Abstract
Warfarin has had a thin margin of benefit over risk for the prevention of stroke and systemic embolism in patients with ESRD because of higher bleeding risks and complications of therapy. The successful use of warfarin has been dependent on the selection of patients with nonvalvular atrial fibrillation at relatively high risk of stroke and systemic embolism and lower risks of bleeding over the course of therapy. Without such selection strategies, broad use of warfarin has not proven to be beneficial to the broad population of patients with ESRD and nonvalvular atrial fibrillation. In a recent meta-analysis of use of warfarin in patients with nonvalvular atrial fibrillation and ESRD, warfarin had no effect on the risks of stroke (hazard ratio, 1.12; 95% confidence interval, 0.69 to 1.82; P=0.65) or mortality (hazard ratio, 0.96; 95% confidence interval, 0.81 to 1.13; P=0.60) but was associated with increased risk of major bleeding (hazard ratio, 1.30; 95% confidence interval, 1.08 to 1.56; P<0.01). In pivotal trials, novel oral anticoagulants were generally at least equal to warfarin for efficacy and safety in nonvalvular atrial fibrillation and mild to moderate renal impairment. Clinical data for ESRD are limited, because pivotal trials excluded such patients. Given the very high risk of stroke and systemic embolism and the early evidence of acceptable safety profiles of novel oral anticoagulants, we think that patients with ESRD should be considered for treatment with chronic anticoagulation provided that there is an acceptable bleeding profile. Apixaban is currently indicated in ESRD for this application and may be preferable to warfarin given the body of evidence for warfarin and its difficulty of use and attendant adverse events.
Keywords: oral anticoagulation, atrial fibrillation, end-stage renal disease, stroke, Anticoagulants, Embolism, Hemorrhage, Humans, Kidney Failure, Chronic, renal dialysis, Warfarin, apixaban
Introduction
The increasing incidence of CKD caused by substrates mirrors the increasing incidence of nonvalvular atrial fibrillation (NVAF) defined as atrial fibrillation not associated with native valvular heart disease or valve replacement. This is the most common cardiac arrhythmia in patients with ESRD. About one in 10 Americans (20 million) have kidney disease, with functional impairment categorized by eGFR as follows: normal, >90 ml/min per 1.73 m2; mild CKD, 60–90 ml/min per 1.73 m2; moderate CKD, 30–59 ml/min per 1.73 m2; severe CKD, 15–29 ml/min per 1.73 m2; and ESRD, <15 ml/min per 1.73 m2 (1). The prevalence of NVAF increases with decreasing renal function (1–5), with rates of NVAF estimated from 3.5% to 27% among patients receiving dialysis for ESRD (2–9).
Thromboembolic stroke and systemic embolism (SSE) are frequent and dangerous complications of NVAF. Strokes caused by NVAF are more severe, more disabling, and more often fatal than strokes caused by other etiologies (10). In patients with NVAF, therefore, it is critical to determine the need for chronic anticoagulation by comparing the relative risks of SSE and bleeding. For >50 years in the United States, the vitamin K antagonist (VKA) warfarin was the only oral anticoagulant and the standard of care for prevention of SSE in NVAF. The limitations with warfarin are well documented. The introduction of novel oral anticoagulants (NOACs) ameliorated several of these challenges, especially by providing favorable dosing predictability. It is important to understand differences among NOACs, with particular regard to pharmacokinetics and renal excretion, in treating patients with severe CKD or ESRD.
The 2005 Kidney Disease Improving Global Outcomes/Kidney Disease Outcomes Quality Initiative guidelines do not recommend warfarin anticoagulation for primary prevention of stroke in patients with ESRD and NVAF receiving dialysis, although previous recommendations for secondary prevention and monitoring in patients on dialysis receiving warfarin anticoagulation remain (11). NOACs offer alternatives to VKA treatment. They are administered in fixed doses, do not require routine anticoagulation monitoring, and present fewer concerns regarding drug or food interactions.
