Abstract
Every four years, the Olympic Games plays host to competitors who have built on their natural talent by training for many years to become the best in their chosen discipline. Similar spirit and endeavour can be found throughout the microbial world, in which every day is a competition to survive and thrive. Microorganisms are trained through evolution to become the fittest and the best adapted to a particular environmental niche or lifestyle, and to innovate when the ‘rules of the game’ are changed by alterations to their natural habitats. In this Essay, we honour the best competitors in the microbial world by inviting them to take part in the inaugural Microbial Olympics.
Welcome to the NRMicro stadium for the opening ceremony of the inaugural Microbial Olympics. The Microbial Olympic Torch (Vibrio fischeri)1 has been passed around the microbial community and will now shine out over the upcoming games. The parade of microbial competitors makes its way around the stadium, waving flagella, cilia and other appendages at the thronging crowd. Our expert panel has selected seven events and designed the rules for each competition in what should prove to be an exciting and entertaining inaugural games. Now, we go straight down to the track, where the first event is getting underway.
Sprint
Merry Youle and Forest Rohwer
In this event, the gold medal goes to the microorganism with the most progeny at the finish line. The eukaryotic entry is a yeast, groomed and selected by bakers and brewers for millennia. Exuberant beer-drinking, bagel-munching fans cheer in the stands of the NRMicro stadium as the first rotund cell rolls across the finish line, separating into mother and daughter in a respectable 90 minutes.
A mighty roar from the previously silent majority reverberates around the stadium as the bacterial contestant approaches the start line. The bacteria are sure of victory. Their candidate is the highly cultured lab rat, Escherichia coli, backed by a staff of nutritionists who have provisioned it with a rich medium. The day is sunny, the temperature in the upper 30s. It is hardly a contest. The bacterium has 90 minutes to show its stuff, but takes only 17 to divide. Victory seems assured. There is plenty of room on the field, and nutrients abound. Both progeny cells in turn promptly divide after another 17 minutes. Now there are four. The population is increasing exponentially with a base of two. Already the more mathematically inclined spectators have predicted that 32 flagellated rods will tumble across the 90 minute finish line — clearly the winners.
But wait, what is happening? A third contestant threatens to steal the medal. The stress of the race combined with the day's intense solar ultraviolet rays has induced the prophage on board one of the E. coli cells. At first, there are no signs that anything is wrong. Then, the cell shudders and loses momentum as the membrane implodes and the proton motive force is lost. Its cell wall is breached, spewing 25 progeny phages onto the track. A chant erupts from the eukaryotic crowd: “Kill the winner! Kill the winner!” (REF. 2.) Some of the newly emergent phages bump into the nearby E. coli cells and induce their prophages to go lytic and join the massacre. The phage population grows exponentially with a base of 25, and soon all the E. coli cells on the race track have succumbed. Their siblings clustered on the sidelines are the next to go as the phages diffuse outwards. The microbiologists in the stands move nervously towards the exits, their intestines churning as the bacterial residents within panic. A voice over the loudspeaker announces, “Gold to the phage.”
The take-home lesson? Yeast and bacteria divide. Phages multiply.
Boxing
Apollo Stacy and Marvin Whiteley
Things are just about to get underway over in the boxing arena with the semi-finals and final of the Microbial Olympics boxing event. We pass you over to your hosts for the day, Sam and Bill.
Bill
Welcome to bacterial boxing! I'm your host, Bacillus Bill.
Sam
And I'm your co-host, Salmonella Sam.
Bill
Today, four ferocious bacteria will go head to head (er, pole to pole?) to determine which is the toughest.
Sam
Entering the ring for our first semi-final are Deinococcus radiodurans and Pseudomonas aeruginosa.
Bill
Some may know Deinococcus for its ability to survive extreme radiation dosages3.
Sam
Deinococcus can take the heat ... radioactive heat, that is!
Bill
But it's not the most aggressive fighter, as it's not known to cause disease in humans.
Sam
It'll have to be careful around Pseudomonas, an opportunistic pathogen who will exploit Deinococcus's weaknesses.
Ding
Bill
The fight has started, but Deinococcus is immotile and can't leave its corner.
