Abstract
Background
Since 2006, the government of Kenya began decentralizing HIV care from secondary health facilities (SHF) to an expanded network, including primary health facilities (PHF). We evaluated the impact of this strategy on enrollment, care, and outcomes among adult patients in Central Province, Kenya, from 2006 to 2010.
Methods
We analyzed electronic patient-level data for 26,690 patients at 15 SHF and 22 PHF. Enrollment, patient, and facility characteristics and patterns in CD4+ testing, World Health Organization staging, and antiretroviral treatment (ART) initiation were compared between SHF and PHF. Survival analysis was used to estimate cumulative death and loss to follow-up (LTF) rates in PHF and SHF. Multivariate competing risks regression and Cox proportional hazards models were constructed to identify correlates of LTF and death.
Results
Enrollment in PHF increased mainly between 2007 and 2009, representing 5% and 25% of all new enrollments, respectively. CD4+ test provision and World Health Organization staging, time to ART initiation, and CD4+ count at ART initiation were for the most part similar between PHF and SHF. In multivariate analyses, pre-ART patients enrolled in PHF had a lower risk of LTF than those enrolled in SHF (SHR = 0.77, 95% confidence interval: 0.61 to 0.96). No differences in risk of death among pre-ART patients or in LTF or death among ART patients were observed.
Conclusions
Enrollment at PHF increased substantially during the period; death rates were comparable between PHF and SHF, whereas LTF among pre-ART patients was lower at PHF. This suggests that decentralization can be a successful strategy for expanding HIV care.
Keywords: Kenya, decentralization, antiretroviral therapy, pre-ART, retention, operations research
INTRODUCTION
Kenya is one of the countries in the world most severely affected by the HIV epidemic. With an estimated adult HIV prevalence of 5.6%, there are an estimated 1.2 million people living with HIV in Kenya.1 According to the 2012 Kenya AIDS Indicator Survey, fewer than half of all HIV-infected adults in Kenya know their HIV status and less than two-thirds of individuals eligible for therapy are on antiretroviral treatment (ART).1 More than 75% of Kenyans live in rural areas, and, in recent years, estimated HIV prevalence in rural areas has begun to reach levels estimated within urban settings—suggesting that the population in need of HIV care in Kenya is largely, and increasingly, rural.2
Initial efforts to provide HIV care and treatment throughout Kenya focused on large secondary health facilities (SHF), such as provincial and district hospitals often located in non-rural areas. To improve patient access to services and reduce staff burden in large facilities, HIV services have been dramatically expanded through a strategy of decentralization from 15 health facilities—mainly SHF—in 2003 to over 700 facilities by December 2008.3 An essential part of this process has been the establishment of HIV clinics at smaller primary health facilities (PHF), such as health centers and dispensaries. The aim of decentralization was to achieve universal HIV care and treatment by providing services close to those in need, in a sustainable fashion. It was also expected that decentralization would foster integration of HIV programs with other services provided at the health facilities and result in improved quality of services and patient care.3
A small body of research has generally found that decentralization of adult HIV care in sub-Saharan Africa has been successful in attracting patients to peripheral facilities, and retaining them in care at least, and to large secondary facilities.4–8 Although encouraging, these analyses have, however, largely relied on routine programmatic data among patients receiving ART and have often not examined key indicators of the quality of care provided, such as routine assessments of patients for ART eligibility and subsequent initiation of patients on ART once eligible; furthermore, the success of decentralizing HIV care in Kenya remains to be characterized. Therefore, more evidence is needed to understand the effectiveness of decentralization in expanding access to quality HIV services.
In this analysis, we compared key adult patient and facility characteristics, measures of the quality of routine care provided (eg, CD4+ testing, time to ART initiation), and patient death and loss to follow-up (LTF) among adults >15 years of age enrolled in HIV care in SHF and PHF in Central Province, Kenya, during the period 2006–2010, with patient follow-up time observed through 2011.
METHODS
Study Design and Study Population
This study was conducted through analyses of clinical program data collected routinely as a part of HIV care and treatment services in Kenya and of data on facility characteristics collected annually between 2006 and 2010. The facilities included in this analysis were supported by ICAP-Columbia University (ICAP) through funding from the President’s Emergency Plan for AIDS Relief.9 Clinics included in this analysis were the 37 Ministry of Health (MOH) facilities in Central Province that routinely entered patient-level HIV care data into an electronic patient-level database. In Central Province, the number of MOH facilities providing HIV care expanded from 14 in 2006 to 52 by the end of 2010; during this time, 42,863 persons were enrolled in HIV care.10 Across the 37 facilities included in this analysis, 26,690 persons enrolled in HIV care between 2006 and 2010, which constitutes 62% of all patients enrolled in care across all 52 MOH sites in Central Province during the period.
