Abstract
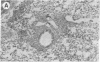
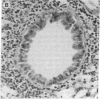
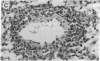
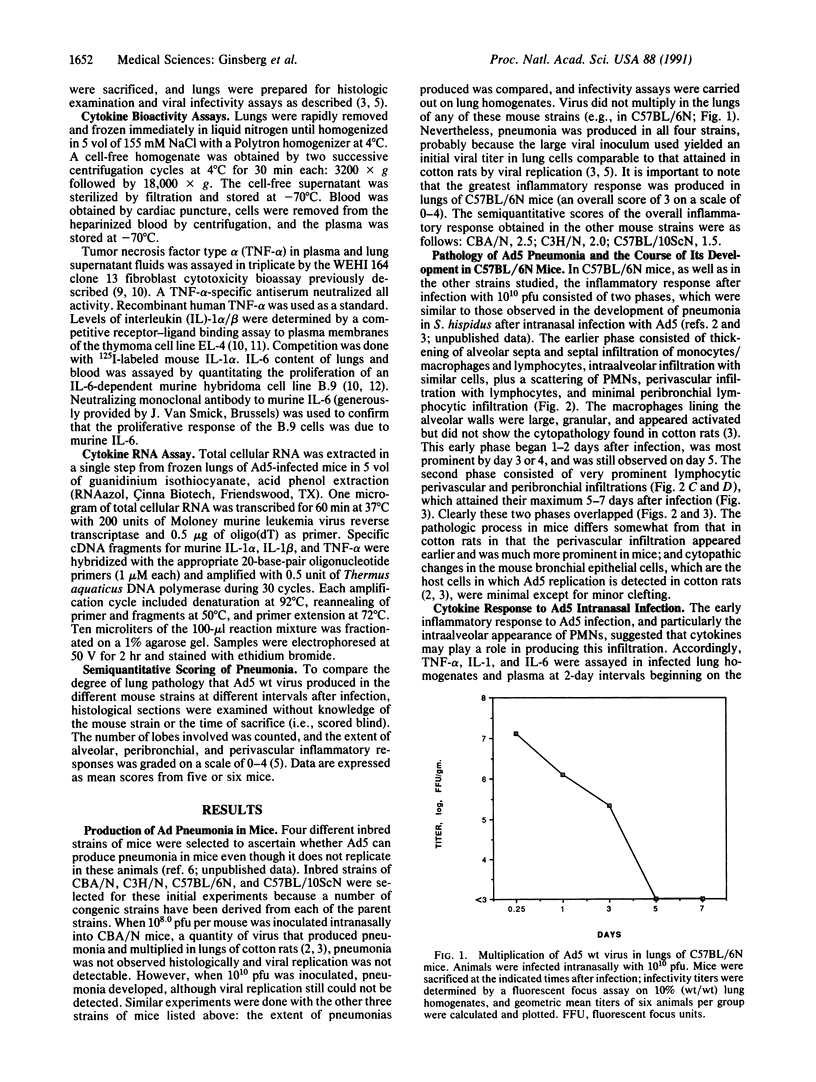
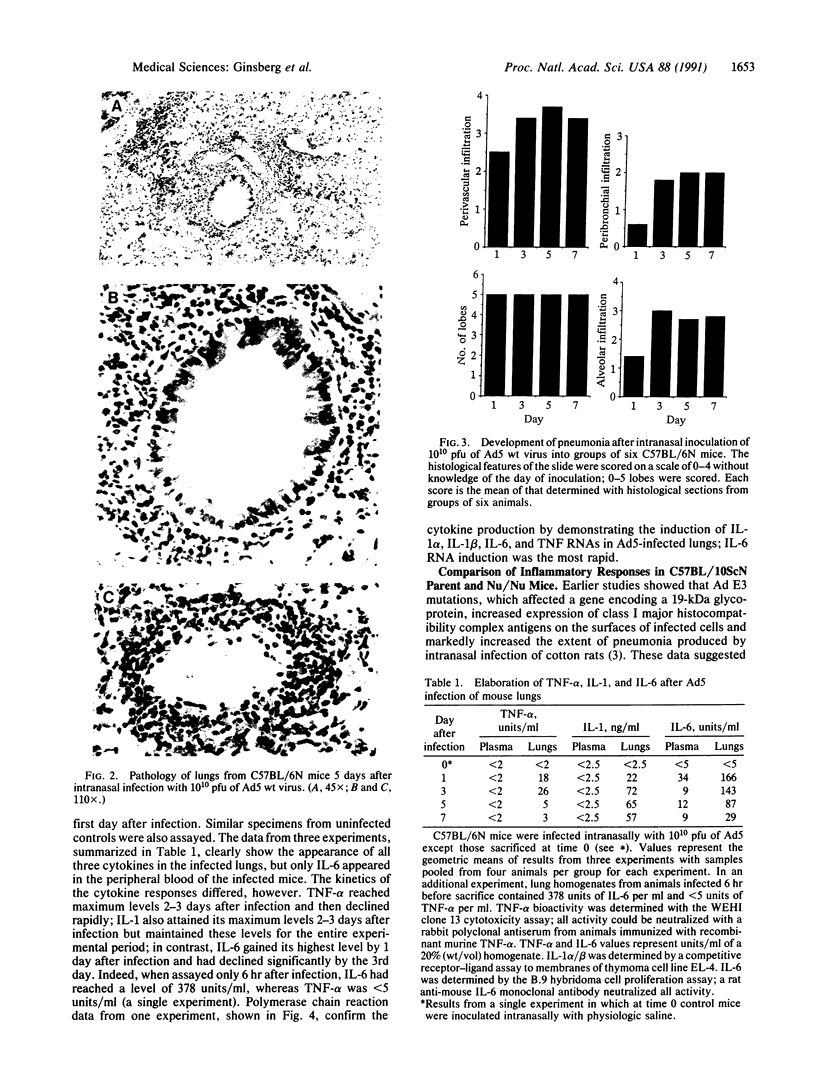
Intranasal inoculation of type 5 adenovirus (Ad5) produced pneumonia in mice even though the virus did not replicate. To induce the pneumonia, however, a large viral infectious dose was required--i.e., 10(10) plaque-forming units. Four strains of inbred mouse were studied (C57BL/6N, C57BL/10ScN, CBA/N, and C3H/N): all showed similar inflammatory responses, although the greatest infiltration occurred in the C57BL/6N mice. The pathological response to Ad5 infection resembled that previously described in cotton rats: it consisted of overlapping early and late phases, and the infiltration contained primarily lymphocytes and monocytes/macrophages with a scattering of polymorphonuclear leukocytes. The prominent early phase and the presence of polymorphonuclear leukocytes suggested that induction of cytokines may play an important role in the pathogenesis of this pneumonia. Assays showed the appearance of tumor necrosis factor alpha (TNF-alpha), interleukin 1 (IL-1), and IL-6 in the infected mouse lungs concomitant with the developing early-phase infiltration. Only IL-6 was found in the peripheral blood. IL-6 reached maximum titers 6-24 hr after infection, whereas maximum levels of TNF-alpha and IL-1 were attained 2-3 days after infection. Specific RNAs for each of these cytokines were demonstrated in the infected lungs. To test the hypothesis that a cytotoxic T-cell response was responsible for the second phase, which primarily consisted of a perivascular and peribronchial infiltration of lymphocytes, Ad5 was used to infect C57BL/10ScN Nu/Nu and parent mice. The nude mice showed a normal early-phase response, but essentially no peribronchial and only minimal perivascular infiltrations occurred.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Berman J. S., Beer D. J., Theodore A. C., Kornfeld H., Bernardo J., Center D. M. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990 Jul;142(1):238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- Brakenhoff J. P., de Groot E. R., Evers R. F., Pannekoek H., Aarden L. A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987 Dec 15;139(12):4116–4121. [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Eggerding F. A., Pierce W. C. Molecular biology of adenovirus type 2 semipermissive infections. I. Viral growth and expression of viral replicative functions during restricted adenovirus infection. Virology. 