With multivariable adjustment, the time to progression was 40% shorter for participants in the highest compared with those in the lowest FGF23 tertile (model 3, Table 3). Each doubling of FGF23 was associated with an 18% shorter time to progression. The associations between FGF23 and outcomes were qualitatively unchanged when the analysis was restricted to the progression end points of onset of dialysis or transplantation only (data not shown).
Table 3.
Relative time to the composite end point according to indices of mineral metabolism
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimally Adjusteda | +Adjusted for GFR, Up/cb | Multivariable Adjustedc | +Mineral Metabolitesd | |||||||||
| RT | 95% CI | P Value | RT | 95% CI | P Value | RT | 95% CI | P Value | RT | 95% CI | P Value | |
| Plasma FGF23 tertiles, RU/ml | ||||||||||||
| <100 | Reference | Reference | Reference | Reference | ||||||||
| 100–169 | 0.56 | 0.39 to 0.79 | 0.001 | 0.70 | 0.51 to 0.95 | 0.02 | 0.68 | 0.51 to 0.92 | 0.01 | 0.69 | 0.50 to 0.94 | 0.02 |
| ≥170 | 0.31 | 0.22 to 0.44 | <0.001 | 0.59 | 0.43 to 0.80 | 0.001 | 0.60 | 0.44 to 0.81 | 0.001 | 0.59 | 0.42 to 0.82 | 0.002 |
| P for linear trend | <0.001 | 0.003 | 0.004 | 0.0 | ||||||||
| Plasma FGF23, per doubling | 0.64 | 0.56 to 0.72 | <0.001 | 0.81 | 0.73 to 0.91 | <0.001 | 0.82 | 0.74 to 0.91 | <0.001 | 0.82 | 0.73 to 0.92 | 0.001 |
| Serum 1,25(OH)2D tertile, pg/ml | ||||||||||||
| >34 | Reference | Reference | Reference | Reference | ||||||||
| 25–34 | 0.66 | 0.45 to 0.96 | 0.03 | 0.81 | 0.59 to 1.1 | 0.18 | 0.84 | 0.61 to 1.14 | 0.26 | 0.88 | 0.65 to 1.19 | 0.41 |
| <25 | 0.42 | 0.29 to 0.61 | <0.001 | 0.71 | 0.52 to 0.97 | 0.03 | 0.80 | 0.58 to 1.09 | 0.15 | 0.83 | 0.61 to 1.13 | 0.24 |
| P for linear trend | <0.001 | 0.09 | 0.34 | 0.50 | ||||||||
| Serum 1,25(OH)2D, per 10 pg/ml lower | 0.66 | 0.57 to 0.76 | <0.001 | 0.84 | 0.74 to 0.95 | 0.0 | 0.89 | 0.79 to 0.99 | 0.03 | 0.90 | 0.80 to 1.00 | 0.06 |
, additionally adjusted; Up/c, urine protein-to-creatinine ratio; RT, relative time; 95% CI, 95% confidence interval; FGF23, fibroblast growth factor 23; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Adjusted for gender, age, and race.
Additionally adjusted for GFR, Up/c.
Additionally adjusted for systolic BP z-score, glomerular diagnosis, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, active vitamin D, and phosphate binder use. This is the primary model of interest.
Additionally adjusted for mineral metabolites [phosphorus z-score, tertiles of FGF23, 25OHD, and 1,25(OH)2D] other than the primary exposure variable.
Mineral Metabolites and Risk of Progression
Serum 25OHD and phosphorus z-score were significantly associated with progression in minimally adjusted models, but the associations were heavily attenuated with full multivariable adjustment (Table 2). When modeled as a continuous variable, each 10 pg/ml lower serum 1,25(OH)2D was associated with a 32% higher risk of progression when adjusted for baseline GFR and proteinuria (model 2, P=0.004); however the association between 1,25(OH)2D and progression was attenuated and no longer significant in fully adjusted analyses (model 3, Table 2). Serum PTH, which was measured in a subgroup of 219 participants, was not associated with progression in any of the models and did not attenuate the significant association between FGF23 and progression (data not shown).