The source of the clinical patient data was the MOH Comprehensive Care Clinic Patient Card (MOH 257), which was completed by facility health workers at every patient visit. This included patient demographic and clinical data, laboratory test results, treatment including ART, and key mortality and morbidity outcomes. Updated contents of the patient card were entered on-site by data clerks into an electronic patient-level database on a routine basis.
Clinic and Program Characteristics
Information on characteristics of facilities and programs was collected annually, beginning in 2006, using a structured questionnaire for each HIV care facility. Questionnaires were completed by ICAP staff, based on observations and on interviews with facility staff. Information collected included the range of clinical and support services provided, HIV clinic staffing levels by cadre, location type, and facility type. Location types could include urban city (officially designated to be a city, with city administration and political bodies), semi-urban (big and small towns, peri-urban areas, growth points, mining communities), and rural (village, subsistence farming areas, large- and small-scale commercial farming areas) settings. Facility types were defined as public primary (health center clinic), public secondary (district/provincial hospital), public tertiary (teaching/national referral hospital), or private (any facility run by private, nongovernmental, or faith-based organization) facilities. In this analysis, public primary facilities were defined as PHF and public secondary facilities as SHF—no public tertiary or private facilities were supported by ICAP and collecting electronic patient-level data during the period examined.
Guidelines for HIV Care and ART
Kenya MOH national ART guidelines released in 2005 recommended initiation of ART for all adult patients evaluated as World Health Organization (WHO) stages 1 and 2 with a CD4+ cell count (CD4+) <200 cells per cubic millimeter; for patients with WHO stage 3 and CD4+ <350 cells per cubic millimeter; and for all patients determined to be WHO stage 4.11 Additionally, where CD4+ testing was not available, all patients assessed as WHO stage 3 or 4 were recommended to initiate ART. Updated ART guidelines were released by the Kenya MOH in November 2011 and therefore largely do not apply to the patient visits observed in our analysis.12
Statistical Methods
Patient- and facility-level characteristics at enrollment and at ART initiation were described and compared between PHF and SHF. Adult patients enrolled at 1 of the 37 facilities between 2006 and 2010 were included in this analysis, with follow-up data for these patients available through 2011.
Description of the Decentralization Process
Total number and percentage of active patients and new enrollments at PHF and SHF were calculated for each calendar year to show the secular trend of decentralization. The total number of the PHF and SHF that initiated HIV care services was also presented.
Patient- and facility-level characteristics at enrollment and at ART initiation were described and compared between PHF and SHF. Facility-level characteristics included calendar year when the facility initiated HIV services, facility location, whether the facility had a CD4 machine on-site and nurses initiating ART or prescribing ART, and the patient-to-provider ratio. Annual provider–patient ratio was calculated for each facility, each year, as the sum of the full-time–equivalent (FTE) number of physicians, nurses, and clinical officers divided by the number of patients who had at least one visit during the respective calendar year at the facility. (Only staff time dedicated to HIV care patients was included in this measure.) Patient-level characteristics included sex, age, point of entry to HIV care, CD4+, and WHO stage at enrollment and at ART initiation for ART patients. The frequencies of categorical variables were compared using χ2 tests, and the mean values of continuous variables were compared using Student t tests.
Quality of Care, Patient Outcomes, and the Impact of Decentralization
The proportion of patients with ART eligibility assessed at enrollment and at 3 and 6 months after enrollment were calculated and compared between patients in PHF and SHF. Among patients who were eligible for ART at enrollment, the proportion of patients who initiated ART and the median time to ART initiation were also compared between the 2 groups.
Lost to follow-up and death curves were estimated using survival analyses. Bivariate and multivariate models were fitted to identify whether decentralization from SHF to PHF had any impact on patient outcomes at both pre-ART and post-ART stages. The pre-ART stage was defined as the time from patient enrollment into care to the end of follow-up or the event, including death, lost to follow-up, transfer out, or ART initiation. The multivariate model adjusted for age, gender, marital status, CD4+ and WHO stage at enrollment or ART initiation, and calendar year of enrollment into HIV care or ART initiation. Kaplan–Meier and Cox regression models were used for patients on ART, and competing risks methods were used for the pre-ART analysis, treating ART initiation as the competing risk for death and LTF. Hazard ratios were calculated for the ART analyses, and subhazard ratios were calculated for the pre-ART analyses.13 A robust variance estimate was used to adjust for within-site correlations.14,15 All analyses were done using SAS 9.3 and Stata 12.1.