Sam
Pseudomonas also can't move across the ring's solid surface. But what's this? Its trainer just gave it a signal, a quorum- sensing autoinducer. Pseudomonas is greasing the ring with surfactant and rapidly swarming4 towards Deinococcus.
Bill
Deinococcus is literally in the ropes — the ropes of Pseudomonas's flagellum!
Sam
Uh-oh. At close range, Pseudomonas can deliver toxins via type VI secretion5.
Bill
Wow! The wind and everything else have been knocked out of Deinococcus. Pseudomonas wins the first semi-final!
Sam
For our next semi-final, ‘clap’ your hands for Neisseria gonorrhoeae and methicillin-resistant Staphylococcus aureus (MRSA).
Bill
I've heard MRSA can't be knocked out! Sam: And Neisseria will be hard to catch. Its antigenic variation means that it's highly evasive of the human immune system6.
Bill
Hmm, looks like MRSA is feeling ‘cocc-y’. It's already draping itself in gold.
Sam
Actually, that's MRSA's gold armour, the antioxidant staphyloxanthin7.
Ding
Bill
Neisseria throws a strong right (grappling) hook, a pilus8. But what's this? Neisseria hit MRSA ‘below the belt’ and is being disqualified!
Sam
The final will be between two arch-nemeses, Pseudomonas and Staphylococcus, who often encounter each other in chronic infections9.
Ding
Bill
Shocking! MRSA launches a super-antigen to recruit the immune system10 into the fight against Pseudomonas.
Sam
But Pseudomonas forms a biofilm and rolls with the punches.
Bill
Uh-oh. Looks like Pseudomonas is getting stronger the more it interacts with MRSA. It's polymicrobial synergy11!
Sam
Too bad for MRSA. It's taking a beating from the cocktail of antimicrobials12,13 that Pseudomonas is whipping up.
Bill
Although MRSA can rapidly acquire antibiotic resistances14, it's no match for this poison. Pseudomonas wins the gold!
Sam
Or does it? Pseudomonas's use of an autoinducer is being reviewed.
Bill
Owing to the ban against performance- enhancing small molecules, the Microbial Olympics Committee is revoking Pseudomonas's gold medal!
Sam
Meaning that MRSA will receive the gold and Deinococcus takes the silver.
Bill
That wraps up the bacterial boxing for these games. Congratulations to the winners!
100 μm freestyle swimming
Bradley C. Steel, Nicolas J. Delalez, Ashley L. Nord, Richard M. Berry and Judith P. Armitage
The 100 μm freestyle final takes place in the Oxford microscopic pool and should be an exciting race. Microorganisms range from less than 1 μm to more than 100 μm in length, so a sporting contest is ensured by limiting contestants to micrometre-sized bacteria that swim by rotating helical flagella. Pre-competition favourite, the tiny but speedy Bdellovibrio bacteriovorus, was disqualified in the heats for eating another competitor. Fans of Salmonella enterica and Helicobacter pylori were left disappointed after they failed their random pathogenicity tests.