Clinical Experience with Warfarin in ESRD
Warfarin has been shown to have a benefit in patients with renal compromise. A nationwide registry study that included 11,128 patients with nonend stage CKD and 1728 receiving RRT found a benefit in both groups. In patients with nonend stage CKD and a CHA2DS2-VASc score of two or more, warfarin was associated with a lower risk of a composite outcome of fatal stroke/fatal bleeding (hazard ratio [HR], 0.71; 95% confidence interval [95% CI], 0.57 to 0.88), a lower risk of cardiovascular death (HR, 0.80; 95% CI, 0.74 to 0.88), and a lower risk of all-cause death (HR, 0.64; 95% CI, 0.60 to 0.69), and in patients on RRT with a CHA2DS2-VASc score of two or more, warfarin was associated with an all–cause mortality benefit (HR, 0.85; 95% CI, 0.72 to 0.99) (12). If patients would have additionally been prospectively selected for lower risk of bleeding using the Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol Concomitantly (HAS-BLED) score, it is possible that the benefit-to-risk ratio could have been leveraged even more in favor of the use of warfarin (13). It is important to realize that, when these scores are applied in datasets post facto, risk scores seem to be useless, because the decision to use anticoagulation has been made; also, SSE will always track with higher CHA2DS2-VASc risk, and bleeding may not trend with the HAS-BLED score, because other clinical parameters, such as the use of aspirin, thienopyridine, or anticoagulation, in the dialysis circuit could have been altered (14).
Although studies do suggest benefit with warfarin, there is universal consensus that consistent positive outcomes are limited by its narrow therapeutic index and the requirement for routine monitoring of anticoagulant effect, labile anticoagulant effect, genetic variances, and drug/food interactions. Some of these limitations may be compounded with renal disease, potentially contributing to the reason for variable success with warfarin in this patient population historically. For example, it is documented that patients with disease states causing fluctuations in volume status are at risk for complications with warfarin management (15,16). The mechanism for variable warfarin responsiveness is triggered by hepatic congestion and redistribution of body fluids. This phenomenon is frequently observed in clinical practice with patients having ESRD requiring hemodialysis. Prescribers are aware of these anomalies; however, the unpredictability makes dosing complicated without the use of risk scores. These concerns may be addressed by having alternative agents, such as the NOACs.
Pharmacodynamics of Novel Anticoagulants in ESRD
Trials for dabigatran (17) and rivaroxaban (18) excluded subjects with creatinine clearance (CrCl) <30 ml/min; the trials comparing apixaban versus warfarin (19) and aspirin (20) excluded subjects with CrCl≤25 ml/min, and the pivotal edoxaban trial (21) excluded subjects with CrCl≤30 ml/min. The pivotal trials evaluated anticoagulant efficacy and safety in patients with renal impairment but excluded patients maintained on hemodialysis. In the absence of data, therefore, authors of guidelines are hesitant to make definitive recommendations and instead, advise the conventional choice of warfarin. Lacking evidence from controlled trials in this fragile population, clinicians must draw on clinical experience to weigh the likely benefits versus risks of warfarin. However, the expanding body of evidence from studies supporting clinical use of NOACs may tip the balance toward these agents.
An open label, parallel group, single–dose (5 mg apixaban) study evaluated the effects of ESRD and hemodialysis on apixaban pharmacokinetics in groups designated as healthy subjects (n=8) and subjects with ESRD (n=8) (22). Subjects with ESRD received two single doses separated by a 7-day washout: the first dose 2 hours before and the second immediately after hemodialysis. Consistent with observations in severe renal impairment, apixaban exposure was modestly higher in subjects with ESRD than in healthy subjects; apixaban area under the plasma concentration curve ([AUC][0–T]) was approximately 20% higher in subjects with ESRD who underwent hemodialysis after dosing (ratio of adjusted geometric means, 1.19; 90% CI, 0.91 to 1.55) and 40% higher in subjects with ESRD without hemodialysis after dosing (1.39; 90% CI, 1.10 to 1.76). Maximum plasma concentration values were approximately 21% (0.79; 90% CI, 0.62 to 1.01) and approximately 10% (0.90; 90% CI, 0.70 to 1.17) lower in subjects with ESRD with and without hemodialysis after dosing, respectively. Renal clearance was 0.02 ml/min in subjects with ESRD compared with 11.26 ml/min in healthy subjects, and dialysis clearance in subjects with ESRD was slightly higher (17.69 ml/min) than renal clearance in healthy subjects. There is an ongoing 9-day trial of apixaban at 2.5 mg orally twice a day in patients with ESRD to assess drug accumulation (23).