Ethical Approval
All data were de-identified before analysis, and the investigators had no access to identifiable patient information. Institutional review board approval was obtained from the Kenya Medical Research Institute; the study was designated nonhuman subject research by the Institutional Review Board of Columbia University; the Center for Global Health at the US Centers for Disease Control and Prevention determined the study to not involve engagement in human subject research.
RESULTS
Facility and Program Characteristics
Beginning in 2007, the number of SHF-enrolling patients remained constant at 15 facilities, whereas the number of PHF-enrolling patients increased from 1 before 2007, to 3 in 2007, 13 in 2008, 21 in 2009, and 22 in 2010 (Table 1). The annual number of patients enrolling in HIV care at SHF peaked in 2007 (5757) and declined each subsequent year. Annual enrollment at PHF plateaued in 2009 and 2010 (1262 and 1265). Over time, the proportion of all new HIV care patients who were enrolled in a PHF also increased, with the majority of increase occurring between 2007 and 2009. For the last year observed, 2010, 26% of new HIV care patients enrolled at a PHF, whereas the remaining 74% of patients enrolled at an SHF.
TABLE 1.
Facility Characteristics, by Facility Type
| PHF (n = 22 Facilities) | SHF (n = 15 Facilities) | |
|---|---|---|
| Number of facilities initiating HIV services, n (%) | ||
| ≤2006 | 1 (4.5) | 13 (86.7) |
| 2007 | 2 (9.0) | 2 (13.3) |
| 2008 | 10 (45.5) | 0 (0.0) |
| 2009 | 8 (36.4) | 0 (0.0) |
| 2010 | 1 (4.5) | 0 (0.0) |
| Number of patients enrolling in HIV care, n (%)* | ||
| 2006 | 148 (3.4) | 4203 (96.6) |
| 2007 | 389 (6.3) | 5757 (93.7) |
| 2008 | 817 (14.0) | 5016 (86.0) |
| 2009 | 1262 (23.0) | 4216 (77.0) |
| 2010 | 1265 (25.9) | 3617 (74.1) |
| Location of facility, n (%) | ||
| Semi-urban | 1 (4.5) | 7 (46.7) |
| Rural | 21 (95.5) | 8 (53.3) |
| Facility had a CD4 machine on-site, n (%) | ||
| 2006 | 1 (4.5) | 3 (20.0) |
| 2007 | 1 (4.5) | 6 (40.0) |
| 2008 | 1 (4.5) | 6 (40.0) |
| 2009 | 2 (9.0) | 6 (40.0) |
| 2010 | 2 (9.0) | 6 (40.0) |
| Nurse provision of ART at facility, n (%)† | ||
| ART eligibility assessed by nurses | ||
| 2009 | 21 (95.5) | 14 (93.3) |
| 2010 | 21 (95.5) | 14 (93.3) |
| ARV prescription by nurses at ART initiation | ||
| 2009 | 22 (100.0) | 14 (93.3) |
| 2010 | 22 (100.0) | 13 (86.7) |
| Provider–patient ratio (total clinical staff FTE/100 patients, mean (range)‡§ | ||
| 2006 | 8.6 (0–25.0) | 1.1 (0.2–6.1) |
| 2007 | 3.0 (0.7–8.3) | 1.3 (0.2–8.7) |
| 2008 | 1.8 (0.4–5.0) | 0.6 (0.1–2.0) |
| 2009 | 2.6 (0.5–6.7) | 0.5 (0.3–1.2) |
| 2010 | 2.6 (0.5–6.7) | 0.5 (0.1–1.3) |
| Provider–patient ratio (physician staff FTE/100 patients, mean (range) | ||
| 2006 | 1.4 (0–3.4) | 0.3 (0–3.0) |
| 2007 | 0 (0–0) | 0.1 (0–0.3) |
| 2008 | 0 (0–0) | 0.1 (0.1–0.2) |
| 2009 | 0 (0–0) | 0.004 (0–0.06) |
| 2010 | 0 (0–0) | 0.03 (0–0.2) |
Percentage shows percentage of total annual enrollment per site type (row percentage).
Nurse provision of ART was not assessed before 2009.