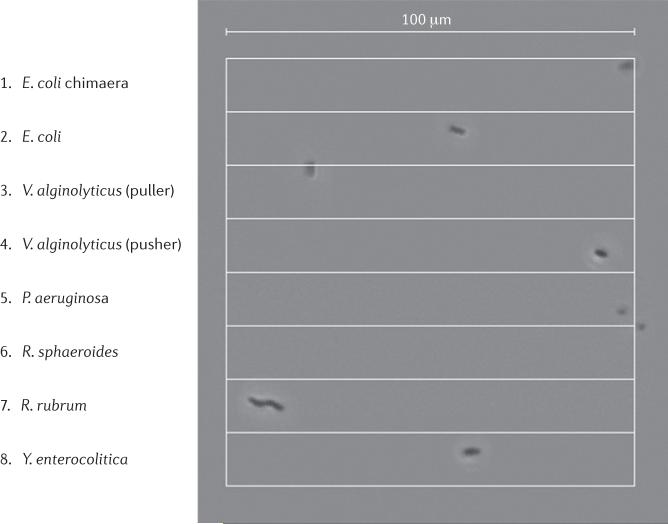
Bacteria swim to optimize their environment or to escape predators. They are typically too small to notice any variations across their length, so instead change swimming direction randomly, but do this less often when thing are getting better (for recent reviews of bacterial motors and chemo-taxis, see REFS 15,16). This is a handicap for a straight sprint, so half of the finalists are genetically modified to lack sensory responses. The singly flagellated contestants, Rhodobacter sphaeroides, P. aeruginosa (competing in its second event of the games) and Vibrio alginolyticus, start well (see Supplementary information S1 (movie)). The multiply flagellated, sodium-driven chimeric E. coli matches their pace, but its proton-driven relative falls behind. Ten micrometres in, the puller V. alginolyticus is attracted to the pool surface and swims in circles, failing to complete the race. In what turns out to be a photo finish (FIG. 1), R. sphaeroides wins by a body length, in 2.02 seconds, taking gold after surviving a post-race challenge for seemingly crossing its lane line. The chimeric E. coli survives a late slowdown to finish in 2.08 seconds, taking silver from P. aeruginosa at 2.12 seconds. The pusher V. alginolyticus comes in a close fourth, and 1 second later proton-driven E. coli edges out Yersinia enterocolitica for fifth place. Rhodospirillum rubrum was never in the contest, taking more than 15 seconds to finish.
Figure 1. The 100 μm freestyle swimming.
Contestants (and country of origin) by lane: (1) sodium-driven chimeric Escherichia coli, with multiple flagella (Japan); (2) proton-driven E. coli, with multiple flagella (USA); (3) sodium-driven Vibrio alginolyticus, with a single polar, clockwise locked ‘puller’ flagellum (that is, the flagellum pulls the cell body along behind it; Japan); (4) sodium-driven V. alginolyticus with a single polar, anticlockwise locked ‘pusher’ flagellum (that is, the flagellum pushes the cell body along in front of it; Japan); (5) proton-driven Pseudomonas aeruginosa, with a single polar flagellum (Australia); (6) proton-driven Rhodobacter sphaeroides, with a single subpolar flagellum (USA); (7) proton- driven Rhodospirillum rubrum, a spiral-shaped bacterium with multiple flagella at each pole to both push and pull (USA); and (8) proton-driven Yersinia enterocolitica, with multiple flagella (Belgium). See Supplementary information S1 (movie) for a video of the race.
As in the early human Olympic Games, contestants for the Microbial Olympics swimming were chosen on the basis of their ability to travel to the event; they were in Oxford freezers and easy to culture. With widening access and more rigorous selection, we expect a steady improvement in performance. B. bacteriovorus could break the 2 second barrier, and a large marine sulphur bacterium such as Thiovulum majus could swim the race within 0.2 seconds, if only it could be easily cultured in the lab and would fit into the lane. Given the vast range of uncharacterized species in the oceans and the promise of synthetic biology, who knows what the 2016 games will bring? In 2012, however, we salute the gold medallist, R. sphaeroides!
Javelin
Sophien Kamoun and Saskia Hogenhout
Back in the NRMicro stadium, the highly anticipated javelin competition is getting underway, with competitors drawn from a range of parasitic microbial backgrounds. Parasitic microorganisms secrete proteins and other molecules — collectively known as effectors — into different host cell compartments in order to modulate host responses and facilitate colonization and dispersal.
Effectors are the direct or indirect products of genes that reside in microbial genomes, but they have a long reach: they exert an effect on host cells that can be distantly located from the microbial cell. In the javelin competition, medals are awarded to the plant and animal pathogens that propel their effectors the furthest from their cells.
First up to throw is the rice blast fungus Magnaporthe oryzae, which causes the most destructive disease of the staple food crop rice. Following penetration of rice tissue, M. oryzae localizes inside a single cell in the rice epidermis during the first 30 hours. During this period, the fungus secretes effector proteins, some of which traffic to neigh-bouring plant cells away from the invading fungus17. This movement of the effectors throughout plant tissue occurs through the plasmodesmata, which are microscopic channels that link adjoining plant cells. M. oryzae throws a respectable distance, but it looks beatable and may not prove enough to win.