To characterize rivaroxaban pharmacokinetics/pharmacodynamics in patients on maintenance hemodialysis without residual renal function (n=18) (24), subjects initially received a single dose of rivaroxaban (10 mg) after each of three consecutive dialysis sessions; AUC and the effect on coagulation parameters were measured for 44 hours thereafter. Next, a single dose of rivaroxaban (10 mg) was given 6–8 hours before a dialysis session, and the effect of dialysis on rivaroxaban concentrations was evaluated. Finally, rivaroxaban (10 mg) was administered once daily, and AUC was measured over 24 hours on days 1 and 7. Mean AUC0–44 of rivaroxaban plasma concentrations after a single 10-mg dose was 2072 μg/L per hour, mean maximum concentration was 172.6 μg/L, and mean terminal elimination half-life was 8.6 hours. Mean trough concentration after multiple daily doses of 10 mg was 20.2 μg/L. Drug exposure was similar to that in published findings after a 20-mg dose in healthy volunteers. Rivaroxaban was not eliminated by dialysis, but there was no accumulation after multiple daily dosing.
An open label study of patients with ESRD on a stable regimen of dialysis three times weekly (n=10) compared the safety and pharmacokinetics of edoxaban (15 mg) dosed on two occasions: 2 hours before a 4-hour hemodialysis session (with hemodialysis) and on a nonhemodialysis day (without hemodialysis); there was a 7-day minimum washout between doses (25). Total clearance values were 24 and 23 L/h with and without hemodialysis, respectively, with AUC0–∞ values of 676±221 and 692±150 ng·h per 1 ml, respectively. Hemodialysis had minimal effects on edoxaban clearance as published and did not effectively remove edoxaban from the bloodstream. Unfortunately, there are no published studies of pharmacodynamics in patients undergoing peritoneal dialysis. Thus, this review of NOAC pharmacodynamics suggests that apixaban is the most likely drug to move forward in the clinical care of patients with ESRD and NVAF.
Results of Apixaban in CKD
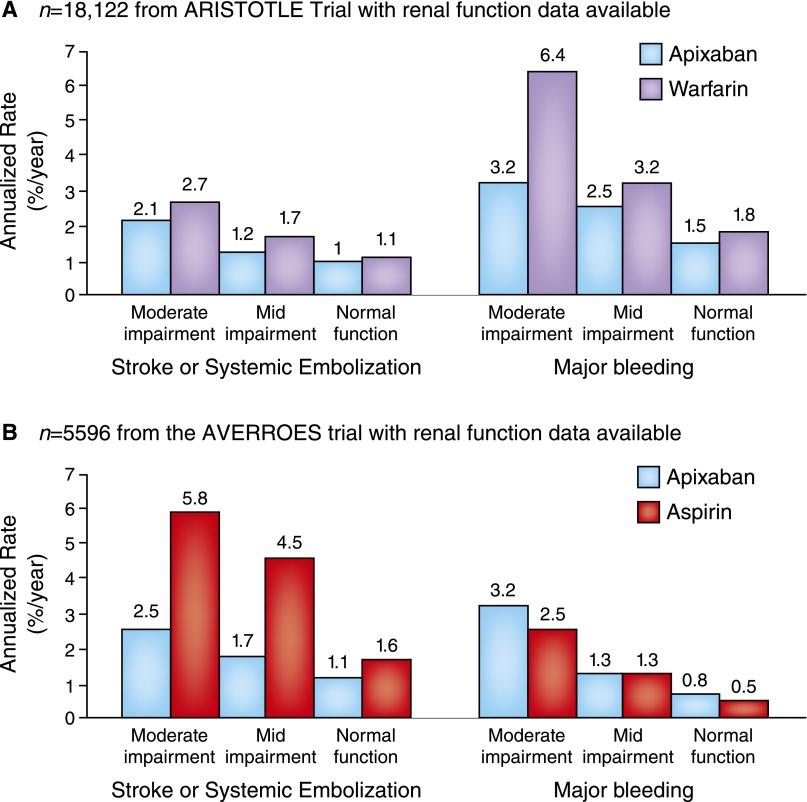
The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial included patients with CrCl<30 ml/min and excluded those with CrCl<25 ml/min (see below) (20,26,27). Percentages of patients experiencing SSE and major bleeding by renal function group and the principal efficacy and safety end points of the pivotal trials are shown for apixaban versus warfarin in Figure 1A (the ARISTOTLE Trial) and apixaban versus aspirin in Figure 1B (the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment [AVERROES] Trial). Data from the ARISTOTLE Trial showed that the rate of major bleeding was 2.13% per year with apixaban versus 3.09% per year with warfarin (HR, 0.69; 95% CI, 0.60 to 0.80; P<0.001) (28). Compared with warfarin, major extracranial hemorrhage associated with apixaban was less likely to result in hospitalization, medical or surgical intervention, transfusion, or change in antithrombotic therapy. Major hemorrhage followed by death within 30 days occurred one half as often in apixaban-treated patients as in warfarin-treated patients (HR, 0.50; 95% CI, 0.33 to 0.74; P<0.001). In a subanalysis of the ARISTOTLE Trial by renal function, apixaban was more effective than warfarin in preventing SSE and reducing mortality, regardless of the degree of renal impairment (29). Apixaban was associated with fewer major bleeding events across the range of renal function, and patients with renal impairment seemed to benefit most from apixaban therapy with regard to major bleeding.
