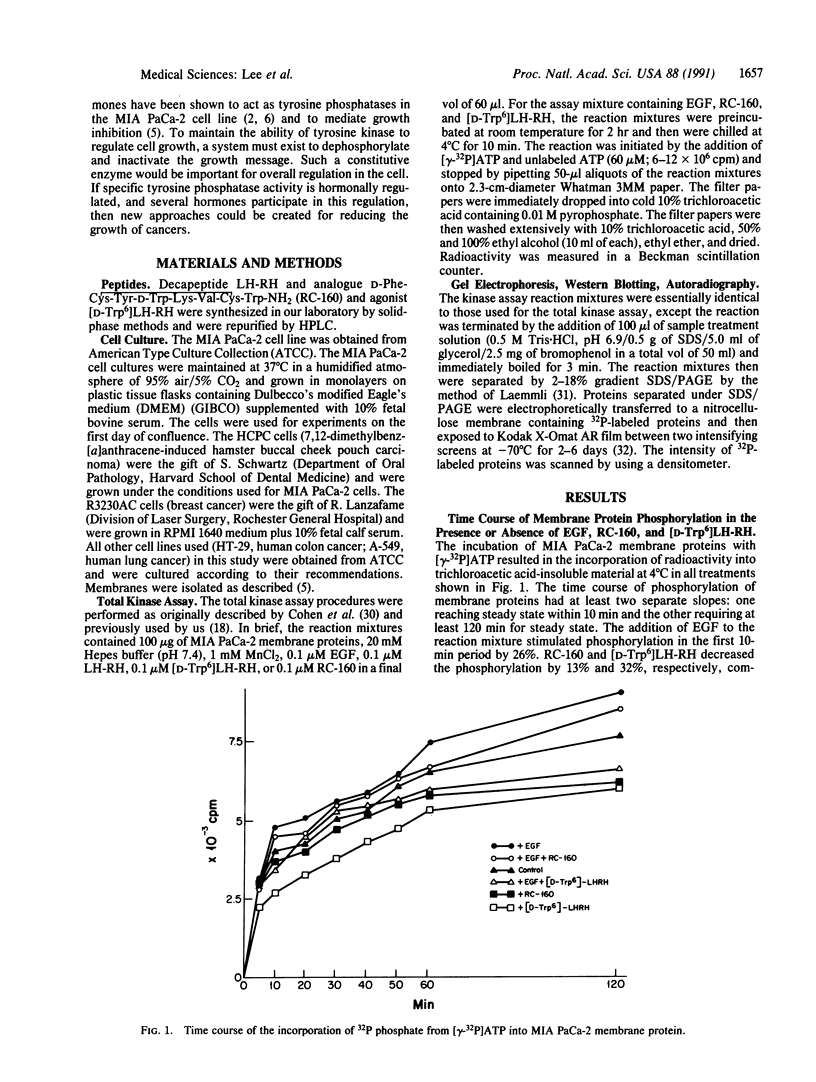
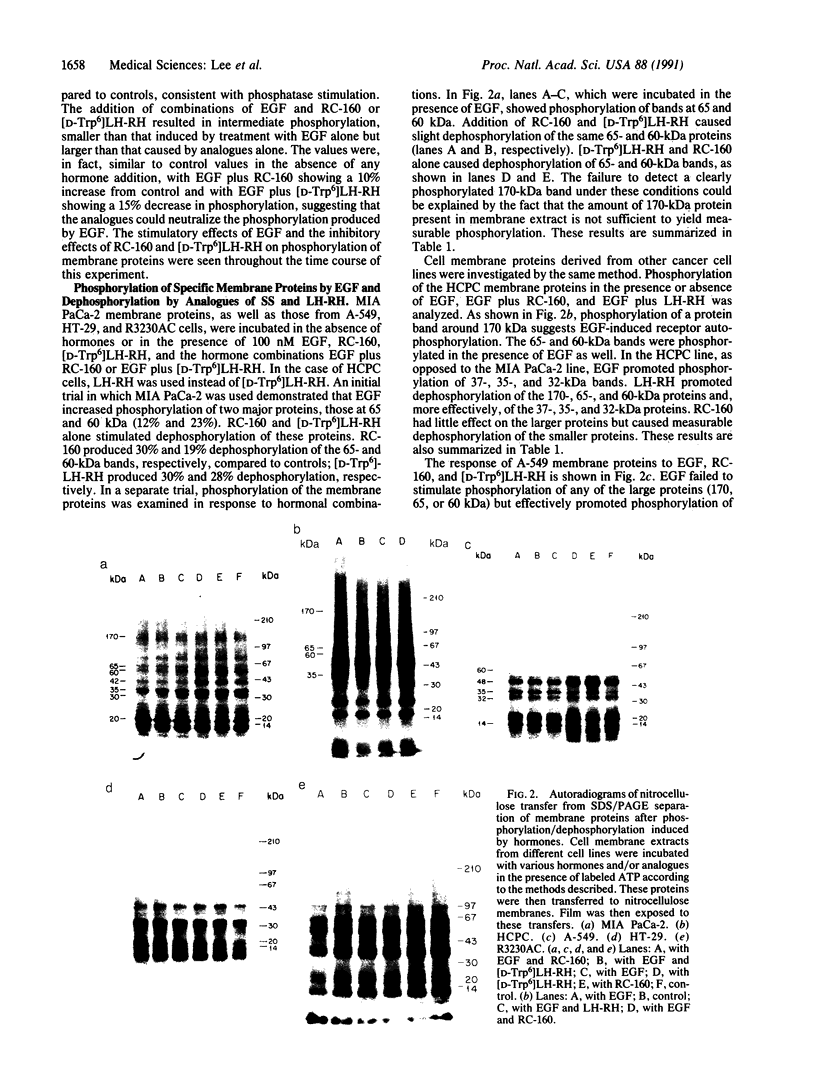
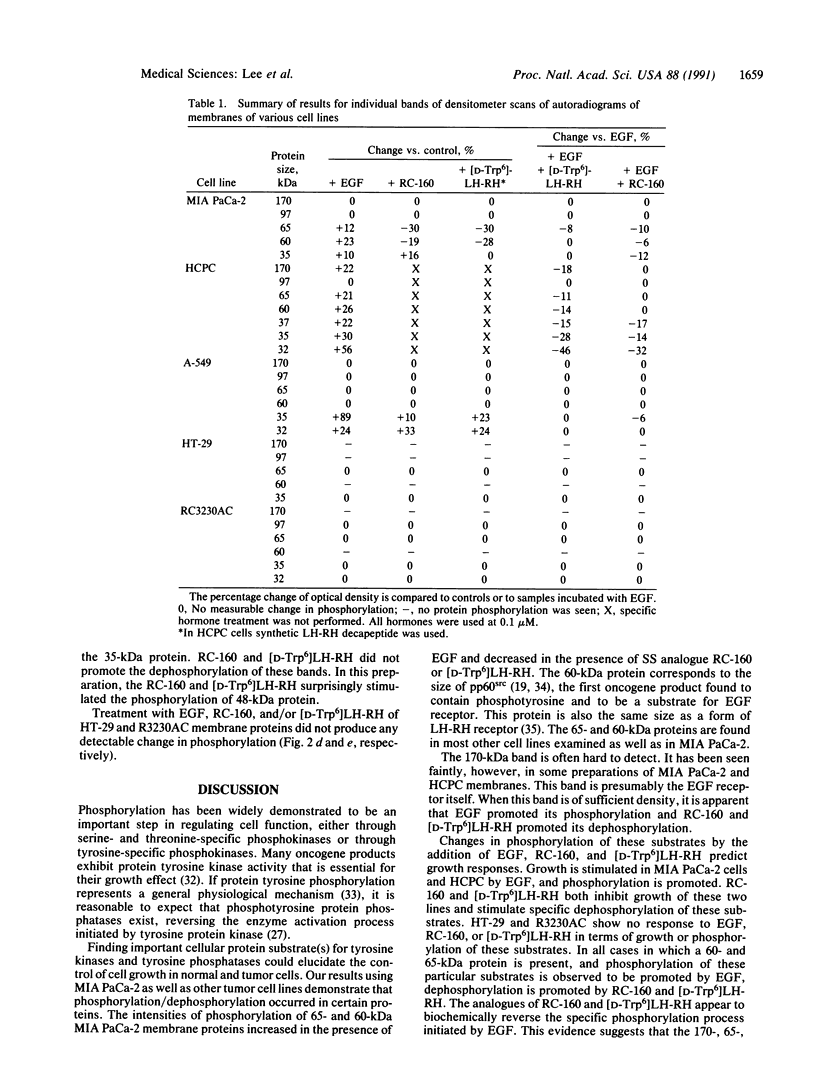
Abstract
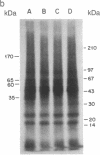
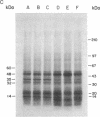
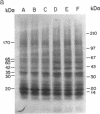
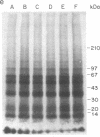
Analogues of somatostatin (SS) and luteinizing hormone-releasing hormone (LH-RH) activate tyrosine phosphatases in MIA PaCa-2 human pancreatic cancer cell line membranes and inhibit growth. We compared the substrates phosphorylated by epidermal growth factor (EGF) to those dephosphorylated by the SS analogue RC-160 (D-Phe-Cys-Tyr-D-Trp-Lys-Val-Cys-Trp-NH2) and [D-Trp6]LH-RH in cancer cell lines such as MIA PaCa-2 (human pancreatic cancer), HCPC (hamster cheek pouch carcinoma), A-549 (human lung cancer), HT-29 (human colon cancer), and R3230AC (breast cancer). EGF phosphorylated proteins of 170, 65, and 60 kDa and analogues of SS and LH-RH promoted the dephosphorylation of these proteins in MIA PaCa-2 and HCPC cell lines. The EGF receptor is 170 kDa. pp60src (60 kDa) is known to be a substrate for EGF receptor. The LH-RH receptor is also 60 kDa. The effects of RC-160 and [D-Trp6]LH-RH were quantitatively different. Examinations of HT-29, A-549, and R3230AC cancer cell lines revealed no phosphorylation by EGF or dephosphorylation by RC-160 and [D-Trp6]LH-RH. In addition to the 170-, 65-, and 60-kDa proteins, 35-kDa proteins were also phosphorylated in some cancer cell lines. This work demonstrates that analogues of SS and LH-RH can reverse the effects of EGF biochemically as well as functionally.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff J. R., Friedman P. N., Marshak D. R., Prives C., Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Shimer S., Johnson K. A., Lawrence J. B., Johnson C., Bruskin A., Green N. R., Hill D. E. Molecular cloning and chromosome mapping of the human gene encoding protein phosphotyrosyl phosphatase 1B. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5148–5152. doi: 10.1073/pnas.87.13.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Ferrar J. A., Cuthbert A. W., Cox H. M. The antisecretory effects of somatostatin and analogues in rat descending colon mucosa. Eur J Pharmacol. 1990 Aug 10;184(2-3):295–303. doi: 10.1016/0014-2999(90)90621-c. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Tonks N. K., Charbonneau H., Cicirelli M. F., Cool D. E., Diltz C. D., Krebs E. G., Walsh K. A. Protein tyrosine phosphatases: a novel family of enzymes involved in transmembrane signalling. Adv Second Messenger Phosphoprotein Res. 1990;24:273–279. [PubMed] [Google Scholar]
- Gill G. N. Regulation of EGF receptor expression and function. Mol Reprod Dev. 1990 Sep;27(1):46–53. doi: 10.1002/mrd.1080270110. [DOI] [PubMed] [Google Scholar]
- Hanks G. E. RE: The risk of distant metastases after transurethral resection of the prostate versus needle biopsy in patients with localized prostate cancer. J Urol. 1990 Sep;144(3):751–752. doi: 10.1016/s0022-5347(17)39583-6. [DOI] [PubMed] [Google Scholar]
- Hierowski M. T., Liebow C., du Sapin K., Schally A. V. Stimulation by somatostatin of dephosphorylation of membrane proteins in pancreatic cancer MIA PaCa-2 cell line. FEBS Lett. 1985 Jan 7;179(2):252–256. doi: 10.1016/0014-5793(85)80529-9. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Lindberg R. A., Middlemas D. S. Novel receptor protein-tyrosine kinases. Adv Second Messenger Phosphoprotein Res. 1990;24:260–265. [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Bilski J., Jaworek J., Tasler J., Schally A. V. Comparison of somatostatin and its highly potent hexa- and octapeptide analogs on exocrine and endocrine pancreatic secretion. Proc Soc Exp Biol Med. 1988 Feb;187(2):241–249. doi: 10.3181/00379727-187-42661. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liebow C., Hierowski M., duSapin K. Hormonal control of pancreatic cancer growth. Pancreas. 1986;1(1):44–48. doi: 10.1097/00006676-198601000-00009. [DOI] [PubMed] [Google Scholar]
- Liebow C., Lee M. T., Schally A. Antitumor effects of somatostatin mediated by the stimulation of tyrosine phosphatase. Metabolism. 1990 Sep;39(9 Suppl 2):163–166. doi: 10.1016/0026-0495(90)90237-7. [DOI] [PubMed] [Google Scholar]
- Liebow C., Reilly C., Serrano M., Schally A. V. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moattari A. R., Cho K., Vinik A. I. Somatostatin analogue in treatment of coexisting glucagonoma and pancreatic pseudocyst: dissociation of responses. Surgery. 1990 Sep;108(3):581–587. [PubMed] [Google Scholar]
- Mulchahey J. J., Neill J. D., Dion L. D., Bost K. L., Blalock J. E. Antibodies to the binding site of the receptor for luteinizing hormone-releasing hormone (LHRH): generation with a synthetic decapeptide encoded by an RNA complementary to LHRH mRNA. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9714–9718. doi: 10.1073/pnas.83.24.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier S. A., Mitchell R., Fink G. Solubilization of a large molecular weight form of the rat LHRH receptor. J Endocrinol. 1987 Oct;115(1):151–159. doi: 10.1677/joe.0.1150151. [DOI] [PubMed] [Google Scholar]
- Qayum A., Gullick W., Clayton R. C., Sikora K., Waxman J. The effects of gonadotrophin releasing hormone analogues in prostate cancer are mediated through specific tumour receptors. Br J Cancer. 1990 Jul;62(1):96–99. doi: 10.1038/bjc.1990.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Maurer R., von Werder K., Torhorst J., Klijn J. G., Lamberts S. W. Somatostatin receptors in human endocrine tumors. Cancer Res. 1987 Jan 15;47(2):551–558. [PubMed] [Google Scholar]