Second to throw is Haptoglossa mirabilis, an oomycete parasite. It's a big throw, and H. mirabilis puts itself in the mix for a medal. But wait, what's this? Haptoglossa has been disqualified for using a gun! H. mirabilis uses an incredibly intricate cell, the gun cell, to shoot infective spores inside a wandering animal host, such as a nematode18. Official result: disqualified, and H. mirabilis heads home empty handed.
Next up to throw is Clostridium botulinum. This Gram-positive bacterium secretes botulin toxin, a potent neurotoxin that, when ingested by humans, can cause a severe paralytic disease known as botulism19. Exposure to botulin toxin happens either after infection with the bacterium or by ingestion of contaminated food. The toxin then moves throughout the body, far away from the bacterium. Interestingly, botulin toxin reaches even further than this, as it is used in several cosmetic and medical procedures under the trade name Botox (Allergan). A big throw from C. botulinum, and it takes the lead from M. oryzae.
Last to step up to take a shot at the javelin title is the rust fungus Puccinia monoica. P. monoica is a spectacular plant pathogen that induces the production of flower-like structures in its Boechera hosts20. The ‘pseudo flowers’ attract insects that are essential for sexual outcrossing and completion of the fungal sexual reproduction cycle. P. monoica pseudoflowers release a unique scent composed of a mixture of volatile effectors that act as insect attractants21. These scent effectors reach far from the pseudoflower to alter the behaviour of the target insect. By far and away the longest distance of the competition, and a change of colour for the rust fungus as it picks up first place and a gold medal. C. botulinum ends up with a silver medal to take with it to its next host, and M. oryzae is awarded the bronze.
Pathogen relay
Stephen P. Diggle, James Gurney and Eric J. G. Pollitt
Staying in the NRMicro stadium, the next event is the pathogen relay. The ‘conventional wisdom’ about virulence is that parasites inevitably evolve towards being benign in their hosts. Indeed, in 1875 it was written that “the parasite makes a profession out of living at its neighbours expense and all its industry consists of exploiting it with economy, without putting its life in danger. It does not kill its chicken in order to have the eggs” (REF. 22). More recently, however, it was shown that if recovery and virulence are linked, then intermediate virulence is favoured23, and it has been suggested that the mechanism of transmission also influences virulence24. Thus, a biological tradeoff has been proposed; in this trade-off, virulence is an unavoidable consequence of transmission, and if a parasite evolves a high transmission rate, there must be a cost in terms of the length of the infection, which can mean either recovery or death of the host.
The pathogen relay differs from other events at the Microbial Olympics in that it is the human hosts that line up at the starting line. Each is infected with a different pathogen, and it is these pathogens, each with a different transmission rate and level of virulence, that are the real Olympians. At the starting line, the infected hosts move down the track to the next transmission point, where they encounter a new single host. Microbial athletes must then transmit to this new host before the newly infected host can move onto the next transmission point. The winner is the pathogen who successfully infects all their hosts at each point along the track.
In lane 1 is the plague (Yersinia pestis), historically a great competitor and still a firm favourite with hosts. For this race, she has chosen her pneumonic form. She is just great at transmitting to new hosts, and boy is she virulent! No wonder the Black Death is hotly tipped to win here. In lane 2, we have chlamydia (Chlamydia trachomatis). Increasingly popular with younger hosts, this microorganism doesn't blow its own trumpet but is looking to cause an upset. Lane 3 sees a potential rising star in bird flu (avian influenza virus H5N1). Training in birds has been going extremely well, but it remains to be seen whether she can carry this form into humans in this race. In lane 4, we have the common cold (rhinovirus). Often easily dismissed by hosts, she is a rank outsider for this event.