Discussion
In the largest longitudinal study of children with predialysis CKD to date, we observed that higher concentrations of plasma FGF23 were associated with a higher risk of progression to dialysis, renal transplant, or 50% decline in GFR. In participants with FGF23 values in the highest tertile (>1.7 times the upper limit of normal), the risk of progression was 2.5-fold higher than in those with FGF23 values in the normal range, and this association remained significant after adjustment for baseline GFR, severity of proteinuria, BP, and medication use including angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers. Further, the association between higher FGF23 and outcomes remained significant when the analysis was limited to those reaching the stricter progression end point of dialysis or transplantation. When analyzed as a continuous variable, each doubling of FGF23 was associated with a 33% higher risk of progression in fully adjusted analyses. In separate time-to-event analyses, progression to the composite end point was 40% faster in children with FGF23 values in the highest versus those in the lowest tertile. Thus, plasma FGF23 was strongly associated with progression before and after adjustment for confounders, showing a graded risk across the full range of values that was robust to several modeling strategies.
Our finding that FGF23 is an independent risk factor for disease progression in children with CKD is consistent with studies of adults in which FGF23 was shown to independently associate with CKD progression in patients with diabetic and nondiabetic renal disease (13,32,33), IgA nephropathy (14), and hypertensive nephrosclerosis (15). The magnitude of the effect of FGF23 on CKD progression in the present study is very similar to that in studies of adults (14,15,17,32). Further, FGF23 independently associated with a higher risk of mortality (17) and allograft dysfunction (16,17) in renal transplant recipients, and the development of ESRD in the general population (34). In some but not all of those studies, PTH (13,15) was an additional risk factor for progression. However, we found no association between risk of progression and PTH, which was measured in approximately half of study participants.
The effect of 25OHD status on CKD progression recently was examined. Scialla et al. found no association between quartiles of 25OHD and CKD progression in black participants with hypertensive nephrosclerosis (15). In a post hoc analysis of data from the Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients trial, Shroff et al. found that 25OHD was an independent predictor of progression in 167 children with CKD of nonglomerular origin (35). Although we observed that low levels of 25OHD associated with a higher risk of progression in minimally adjusted analyses, the association was heavily attenuated after further adjustment for GFR, proteinuria, and BP. There are some differences between the two studies: Shroff et al. studied fewer children and the prevalence of vitamin D deficiency was higher than in the present study. Also, higher systolic BP was significantly associated with progression in our analyses (data not shown) but not in those of Shroff et al. (35). They also found that FGF23 was associated with progression in adjusted analyses (HR, 1.03 per 10 RU/ml higher FGF23; 95% confidence interval 1.005 to 1.057).
Higher baseline levels of proteinuria (22,36) and BP (22,36) have been associated with greater risk or faster rate of progression in children with glomerular and nonglomerular etiology of CKD. Children with glomerular diseases have higher levels of proteinuria and plasma FGF23 (3), both of which might contribute to more rapid progression than in those with nonglomerular etiology. In the present study, the association between baseline FGF23 and outcome remained significant even after adjustment for proteinuria and glomerular etiology; indeed, glomerular etiology did not significantly associate with progression in any of the models that adjusted for baseline GFR and proteinuria.
A renoprotective effect of active vitamin D metabolites has been identified in some experimental (37,38) and clinical settings (39,40). However, we failed to observe an association between serum 1,25(OH)2D and progression after multivariable adjustment that included proteinuria, medication use, and systolic BP, the latter a predictor of CKD progression in children (22,36).
We (22) and others (41) previously observed that hyperphosphatemia was a risk factor for CKD progression in children, using analyses that adjusted for GFR alone (22) and not for proteinuria (22,41). Indeed, we identified proteinuria as the strongest predictor for CKD progression in children (22). In the present multivariable analyses that included adjustment for proteinuria, serum phosphorus did not significantly associate with progression, suggesting residual confounding in prior studies of children (22,41). Some studies of adults with CKD report an association between serum phosphorus and progression (15,32,42,43), whereas others do not (13,33,44).