FTE staff = number of staff hours for nurses, physicians, and clinical officers/40 hours.
Differences in total clinical staff provider–patient ratio between PHF and SHF were significant (P < 0.001) for each year except 2007.
ARV, antiretroviral.
The SHF were nearly evenly divided between rural (53%) and semi-urban areas of Central Province (Table 1). The PHF were almost exclusively (95%) located in rural areas. Since 2007, 40% of SHF had a CD4 machine located on-site; during any given year, a maximum of only 2 (10%) PHF reported having a CD4+ machine on-site. Nearly all SHF (93%) and all PHF (100%) reported nurse-led ART eligibility assessment and prescription of ART at some point across the period observed (2009–2010). The mean annual provider-to-patient ratio for total clinical staff in SHF ranged from 0.5 to 1.3 FTE providers per 100 patients for all periods. The mean ratio for PHF each year except one was dramatically higher than SHF, ranging from 1.8 to 8.6 since 2008 (as compared with 0.5–0.6 for SHF) (P < 0.001 for each year except 2007). The mean annual provider-to-patient ratio limited to physicians ranged from 0.004 to 0.3 FTE providers per 100 patients in SHF. No physician staffing was reported for any PHF between 2007 and 2010.
Patient Characteristics, Quality of Care, and Outcomes
Across the period observed, a total of 26,690 patients enrolled in HIV care and 15,877 initiated ART in 1 of the 37 facilities. For both SHF and PHF, the populations enrolling in care were predominately female (69.3%); the age structure of the 2 types of facilities was also similar (Table 2). Patients of PHF were, however, more likely to have transferred into the facility than were patients of SHF (19.8% at PHF vs. 12.1% at SHF; P < 0.0001).
TABLE 2.
Patient Characteristics at Enrollment in HIV Care and at ART Initiation, by Facility Type
| PHF (n = 3881)
|
SHF (n = 22,809)
|
Total (n = 26,690)
|
||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | P | |
| Sex | ||||
| Female | 2807 (72.3) | 15,684 (68.8) | 18,491 (69.3) | <0.0001 |
| Male | 1074 (27.7) | 7121 (31.2) | 8195 (30.7) | |
| Age group | ||||
| 15–20 | 82 (2.1) | 353 (1.6) | 435 (1.6) | 0.0013 |
| 20–30 | 789 (20.3) | 5205 (22.8) | 5994 (22.5) | |
| 30–40 | 1647 (42.4) | 9491 (41.6) | 11,138 (41.7) | |
| 40–50 | 951 (24.5) | 5337 (23.4) | 6288 (23.6) | |
| 50–60 | 307 (7.9) | 1725 (7.6) | 2032 (7.6) | |
| 60+ | 105 (2.7) | 698 (3.1) | 803 (3.0) | |
| Point of entry to HIV care | ||||
| Transferred in | 769 (19.8) | 2749 (12.1) | 3518 (13.2) | <0.0001 |
| VCT | 731 (18.8) | 6527 (28.6) | 7258 (27.2) | |
| PMTCT | 489 (12.6) | 1971 (8.6) | 2460 (9.2) | |
| TB/HIV | 178 (4.6) | 1364 (5.8) | 1542 (5.8) | |
| PITC (in/outpatient) | 135 (3.5) | 2431 (6.0) | 2566 (9.6) | |
| Unknown/other | 1579 (40.7) | 7767 (34.1) | 9346 (35.0) | |
| CD4 cell count at enrollment | ||||
| CD4 cell count recorded | 2312 (59.6) | 13,377 (58.7) | 15,689 (58.8) | |
| <100 | 577 (25.0) | 4201 (31.4) | 4778 (30.5) | <0.0001 |
| 100–199 | 504 (21.8) | 2880 (21.5) | 3384 (21.6) | |
| 200–349 | 504 (21.8) | 2686 (20.1) | 3190 (20.3) | |
| 350+ | 727 (31.4) | 3610 (27.0) | 4337 (27.6) | |
| WHO stage at enrollment | ||||
| WHO stage recorded | 3450 (88.9) | 17,336 (76.0) | 20,786 (77.9) | |
| 1 | 1355 (39.3) | 4559 (26.3) | 5914 (28.5) | <0.0001 |
| 2 | 1018 (29.5) | 5865 (33.8) | 6883 (33.1) | |
| 3 | 958 (27.8) | 5935 (34.2) | 6893 (33.2) | |
| 4 | 119 (3.5) | 977 (5.6) | 1096 (5.3) | |
| Patients on ART | 2391 (61.6) | 13,486 (59.1) | 15,877 (59.5) | |
| CD4 cell count at ART initiation | ||||
| With CD4 | 1932 (80.8) | 11,063 (82.0) | 12,995 (81.9) | |
| <100 | 609 (31.5) | 4257 (38.5) | 4866 (37.5) | <0.0001 |
| 100–199 | 582 (30.1) | 3352 (30.3) | 3934 (30.3) | |
| 200–349 | 612 (31.7) | 2917 (26.4) | 3529 (27.2) | |
| 350+ | 129 (6.7) | 537 (4.9) | 666 (5.1) | |
| WHO stage at ART initiation | ||||
| With WHO stage | 1833 (76.7) | 9721 (72.1) | 11,554 (72.8) | |
| 1 | 349 (19.0) | 1565 (16.1) | 1914 (16.6) | 0.007 |
| 2 | 600 (32.7) | 3477 (35.8) | 4077 (35.3) | |
| 3 | 768 (41.9) | 4072 (41.9) | 4840 (41.9) | |
| 4 | 116 (6.3) | 607 (6.2) | 723 (6.3) | |
PMTCT, prevention of mother-to-child transmission; PITC, Provider-initiated testing and counseling; TB, tuberculosis; VCT, voluntary testing and counseling.