In a stunning upset, rhinovirus comes home to take the gold medal, leaving chlamydia to take silver and the plague, the bronze (FIG. 2). The virus's winning strategy is to combine a high transmission rate with low virulence, allowing for easy infection and low morbidity. This effective combination results in the wide dispersion of the hosts carrying our champion. Chlamydia takes the silver, with her low virulence being hampered by a low transmission rate, as it relies on sexual interactions between hosts, which are not always guaranteed! The chances of transmission are reduced when host density is low, as is the case in our race. For all her infecting power, pneumonic plague is too virulent for her own good. Her hosts are extremely sick and unable to rapidly disperse. Indeed, she is lucky to even finish the race, given the low host density, as many of her hosts die before the chance of transmission arises. Bird flu is hampered by a poor ability to transmit between human hosts coupled with a high virulence when she is successful. However, she will be training hard and hopes to improve her human transmission rate in time for the 2016 games.
Figure 2. The pathogen relay.
The trade-off between virulence and transmission, represented as a relay race.
This race was very simplistic, taking into account only the virulence of a particular disease (in terms of morbidity and mortality) and a hypothesized rate of transmission from one single host to another. In reality, the situation is much more complicated. Host density is an important factor, and if this had been high in the race, the overall outcome may have been different. Different modes of transmission (for example, vector-borne versus waterborne pathogens) are also important in determining the evolution of virulence. A good general read on this topic is Paul Ewald's Evolution of Infectious Disease25.
Diving
Antje Boetius
For the second event at the aquatic centre, we head over to the diving pool. Diving requires outstanding physiological excellence, supreme coordination skills and beauty in execution. The more difficult a dive, the higher the potential score if it is performed correctly — and if you are a tiny microorganism, of course there is no greater achievement than synchronous diving with your buddies or hosts. Dives are divided into three stages: the take-off, the dive itself and the impact on the aquatic world. Divers submit in advance the choreography of the dives they will perform. Each of the stages is judged against a strict set of criteria, and the winning divers perform difficult and ambitious dives that have to make a large splash. Competitive diving developed almost 4 billion years ago in a hot pond in Greenland, when the first prototype cells began to perform tumbling routines in water. Note that recently the jury of the diving competition convinced the Microbial Olympics Committee to include candidates that have not yet been given a name, in recognition of the unnamed majority of aquatic microorganisms.
The bacterium Photobacterium phosphoreum is an extreme diver, mastering a huge depth range down to the abyssal plains26. It fascinates the committee with the most glamorous synchronous diving performance. P. phosphoreum glows in the dark, having tamed deep-sea fish as dive partners. The fish make specific light organs for P. phosphoreum to shelter in, and the bacterium helps the ugly beasts to look flashy and attractive to their few potential partners roaming the dark empty deep-sea space. This achievement in synchronous diving has certainly needed endless training hours on evolutionary time scales, with the most beautiful result.
Our next contestant, the diatom Rhizosolenia, has the ability to synchronize thousands of fellow divers of its own cohorts and those of related species into one giant delicate, vertically migrating mat structure. Rhizosolenia is a rod-shaped, cylindrical cell, able to form dense blooms that contribute significantly to global silica production27. The special competence of this diver is synchronized diving by buoyancy regulation. All divers are organized in a mat floating atop in sunlight. Then, nutrients are depleted, and they decide in a rapid, democratic decision process to make themselves heavy in order to dive down and tap into subsurface nutrient pools. Their trick is to regulate carbohydrate production for buoyancy, so that the mat makes a beautifully coordinated dive of 3–4 days duration before resurfacing for sunlight. The committee is impressed.
The candidate Escherichia coli cameroni is next and has submitted an extremely interesting dive choreography in the synchronous diving category, stating that it can dive with its human host from the surface down to the deepest point on Earth, in water 11 km deep, and then back up to the surface, all in one day. This risky dive was carried out successfully in March 2012 and made a big splash28. However, the Microbial Olympics Committee now comes to the conclusion that, despite the significant media attention, this E. coli candidate is free-riding and cannot provide much evidence for synchronizing with its host. However, for mastering at least the metabolic challenge of fearlessness and hanging on in the host gut for 8 hours, the candidate is awarded the bronze medal. Rhizosolenia is deservedly rewarded with the silver medal, and P. phosphoreum takes gold.
Winter Games
S. Craig Cary
Microorganisms can be found in environments in which the temperature can vary widely, with extremes of hot and cold. In light of this, for our last event of the Microbial Olympics we move to the NRMicro-On-Ice facility for the Winter Games.