Figure 1.
Bleeding and stroke/systemic embolism by renal function group in novel oral anticoagulant pivotal trials. (A and B) Bleeding and stroke/systemic embolism by renal function in novel oral anticoagulant pivotal trials. For the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial according to baseline Cockcroft–Gault calculated creatinine clearance, there were 7518 patients (42%) with an eGFR of >80 ml/min, 7587 patients (42%) with an eGFR between >50 and 80 ml/min, and 3017 patients (15%) with an eGFR of ≤50 ml/min. For the Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) Trial, among 5596 patients, there were 1628 patients (29%) with an eGFR of >80 ml/min, 3084 patients (55%) with an eGFR between >50 and 80 ml/min, and 884 patients (16%) with an eGFR of ≤50 ml/min.
The AVERROES Trial subgroup analyses showed that apixaban effects versus aspirin were consistent for SSE (P for interaction =0.13) and major bleeding (P for interaction =0.74) and consistent among subgroups of patients in whom warfarin therapy had previously failed as defined by reasons for warfarin unsuitability, including renal impairment (30). A subanalysis of the AVERROES Trial compared the efficacy and safety of apixaban (5 mg twice daily; 2.5 mg twice daily for selected patients) versus aspirin (81–324 mg daily) in patients with moderate CKD (n=1697). The primary outcome was stroke and noncentral nervous system emboli. The presence of moderate CKD independently predicted primary events and major bleeding. Apixaban reduced primary events by 68% (5.6% per year with aspirin versus 1.8% per year with apixaban; HR, 0.32; 95% CI, 0.18 to 0.55; P<0.001). Rates of major bleeding did not differ significantly between apixaban and aspirin with regard to major bleeding: 2.2% and 2.5% per year, respectively (HR, 1.2; 95% CI, 0.65 to 2.1) (24). Although severe CKD independently predicted stroke in patients with NVAF taking aspirin, among patients with severe CKD, apixaban significantly reduced stroke relative to aspirin without significantly increasing the risk of major hemorrhage (24) (Figure 2, Table 1).
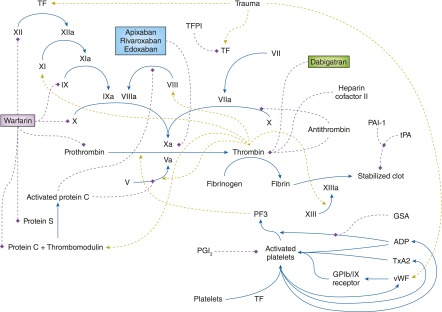
Figure 2.
Coagulation cascade influenced by renal dysfunction and mechanisms of action of oral anticoagulants. The dashed red lines show a negative effect, whereas the dashed green lines show a positive effect on the indicated reaction. The factors in red are decreased in CKD, whereas the factors in green are increased. The new oral anticoagulants and warfarin are boxed. GP1b/IX, glyoprotein 1b/factor IX; GSA, guanidinosuccinic acid; PAI-1, plasminogen activator inhibitor-1; PF3, platelet factor 3; PGI2, prostacyclin; TF, tissue factor; TFPI, tissue factor pathway inhibitor; tPA, tissue plasminogen activator; TxA2, thromboxane A2.
Table 1.