Overall, 41% of patients did not have any CD4+ test results at the time of enrollment in care recorded. The proportion of patients missing CD4+ test results was highest in the earlier years examined (including 73% of PHF patients and 67% of SHF patients missing CD4+ results in 2006) and declined each year to approximately 25% in 2011. Among patients with CD4+ test results at enrollment, a somewhat higher proportion of SHF patients had a CD4+ <100 cells per cubic millimeter (31% vs. 25%) (P < 0.001 comparing all categories). Patients who had transferred in from another facility were less likely than non–transfer-in patients to have an enrollment (post-transfer) CD4+ >350 cells per cubic millimeter (18% vs. 29%); this general pattern was consistent for patients transferring into PHF (18% vs. 34%) and SHF (18% vs. 28%). Among the 77% of patients with a WHO stage at enrollment, a substantially higher proportion of PHF enrollees were recorded as stage 1 (39% vs. 26%), whereas SHF had higher proportions recorded for each stage 2–4 (P < 0.0001). By 6 months after enrollment in care, a somewhat greater proportion of patients in PHF (71%) compared with SHF (65%) had adequate immunologic or clinical staging information to determine ART eligibility (P < 0.001) (Table 3). Among patients who were assessed for ART eligibility in the first 6 months of care, a lower proportion of PHF (55%) than SHF (65%) were found to be ART eligible (P < 0.001), and, among those patients, an equal proportion (37%) of PHF and SHF patients initiated ART within 30 days. By 6 months after ART eligibility was determined, 71% of these patients in PHF and 65% in SHF had started ART (P < 0.001).
TABLE 3.
ART Eligibility Determination and Timing of ART Initiation, by Facility Type
| PHF (n = 3881) | SHF (n = 22,809) | ||
|---|---|---|---|
| n (%) | n (%) | P | |
| Patients with ART eligibility ascertained: | |||
| At enrollment in HIV care* | 2246 (57.9) | 12,402 (54.4) | <0.001 |
| Within first 3 mo of HIV care | 2601 (67.0) | 14,162 (62.1) | <0.001 |
| Within first 6 mo of HIV care | 2768 (71.3) | 14,854 (65.1) | <0.001 |
| Patients determined to be ART eligible within first 6 mo of HIV care | 1521 (54.9†) | 9647 (64.9†) | 0.0003 |
| Among patients who were ART eligible at enrollment* | 1268 (56.5‡) | 8208 (66.2‡) | <0.0001 |
| Initiated ART within 30 days | 472 (37.2) | 3060 (37.3) | 0.97 |
| Initiated ART within 6 mo | 910 (71.8) | 5181 (63.1) | <0.0001 |
ART eligibility at enrollment was ascertained using CD4 and WHO staging information collected within 90 days before and 30 days after the enrollment date.
Percentage among patients with eligibility ascertained.
Percentage of patients screened for ART eligibility who were determined to be eligible at enrollment.
Among all patients who ultimately initiated ART, only a minority (18%) had missing CD4+ test results at the time of ART initiation (Table 2). Annual median CD4+ at ART initiation also increased across the years observed; however, these did not differ significantly between SHF and PHF, with the exception of 2009, when the median value for PHF was higher (P < 0.0001) (data not shown).