Earth's coldest environments, where temperatures permanently exist close to or below the freezing point of water, constitute over 75% of the global biosphere. The cryo-sphere collectively describes those cold areas where water exists primarily in solid form, an area encompassing all the glaciers, ice caps, ice sheets, sea and lake ice, and snow cover on the planet. These environments support a range of cold-adapted organisms that is clearly dominated, in terms of diversity and biomass, by bacteria. Although the conditions and evolutionary steps that led to the origin of cellular life remain unknown and are still an active area of scientific research, it is becoming increasingly clear that adaptations to the cold are a pervasive element in the cryosphere. What are the mechanisms that make a cell cold adapted? The race to sequence the genomes of psychro philic microorganisms has resulted in some true cold-adapted winners.
The bronze medal goes to a classic psychro phile that was described more than 30 years ago, Polaribacter irgensii 23-P, a gasvacuole-containing marine flavobacterium found in both Arctic and Antarctic sea ice29. This bacterium appears to move to the upper water column to dominate after the seasonal melt. P. irgensii 23-P is among the most psychro philic marine bacteria known, exhibit ing no growth at temperatures above 12 °C. Recent genome analysis has revealed that this species has a bacteriorhodopsin and an abundance of desaturases that contribute to its psychrophilic life strategy (A. Murray, personal communication).
In this fiercely contested competition, the silver medal is awarded to Psychrobacter arcticus str. 273-4, originally isolated from 20,000–30,000-year-old Kolyma Siberian permafrost, arguably making it one of the oldest extant life forms on the planet30. This organism resides in subnanolitre biofilms in ice veins of ancient permafrost and can grow at temperatures as low as −10 °C30. P. arcticus 273-4 has been shown to conduct selective protein and DNA synthesis at an astonishing −15 °C; this is believed to primarily target cell maintenance and the repair of DNA damage resulting from prolonged ionizing γ-radiation from the soil and entropic degradation over geological time31. Genome analysis has revealed identifiable cold adaptation in almost all gene categories, with over 50% of the proteome having psychrophilic structural modifications32.
Just pipping the rest of the competitors to the top spot, the gold medal is won by a psychrophilic gammaproteobacterium, Colwellia psychrerythraea str. 34H, isolated from Arctic marine sediments. This bacterium holds the low-temperature-growth record at −12 °C33, a record shared with Psychro monas ingrahamii. However, C. psych reryth raea str. 34H trumps P. ingrahamii by maintaining motility down to −10 °C34, and can also produce active enzymes at as low as −20 °C35. Analysis of this genome has revealed the cosmopolitan use of a full suite of adaptations that confer cryotolerance36, reinforcing the position of this organism as a model for the study of life in permanently cold environments.
As with most competitions, these medals are only won on the day. Studies of psychrophile biology and cold adaptation are thriving in laboratories worldwide, motivated by a need to enhance our basic knowledge of life in ultra-low temperatures here on Earth, and to apply this knowledge to the search for life in other icy worlds of our solar system and beyond. Ongoing cultivation-based and direct-sequencing approaches, such as single-cell genomics and metagenomic studies, are revealing the universal nature of the previously described and novel adaptations used by many residents of the cryosphere. It is clear that our current understanding of these seemingly quiescent communities is only the tip of the iceberg.
Closing ceremony
With the games over and the medals handed out, the winners and losers return to their natural habitats to continue training and improve their chances of competing at the next Microbial Olympics. The Vibrio fischeri torch is extinguished, the fireworks fade into memory, and the stadium empties to sit dormant for the next 4 years. However, as with the human games, the Microbial Olympics can have a real legacy over the coming months and years. In the sporting world, the Olympics inspires young people to try out a new sport or activity, and in doing so not only breeds the next generation of Olympians, but also improves the general health of the population. The organizers and panel of experts for the Microbial Olympics sincerely hope that the microbial games can have a similar effect, either by inspiring the next generation of microbiologists to enter the field or simply by spreading appreciation of the importance of microorganisms, in all shapes and forms, to every aspect of life on Earth. Just as schools all around the world have sports days, we encourage you to consider introducing a Microlympics day into your curriculum and to share the resulting events and medal awardees that you come up with. You never know, they just may be selected for the 2016 Microbial Olympics.