The mechanisms underlying the association between plasma FGF23 concentration and CKD progression, cardiovascular events, and death are unknown. Experimental data favor a direct pathophysiologic role of FGF23 in cardiac toxicity, specifically ventricular hypertrophy, via an FGF receptor 4–dependent pathway (11,45,46). Alternatively, the association between FGF23 and CKD progression might not be direct but rather mediated or confounded by other factors. FGF23 can aggravate other parameters of mineral metabolism that could modulate progression, such as suppression of 1,25(OH)2D synthesis and worsening of hyperparathyroidism (1). The independent effect of FGF23 in our analyses that adjusted for vitamin D and other mineral metabolites argues against this potential mechanism.
Our findings suggest that plasma FGF23 has the potential to be a clinically applicable therapeutic target, as it increases early in the course of GFR decline and is strongly associated with disordered phosphorus metabolism. Higher plasma FGF23 might identify patients with CKD who may benefit from various strategies to lower serum phosphorus concentration, even if values are in the nominally normal range.
A major strength of the present study is the recruitment of a large cohort of children from multiple centers across North America whose cause of CKD is broadly representative of that in children. GFR was determined longitudinally by direct measurement in the majority of participants or using a highly accurate estimating equation in the remainder. Follow-up of participants extended for a median of 5.5 years, and associations between FGF23 and progression were robust to analysis using both proportional hazards regression and parametric time-to-event regression, the latter not requiring assumption of proportional hazards, and when using the strict progression end points of dialysis and transplantation.
The study has several limitations. Our data do not demonstrate causation or reveal mechanisms that might mediate the association between FGF23 and progression. FGF23 was determined 3–6 months after baseline GFR was measured; however, GFR is predicted to decline by <1 ml/min per 1.73 m2 over this period (47). GFR determined by iohexol clearance might underestimate GFR as determined by inulin clearance (48,49). Such underestimation should not bias our results as we defined progression as dialysis, transplantation, or a within-person decrease of 50% in GFR rather than an absolute value of GFR. Further, the CKiD study estimating equation itself was derived from the plasma clearance of iohexol; thus, comparisons between measured and estimated GFR are unbiased. Although only measured in approximately half of participants, PTH did not associate with progression or attenuate the association between FGF23 and progression. Finally, we used a single baseline measurement of FGF23 to predict follow-up events; whether changes in FGF23 over time add incremental predictive value is unknown.
In summary, we demonstrate that higher plasma FGF23 is an independent risk factor for CKD progression in children. Interventional studies are needed to determine whether therapeutic strategies that reduce or attenuate the increase in plasma FGF23 can prevent or delay the progressive course in children with CKD.
Disclosures
A.A.P. has received honoraria from Sanofi US (Bridgewater, NJ) and Ultragenyx (Novato, CA). M.W. has served as a consultant or received honoraria from Amgen (Thousand Oaks, CA), Ardelyx (Fremont, CA), Diasorin (Saluggia, Italy), Keryx (Boston, MA), Lilly (Indianapolis, IN), Pfizer (New York, NY), and Ultragenyx. I.B.S. has received honoraria from Cytochroma (Miami, FL), AbbVie (Redwood City, CA), Amgen, and Sanofi. H.J. is named on a patent describing the FGF23 assay that was used for this study. S.L.F. has received honoraria from Johnson and Johnson (New Brunswick, NJ). B.A.W. has received honoraria from Amgen, Sanofi, and AbbVie.
Acknowledgments
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK; R01-DK-084978 to A.A.P., PO1-DK- 11794 (subproject IV) to H.J.], grants from AbbVie and Genzyme Corporation (to A.A.P.), and the Pediatric Nephrology Innovative Research Fund (to A.A.P.). The CKiD study is funded by the NIDDK, Eunice Kenney Shriver National Institute of Child Health and Human Development, and National Heart, Lung and Blood Institute [UO1-DK- 66143, UO1-DK-66174, U01-DK-082194, and UO1-DK-66116 (Principal Investigators: S.L.F. and B.A.W.)].