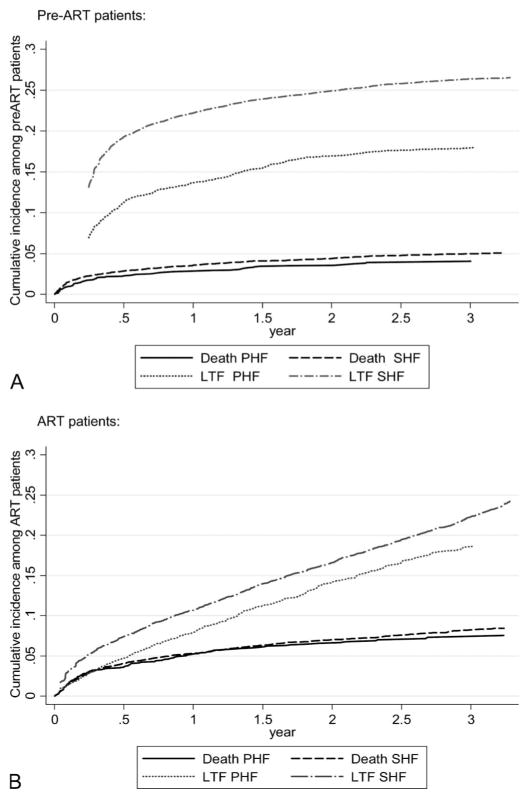
Across the period examined, cumulative mortality among patients in pre-ART did not exceed 8% and no significant difference was observed between SHF and PHF (Fig. 1A). Large differences were, however, recorded in the frequency of patients identified as LTF after enrollment in care—with cumulative LTF reaching 19.3% in SHF by 6 months and 11.3% at this same point in time among PHF patients. This absolute gap in cumulative LTF of about 10% between PHF and SHF established in the first 6–9 months after enrollment was observed to remain roughly constant across the remaining years observed.
FIGURE 1.
Kaplan–Meier graphs showing probability of death and LTF for 3 years after enrollment in HIV care among (A) pre-ART and (B) ART patients, by facility type, PHF, or SHF.
Among patients initiating ART, slightly higher cumulative mortality was observed (approximately 5% at 12 months) than among pre-ART patients, again with no difference in mortality between SHF and PHF (Fig. 1B). LTF after ART initiation, unlike the period after enrollment in care, did not occur mainly within the first 6 months. Rather, the rate of LTF seemed to be relatively constant. Six-month cumulative LTF after ART initiation was 7.4% for SHF patients and 4.7% for PHF patients; this small absolute gap remained roughly constant across the period observed.
Association of Site Type With LTF and Death
Results of multivariate regression models (Table 4) indicate that the facility type (PHF vs. SHF) was not substantially correlated with death among pre-ART patients. However, enrollment in a PHF was significantly associated with a lower risk of LTF among pre-ART patients [adjusted subhazard ratio (SHR) = 0.77, 95% confidence interval: 0.62 to 0.97]. Among ART patients, site type was not clearly associated with death or LTF.
TABLE 4.
Associations Between Attending a PHF and Death Among Pre-ART and ART Patients (N = 15,877)
| Crude SHR* | 95% CI | Adjusted SHR* | 95% CI | |
|---|---|---|---|---|
| Pre-ART | ||||
| Death | 0.82 | 0.59 to 1.13 | 1.28 | 0.90 to 1.82 |
| LTF | 0.62 | 0.42 to 0.93 | 0.77 | 0.62 to 0.97 |
| ART | ||||
| Death | 0.91 | 0.66 to 1.25 | 0.94 | 0.67 to 1.32 |
| LTF | 0.72 | 0.34 to 1.55 | 0.67 | 0.27 to 1.65 |
Reference category: SHF. Adjusted models control for WHO stage, CD4 count, age group, gender, year of patient enrollment in care, or ART initiation.
CI, confidence interval; SHR, subhazard ratio.