Acknowledgements
S. Kamoun and S. Hogenhout are supported by The Gatsby Charitable Foundation and the UK Biotechnology and Biological Sciences Research Council.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Marvin Whiteley's homepage: http://web.biosci.utexas.edu/whiteley_lab/pages/home.html
Sophien Kamoun's homepage: http://www.KamounLab.net Microbial Olympic Torch relay: http://storify.com/naturerevmicro/microbial-olympics-torch-relay Keep up on plant–microorganism interactions research: http://www.scoop.it/mpmi
SUPPLEMENTARY INFORMATION
See online article: S1 (movie)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Merry Youle, Rainbow Rock, Ocean View, Hawaii 96737, USA.
Forest Rohwer, Department of Biology, San Diego State University, San Diego, California 92182, USA.
Apollo Stacy, Section of Molecular Genetics and Microbiology, The University of Texas at Austin, 1 University Station, Austin, Texas 78712, USA.
Marvin Whiteley, Section of Molecular Genetics and Microbiology, The University of Texas at Austin, 1 University Station, Austin, Texas 78712, USA.
Bradley C. Steel, Clarendon Laboratory, Physics Department, University of Oxford, Parks Road, Oxford OX1 3PU, UK
Nicolas J. Delalez, Clarendon Laboratory, Physics Department, University of Oxford, Parks Road, Oxford OX1 3PU, UK
Ashley L. Nord, Clarendon Laboratory, Physics Department, University of Oxford, Parks Road, Oxford OX1 3PU, UK
Richard M. Berry, Clarendon Laboratory, Physics Department, University of Oxford, Parks Road, Oxford OX1 3PU, UK
Judith P. Armitage, Biochemistry Department, University of Oxford, South Parks Road, Oxford OX1 3QU, UK
Sophien Kamoun, The Sainsbury Laboratory, Norwich Research Park, Norwich NR4 7UH, UK.
Saskia Hogenhout, The John Innes Centre, Norwich Research Park, Norwich NR4 7UH, UK.
Stephen P. Diggle, School of Molecular Medical Sciences, Centre for Biomolecular Sciences, University Park, University of Nottingham, Nottingham NG7 2RD, UK
James Gurney, School of Molecular Medical Sciences, Centre for Biomolecular Sciences, University Park, University of Nottingham, Nottingham NG7 2RD, UK.
Eric J. G. Pollitt, School of Molecular Medical Sciences, Centre for Biomolecular Sciences, University Park, University of Nottingham, Nottingham NG7 2RD, UK
Antje Boetius, HGF–MPG Research Group on Deep Sea Ecology and Technology, Alfred Wegener Institute of Polar and Marine Research, D-27515 Bremerhaven, Germany.
S. Craig Cary, Department of Biological Sciences, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand.
References
- 1.Nyholm SV, McFall-Ngai M. The winnowing: establishing the squid–vibrio symbiosis. Nature Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 2.Thingstad TF, Lignell R. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat. Microb. Ecol. 1997;13:19–27. [Google Scholar]
- 3.Cox MM, Battista JR. Deinococcus radiodurans — the consummate survivor. Nature Rev. Microbiol. 2005;3:882–892. doi: 10.1038/nrmicro1264. [DOI] [PubMed] [Google Scholar]
- 4.Kearns DB. A field guide to bacterial swarming motility. Nature Rev. Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell AB, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nature Rev. Microbiol. 2009;7:274–286. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 7.Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nature Rev. Microbiol. 2008;6:466–476. doi: 10.1038/nrmicro1900. [DOI] [PubMed] [Google Scholar]
- 9.Mashburn LM, Jett AM, Akins DR, Whiteley M. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 2005;187:554–566. doi: 10.1128/JB.187.2.554-566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Bakaletz LO. Developing animal models for polymicrobial diseases. Nature Rev. Microbiol. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koley D, Ramsey MM, Bard AJ, Whiteley M. Discovery of a biofilm electrocline using real-time 3D metabolite analysis. Proc. Natl Acad. Sci. USA. 2011;108:19996–20001. doi: 10.1073/pnas.1117298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 14.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowa Y, Berry RM. Bacterial flagellar motor. Q. Rev. Biophys. 2008;41:103–132. doi: 10.1017/S0033583508004691. [DOI] [PubMed] [Google Scholar]