Clinical pharmacokinetics of novel oral anticoagulants and renal impairment
| Characteristic | Dabigatran | Apixaban | Rivaroxaban | Edoxaban |
| Bioavailability, % | 6 | 50 | 80 | Approximately 62 |
| Onset of effect, min | ≤30 | NA | ≤30 | 30 |
| Duration of effect, h | 24–36 | ≥24 | 24 | ≤24 |
| Tmax, h | 1.0–2.0 | 3.0 | 2.5–4.0 | 1.0–2.0 |
| Renal excretion, % of dose | 85 | 27 | 36a | 35 |
| Protein binding, % | 35 | 87 | 92–95 | 55 |
| Elimination half-life by renal impairment,b h | ||||
| Normal (CrCl>80 ml/min) | 13.8 | 7.6 | 8.3 | 10–14 |
| Mild (CrCl=50–79 ml/min) | 16.6 | 7.3 | 8.7 | 8.6 |
| Moderate (CrCl=30–49 ml/min) | 18.7 | 17.6 | 9.0 | 9.4 |
| Severe (CrCl<30 ml/min) | 27.5 | 17.3 | 9.5 | 16.9 |
| AUC0–∞ increase relative to renal impairment | ||||
| Mild | 1.5× | 1.16× | 1.5× | 1.32× |
| Moderate | 3.2× | 1.29× | 1.86× | 1.74× |
| Severe | 6.3× | 1.44× | 2.0× | 1.72× |
| ESRD | Estimated 6×c | 1.39× | 2×d | 1. 93×e |
Data are from ref. 37. NA, not available; Tmax, time to maximum concentration; CrCl, creatinine clearance; AUC0–∞, area under the concentration time curve 0–∞.
In total, 66% renal excretion: 36% unchanged and 30% inactivated metabolites.
For ESRD (CrCl≤15 ml/min), no data were available from pivotal trials of approved novel oral anticoagulants.
Exposure was doubled in subjects with ESRD when receiving one third of the normal dose (50 mg dabigatran).
Exposure in subjects with ESRD receiving 10 mg rivaroxaban was equivalent to that in healthy subjects receiving 20 mg rivaroxaban.
Patients undergoing peritoneal dialysis.
Meta-Analyses
A metaregression analysis to determine the effect of mild to moderate renal impairment on NOAC efficacy and safety versus warfarin included five studies comprising 72,845 patients with NVAF randomized to an NOAC or warfarin (31). Results showed a nonsignificant effect of renal impairment on bleeding and SSE (32). Apixaban was associated with less major bleeding than dabigatran and rivaroxaban but not edoxaban in patients with moderate renal impairment. Results of meta-analyses on the basis of real world clinical experience indicated that, compared with conventional anticoagulants (VKA/warfarin, low molecular weight heparin, and aspirin), NOACs at recommended doses seemed noninferior and safe to reduce the risk of SSE in renally impaired patients (31,33). However, indirect comparisons derived from different trials must be interpreted with caution, and patients with ESRD were excluded from these trials. Additionally, we recognize that the advent of antidotes for NOACs, including idarucizumab (humanized antibody fragment against dabigatran) and decoy proteins (andexanet alfa for apixaban and rivaroxaban), may dramatically change the risk-to-benefit ratio for these agents, particularly in patients with ESRD, although no data in this population are expected from the development programs (34,35).
Conclusions
Given the current equipoise on the risk-benefit profile of warfarin in patients with atrial fibrillation on dialysis, physicians must carefully analyze the emerging data on risk scores and the outcomes with NOAC medications to determine their role in ESRD. It is very likely that specific risk scores for patients with ESRD are needed for SSE and bleeding in ESRD. Early safety and pharmacodynamic data on NOACs are tipping the balance in favor of treatment. Because patients with NVAF and severe renal impairment or ESRD were excluded from the NOAC pivotal trials, clinical data for such patients are limited, and studies are in progress to investigate the NOACs in this population (36). For patients with NVAF and severe renal impairment, apixaban seems to be favored among the NOACs and offers a better risk-benefit profile compared with warfarin. Additional evidence from clinical trials will be required before definitive guidance can be given regarding use of the NOACs in patients with ESRD. In particular, a three-arm trial in patients with NVAF and ESRD would be ideal: aspirin (325 mg po every day) versus warfarin adjusted to an international normalized ratio of 2–3 versus apixaban (2.5 mg orally twice a day every day with doses given after hemodialysis on dialysis days). Only when such a trial is performed will we have guidance on the optimal approach in patients with ESRD.
Disclosures
None.