DISCUSSION
These findings provide the first broad assessment of decentralization process in Kenya. They also present a unique contribution to the literature on decentralization in that they evaluate patient enrollment and outcomes not only among patients on ART but also for patients during the pre-ART stage of care. The results at the same time provide support for some of the key themes in an emerging body of literature on decentralization of HIV care. Namely, we observed a substantial increase in patient enrollment at PHF and generally comparable or reduced rates of death and LTF at these facilities as compared with SHF during 2006–2011. Measures of the quality of care provided—including CD4+ test provision and WHO staging, time to ART initiation, and CD4+ count at ART initiation—were for the most part similar between PHF and SHF, though a higher proportion of eligible PHF patients initiated ART within 6 months. Differences may have been in part facilitated by the higher staffing levels (provider–patient ratios) observed at PHF. We also observed that patients enrolling in HIV care at PHF on average were less immunosuppressed than new patients at SHF, as measured by CD4+ cell count at care initiation, and a substantial proportion of new PHF patients were transfers-in from another HIV care facility.
Although our findings are in general consistent with the results showing comparable or superior retention in decentralized clinics, all previous published studies have shown reduced LTF and several showed lower death rates among ART patients,4–8 which our findings did not. In this case, we observed no difference in LTF or death rates among ART patients but rather lower LTF at PHF only among pre-ART patients (previous studies were limited to ART patients). One likely contributing factor to our study not finding lower LTF in PHF among ART patients is that, which 12-month LTF among PHF patients in our study was similar to that observed in several other studies, LTF among SHF patients was at least slightly lower (in some cases substantially lower) than that reported for other studies.4–8 This may in part have to do with programmatic differences—for example, Bedelu et al6 reported that the SHF included in their analysis did not at the time employ staff to follow-up with patients who defaulted. In addition, 12-month death rates among ART patients of SHF in our analysis seem to be in general substantially lower than the rates at comparable facilities reported in other studies.4–8
Our findings on staffing levels concur with Boyer et al,16 who also reported dramatically smaller patient loads for providers in decentralized clinics. The potential impact of the overall staffing-level disparities between PHF and SHF on quality of patient care and patient outcomes is unclear. The research by Boyer et al16 also found that patients at peripheral sites are more likely to encounter shorter wait times, which may in part be a function of higher provider-to-patient ratios. Numerous other studies have identified long clinic wait times as a key barrier to retention in HIV care.17 Other key factors promoting retention in PHF may be related to the presumably shorter distance between patient homes and facilities, including reduced travel time and transport costs, which have also been cited as main contributors toward LTF.17,18
The existing published research on HIV care decentralization does not present information on levels of immunosuppression at entry to HIV care, and only one published study compared CD4+ at ART initiation.7 The somewhat healthier profile of newly enrolling patients at PHF we observed is consistent with the premise that complicated advanced HIV cases are seen at larger better capacitated facilities.3,5 It is unclear to what extent the lower frequency of advanced HIV cases enrolling at PHF is a result of patient self-selection, with sicker patients choosing to attend SHF, and of PHF providing more accessible care (possibly because of location, size, staffing, and other factors) resulting in earlier HIV care initiation after diagnosis. Our observation that patients initiating ART at a PHF were less likely to have a CD4+ <100 cells per cubic millimeter may be a result of patients presenting for care at PHF somewhat healthier; more routine ascertainment of ART eligibility at PHF —with a slightly higher proportion of patients in PHF having their eligibility for ART determined within the first 6 months of care; and/or PHF patients having a greater likelihood of starting ART in the months after determination of eligibility, as we also observed. It does not, however, seem to be a result of healthier patients being transferred disproportionately to PHF.
Perhaps surprising is the lack of clear relationship between availability of a CD4+ machine on-site at SHF compared with PHF—SHF far more commonly had CD4+ machines—and the percentage of patients with CD4+ test results at enrollment and ART initiation, which did not differ by site type. This may reflect a level of success in establishing an effective laboratory network, as was called for in the Kenya decentralization guidelines,3 in Central Province. This success should, however, be noted within the context of a large proportion of CD4+ test results not available for new patients in SHF and PHF; it is possible that the burden of supporting CD4+ testing for patients enrolled elsewhere has impeded routine CD4+ testing at facilities with CD4+ machines, perhaps through issues such as rationing of equipment and staff time and stock-outs of supplies such as reagents.
The data included in this analysis were limited to 37 of the 52 MOH facilities in Central Province, and therefore, findings may not be generalizable to all MOH facilities in the province. This analysis relied on program data as opposed to rigorously collected research data, and although data quality assessments were conducted routinely to improve data completeness and accuracy, the data had a degree of incompleteness and inaccuracy. In particular, it is recognized that programmatic data may commonly overstate the degree of patient LTF and understate transfers-out and deaths.19 Furthermore, it is also possible that systems for collecting and recording information affecting measures of retention—including documentation of transfers-out of facilities and routine tracing of defaulters and updating of outcome status (eg, death)—are not of comparable quality across SHF and PHF, which would affect the validity of our findings. Lastly, some proportion of missing CD4+ results were likely a result of incompleteness in data entry rather than reflecting tests not done; whether this problem occurred with differing frequency in PHF and SHF is not known.