- 16.Porter SL, Wadhams GH, Armitage JP. Signal processing in complex chemotaxis pathways. Nature Rev. Microbiol. 2011;9:153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- 17.Valent B, Khang CH. Recent advances in rice blast effector research. Curr. Opin. Plant Biol. 2010;13:434–441. doi: 10.1016/j.pbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Robb E, Barron G. Nature's ballistic missile. Science. 1982;218:1221–1222. doi: 10.1126/science.218.4578.1221. [DOI] [PubMed] [Google Scholar]
- 19.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 20.Roy BA. Floral mimicry by a plant pathogen. Nature. 1993;362:56–58. [Google Scholar]
- 21.Raguso RA, Roy BA. ‘Floral’ scent production by Puccinia rust fungi that mimic flowers. Mol. Ecol. 1998;7:1127–1136. doi: 10.1046/j.1365-294x.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 22.van Beneden P. Les Commensaux et les Parasites dans le Regne Animal. G. Ballière; 1875. [Google Scholar]
- 23.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 24.Ewald P. Host-parasite relations, vectors, and the evolution of disease severity. Annu. Rev. Ecol. Syst. 1983;14:465–485. [Google Scholar]
- 25.Ewald P. Evolution of Infectious Disease. Oxford University Press; 1997. [Google Scholar]
- 26.Ruby E, Morin JG. Specificity of symbiosis between deep-sea fishes and psychrotrophic luminous bacteria. Deep Sea Res. 1978;25:161–167. [Google Scholar]
- 27.Villareal TA, Woods S, Moore JK, Culver-Rymsza K. Vertical migration of Rhizosoleniamats and their significance to NO3− fluxes in the central North Pacific gyre. J. Plankton Res. 1996;18:1103–1121. [Google Scholar]
- 28.Than K. James Cameron completes record-breaking Mariana Trench dive. National Geographic News; 2012. [online] http://news.nationalgeographic.com/news/2012/03/120325-james-cameron-mariana-trench-challenger-deepest-returns-science-sub/ [Google Scholar]
- 29.Irgens RL, Suzuki I, Staley JT. Gas vacuolate bacteria obtained from marine waters of Antarctica. Curr. Microbiol. 1989;18:261–265. [Google Scholar]
- 30.Bakermans CH, et al. Psychrobacter cryohalolentissp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol. 2006;56:1285–1291. doi: 10.1099/ijs.0.64043-0. [DOI] [PubMed] [Google Scholar]
- 31.Amato P, Doyle SM, Battista JR, Christner BC. Implications of subzero metabolic activity on long-term microbial survival in terrestrial and extraterrestrial permafrost. Astrobiology. 2010;10:789–798. doi: 10.1089/ast.2010.0477. [DOI] [PubMed] [Google Scholar]
- 32.Ayala-del-Río HL, et al. The genome sequence of Psychrobacter arcticus 273–4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl. Environ. Microbiol. 2010;76:2304–2312. doi: 10.1128/AEM.02101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells LE, Deming JW. Characterization of a cold-active bacteriophage on two psychrophilic marine hosts. Aquat. Microb. Ecol. 2006;45:15–29. [Google Scholar]
- 34.Junge K, Eicken H, Deming JW. Motility of Colwellia psychrerythraea strain 34H at subzero temperatures. Appl. Environ. Microbiol. 2003;69:4282–4284. doi: 10.1128/AEM.69.7.4282-4284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junge K, Eicken, Swanson BD, Deming JW. Bacterial incorporation of leucine into protein down to −20 °C with evidence for potential activity in subeutectic saline ice formations. Cryobiology. 2006;52:417–429. doi: 10.1016/j.cryobiol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Methe BA, et al. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl Acad. Sci. 2005;102:10913–10918. doi: 10.1073/pnas.0504766102. [DOI] [PMC free article] [PubMed] [Google Scholar]