1986 Jan 15;148(1):97–113. doi: 10.1016/0042-6822(86)90406-x. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Frei K., Malipiero U. V., Leist T. P., Zinkernagel R. M., Schwab M. E., Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989 Apr;19(4):689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Gershenwald J. E., Fong Y. M., Fahey T. J., 3rd, Calvano S. E., Chizzonite R., Kilian P. L., Lowry S. F., Moldawer L. L. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4966–4970. doi: 10.1073/pnas.87.13.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Horswood R. L., Chanock R. M., Prince G. A. Role of early genes in pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6191–6195. doi: 10.1073/pnas.87.16.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. P., Pitha P. M., Shin H. S., Shin M. L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nain M., Hinder F., Gong J. H., Schmidt A., Bender A., Sprenger H., Gemsa D. Tumor necrosis factor-alpha production of influenza A virus-infected macrophages and potentiating effect of lipopolysaccharides. J Immunol. 1990 Sep 15;145(6):1921–1928. [PubMed] [Google Scholar]
- Pacini D. L., Dubovi E. J., Clyde W. A., Jr A new animal model for human respiratory tract disease due to adenovirus. J Infect Dis. 1984 Jul;150(1):92–97. doi: 10.1093/infdis/150.1.92. [DOI] [PubMed] [Google Scholar]
- Paganelli K. A., Stern A. S., Kilian P. L. Detergent solubilization of the interleukin 1 receptor. J Immunol. 1987 Apr 1;138(7):2249–2253. [PubMed] [Google Scholar]
- Ray A., Tatter S. B., May L. T., Sehgal P. B. Activation of the human "beta 2-interferon/hepatocyte-stimulating factor/interleukin 6" promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Helfgott D. C., Santhanam U., Tatter S. B., Clarick R. H., Ghrayeb J., May L. T. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988 Jun 1;167(6):1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsson L., Peterson P. A. Abrogation of cell surface expression of human class I transplantation antigens by an adenovirus protein in Xenopus laevis oocytes. J Cell Biol. 1985 Aug;101(2):540–547. doi: 10.1083/jcb.101.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]