CONCLUSIONS
The first few years of decentralization of HIV care in Central Province have shown increased access to crucial HIV care and treatment services. In addition, the data presented here suggest that the quality of services at PHF is in the very least comparable to the quality of services provided at SHF. Further studies are needed to confirm these findings and assess the long-term effects of decentralization on provision of care, quality of services, and acceptance by persons living with HIV.
Acknowledgments
The authors gratefully acknowledge the health-care workers and program and strategic information teams from the Kenyan MOH and ICAP staff in New York and Kenya.
Supported by the President’s Emergency Plan for AIDS Relief through the US Centers for Disease Control and Prevention under the terms of Cooperative Agreement Number U62/CCU223540-07.
Footnotes
M.S., W.J.R., M.H., C.W., D.K., and E.J.A. contributed to the conception of the analysis idea; M.S., M.H., E.K., and W.J.R. contributed to data collection and cleaning; W.J.R. and B.E. advised on the analysis; C.W. conducted the analyses; W.J.R., M.S., and C.W. wrote the manuscript; M.H., E.K., B.E., D.K., and E.J.A. reviewed and contributed to the manuscript, E.J.A. gave overall technical oversight for the analytic process and manuscript writing. All authors read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.National AIDS and STI Control Programme, Ministry of Health, Kenya. Kenya AIDS Indicator Survey 2012: Preliminary Report. Nairobi, Kenya: 2013. [Accessed July 9, 2014]. Available at: http://www.nacc.or.ke/attachments/article/366/KAIS_Preliminary_Report_2012.pdf. [Google Scholar]
- 2.National AIDS Control Commission and National AIDS and STI Control Programme. Kenya AIDS Epidemic Update 2011. Nairobi, Kenya: 2012. [Accessed July 9, 2014]. Available at: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_KE_Narrative_Report.pdf. [Google Scholar]
- 3.National AIDS and STI control Programme, Ministry of Health. HIV/AIDS Decentralization Guidelines. Nairobi, Kenya: 2009. [Accessed July 9, 2014]. http://nascop.or.ke/library/ART%20guidelines/Decentralization%20Guidelines%20for%20HIV%20Care%20and%20Treatment.pdf. [Google Scholar]
- 4.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103:594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Chan AK, Mateyu G, Jahn A, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Trop Med Int Health. 2010;15(suppl 1):90–97. doi: 10.1111/j.1365-3156.2010.02503.x. [DOI] [PubMed] [Google Scholar]
- 6.Bedelu M, Ford N, Hilderbrand K, et al. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;196(suppl 3):S464–S468. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 7.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5:e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bemelmans M, van den Akker T, Ford N, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–1420. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 9.McNairy ML, Lamb MR, Carter RJ, et al. Identifying Optimal Models of HIV Care and Treatment in Sub-Saharan Africa Consortium. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr. 2013;62:e70–e81. doi: 10.1097/QAI.0b013e318278bcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ICAP Unified reporting system website. [Accessed October 1, 2013]; Available at: https://urs2.icap.columbia.edu/
- 11.Kenya Ministry of Health. Guidelines for Antiretroviral Therapy in Kenya. 3. Nairobi, Kenya: 2005. [Accessed July 9, 2014]. Available at: http://www.who.int/hiv/pub/guidelines/kenya_art.pdf. [Google Scholar]
- 12.Kenya Ministry of Medical Services. Guidelines for Antiretroviral Therapy in Kenya. 4. Nairobi, Kenya: 2011. [Accessed July 9, 2014]. Available at: http://www.faces-kenya.org/files/Kenya%20Treatment%20Guidelines%202011.pdf. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Rogers WH. Regression standard errors in clustered samples. Stata Tech Bull. 1993;13:19–23. [Google Scholar]
- 15.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyer S, Protopopescu C, Marcellin F, et al. EVAL Study Group. Performance of HIV care decentralization from the patient’s perspective: health-related quality of life and perceived quality of services in Cameroon. Health Policy Plan. 2012;27:301–315. doi: 10.1093/heapol/czr039. [DOI] [PubMed] [Google Scholar]
- 17.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 18.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in africa: estimation via a sampling-based approach. PLoS One. 2011;6:e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]