Acknowledgments
This paper was supported, in part, by the Baylor Healthcare System Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Centers for Disease Control and Prevention NCfHS: National Chronic Kidney Disease Fact Sheet, 2014. Available at: http://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. Accessed May 6, 2015
- 2.Ananthapanyasut W, Napan S, Rudolph EH, Harindhanavudhi T, Ayash H, Guglielmi KE, Lerma EV: Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 173–181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P: Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 4: 26–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS: Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 127: 569–574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102–1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 7.Das M, Aronow WS, McClung JA, Belkin RN: Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiol Rev 14: 14–17, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S: The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 22: 349–357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahal K, Kunwar S, Rijal J, Schulman P, Lee J: Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: A meta-analysis of observational studies. Chest 149: 951–959, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D’Agostino RB: Stroke severity in atrial fibrillation. The Framingham Study. Stroke 27: 1760–1764, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Bonde AN, Lip GY, Kamper AL, Hansen PR, Lamberts M, Hommel K, Hansen ML, Gislason GH, Torp-Pedersen C, Olesen JB: Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: A nationwide observational cohort study. J Am Coll Cardiol 64: 2471–2482, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Lip GY: Implications of the CHA(2)DS(2)-VASc and HAS-BLED scores for thromboprophylaxis in atrial fibrillation. Am J Med 124: 111–114, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Wang TK, Sathananthan J, Marshall M, Kerr A, Hood C: Relationships between anticoagulation, risk scores and adverse outcomes in dialysis patients with atrial fibrillation. Heart Lung Circ 25: 243–249, 2016 [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly RA, Aggeler PM: Determinants of the response to oral anticoagulant drugs in man. Pharmacol Rev 22: 35–96, 1970 [PubMed] [Google Scholar]
- 16.Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119[1 Suppl]: 8S–21S, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators: Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators: Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators: Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981–992, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators: Apixaban in patients with atrial fibrillation. N Engl J Med 364: 806–817, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators: Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369: 2093–2104, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, Song Y, Pursley J, Boyd RA, Frost C: Apixaban pharmacokinetics in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol 56: 628–636, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Apixaban in Hemodialysis, 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02672709?term=NCT02672709&rank=1. Accessed May 26, 2016
- 24.De Vriese AS, Caluwé R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, Vandecasteele SJ, Emmerechts J: Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 66: 91–98, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Parasrampuria DA, Marbury T, Matsushima N, Chen S, Wickremasingha PK, He L, Dishy V, Brown KS: Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb Haemost 113: 719–727, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Connolly SJ, Gao P, Paolasso E, De Caterina R, Husted S, O’Donnell M, Yusuf S, Hart RG: Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis 21: 429–435, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, Paolini JF, Hankey GJ, Mahaffey KW, Patel MR, Singer DE, Califf RM: Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 32: 2387–2394, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, Huber K, Jansky P, Steg PG, Hanna M, Thomas L, Wallentin L, Granger CB: Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, characteristics, and clinical outcomes. J Am Coll Cardiol 63: 2141–2147, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L: Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Eur Heart J 33: 2821–2830, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Coppens M, Synhorst D, Eikelboom JW, Yusuf S, Shestakovska O, Connolly SJ: Efficacy and safety of apixaban compared with aspirin in patients who previously tried but failed treatment with vitamin K antagonists: Results from the AVERROES trial. Eur Heart J 35: 1856–1863, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Pathak R, Pandit A, Karmacharya P, Aryal MR, Ghimire S, Poudel DR, Shamoun FE: Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol 115: 323–327, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Nielsen PB, Lane DA, Rasmussen LH, Lip GY, Larsen TB: Renal function and non-vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: A systemic review and meta-regression analysis. Clin Res Cardiol 104: 418–429, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Sardar P, Chatterjee S, Herzog E, Nairooz R, Mukherjee D, Halperin JL: Novel oral anticoagulants in patients with renal insufficiency: A meta-analysis of randomized trials. Can J Cardiol 30: 888–897, 2014 [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration: Idarucizumab. Available at: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm467396.htm. Accessed November 30, 2015
- 35.Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, Mathur VS, Castillo J, Bronson MD, Leeds JM, Mar FA, Gold A, Crowther MA: Andexanet alfa for the reversal of Factor Xa inhibitor activity. N Engl J Med 373: 2413–2424, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Fukamizu S, Oyama JI, Mizukami A, Matsumura A, Hashimoto Y, Node K: Rationale and design of the efficacy of rivaroxaban on renal function in patients with non-valvular atrial fibrillation and chronic kidney disease: The X-NOAC study. Int J Cardiol 188: 52–53, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Kaatz S, Mahan CE: Stroke prevention in patients with atrial fibrillation and renal dysfunction. Stroke 45: 2497–2505, 2014 [DOI] [PubMed] [Google Scholar]