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB: Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N: Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J Biol Chem 278: 2206–2211, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Perwad F, Zhang MY, Tenenhouse HS, Portale AA: Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293: F1577–F1583, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Liang N, Chen TH, Li A, Santa Maria C, You M, Ho H, Song F, Bikle D, Tu C, Shoback D, Chang W: Sex and age modify biochemical and skeletal manifestations of chronic hyperparathyroidism by altering target organ responses to Ca2+ and parathyroid hormone in mice. J Bone Miner Res 28: 1087–1100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P; MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE: FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol 7: 727–734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesseling-Perry K, Tsai EW, Ettenger RB, Jüppner H, Salusky IB: Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: a role for FGF-23? Nephrol Dial Transplant 26: 3779–3784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association : Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Warady BA, Chadha V: Chronic kidney disease in children: the global perspective. Pediatr Nephrol 22: 1999–2009, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ: Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA 309: 1921–1929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 63[Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 25.Schwartz GJ, Furth S, Cole SR, Warady B, Muñoz A: Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Muñoz A: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner D, Hanwell HE, Vieth R: An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 42: 1549–1556, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Ersfeld DL, Rao DS, Body JJ, Sackrison JL Jr, Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD: Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 37: 867–874, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL: Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42: 586–592, 1996 [PubMed] [Google Scholar]
- 30.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 31.Lockitch G, Halstead AC, Wadsworth L, Quigley G, Reston L, Jacobson B: Age- and sex-specific pediatric reference intervals and correlations for zinc, copper, selenium, iron, vitamins A and E, and related proteins. Clin Chem 34: 1625–1628, 1988 [PubMed] [Google Scholar]
- 32.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M; HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moysés RM: FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 6: 241–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebholz CM, Grams ME, Coresh J, Selvin E, Inker LA, Levey AS, Kimmel PL, Vasan RS, Eckfeldt JH, Feldman HI, Hsu CY, Lutsey PL; Chronic Kidney Disease Biomarkers Consortium : Serum fibroblast growth factor-23 is associated with incident kidney disease. J Am Soc Nephrol 26: 192–200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shroff R, Aitkenhead H, Costa N, Trivelli A, Litwin M, Picca S, Anarat A, Sallay P, Ozaltin F, Zurowska A, Jankauskiene A, Montini G, Charbit M, Schaefer F, Wuhl E; ESCAPE Trial Group: Normal 25-Hydroxyvitamin D Levels Are Associated with Less Proteinuria and Attenuate Renal Failure Progression in Children with CKD. J Am Soc Nephrol 27: 314–322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, Moxey-Mims MM, Warady BA, Furth SL, Wong CS: Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 10: 571–577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B: Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 74: 1394–1402, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 40.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS: Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 5: 2172–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, Appel LJ: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 17: 2928–2936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825–831, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Mehrotra R, Peralta CA, Chen SC, Li S, Sachs M, Shah A, Norris K, Saab G, Whaley-Connell A, Kestenbaum B, McCullough PA; Kidney Early Evaluation Program (KEEP) Investigators : No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Kidney Int 84: 989–997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Marco GS, Reuter S, Kentrup D, Grabner A, Amaral AP, Fobker M, Stypmann J, Pavenstädt H, Wolf M, Faul C, Brand M: Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol Dial Transplant 29: 2028–2035, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C: Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab 22: 1020–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, Wong C, Mak RH, Moxey-Mims M, Warady BA: Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol 6: 2132–2140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seegmiller JC, Burns BE, Schinstock CA, Lieske JC, Larson TS: Discordance Between Iothalamate and Iohexol Urinary Clearances. Am J Kidney Dis 67: 49–55, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Soveri I, Berg UB, Björk J, Elinder CG, Grubb A, Mejare I, Sterner G, Bäck SE; SBU GFR Review Group : Measuring GFR: a systematic review. Am J Kidney Dis 64: 411–424, 2014 [DOI] [PubMed] [Google Scholar]