- Robinson I. C., Jeffery S., Clark R. G. Somatostatin and its physiological significance in regulating the episodic secretion of growth hormone in the rat. Acta Paediatr Scand Suppl. 1990;367:87–92. doi: 10.1111/j.1651-2227.1990.tb11640.x. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Sharoni Y., Bosin E., Miinster A., Levy J., Schally A. V. Inhibition of growth of human mammary tumor cells by potent antagonists of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1648–1651. doi: 10.1073/pnas.86.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srkalovic G., Cai R. Z., Schally A. V. Evaluation of receptors for somatostatin in various tumors using different analogs. J Clin Endocrinol Metab. 1990 Mar;70(3):661–669. doi: 10.1210/jcem-70-3-661. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Viguerie N., Tahiri-Jouti N., Ayral A. M., Cambillau C., Scemama J. L., Bastié M. J., Knuhtsen S., Estève J. P., Pradayrol L., Susini C. Direct inhibitory effects of a somatostatin analog, SMS 201-995, on AR4-2J cell proliferation via pertussis toxin-sensitive guanosine triphosphate-binding protein-independent mechanism. Endocrinology. 1989 Feb;124(2):1017–1025. doi: 10.1210/endo-124-2-1017. [DOI] [PubMed] [Google Scholar]
- Vécsei L., Widerlöv E., Alling C., Zsigó J., Pávó I., Penke B. Somatostatin28(15-28), but not somatostatin28(1-12), affects central monoaminergic neurotransmission in rats. Neuropeptides. 1990 Aug;16(4):181–186. doi: 10.1016/0143-4179(90)90060-c. [DOI] [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K., Russin D. J. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: sensitivity to asparaginase. Int J Cancer. 1977 Jan;19(1):128–135. doi: 10.1002/ijc.2910190118. [DOI] [PubMed] [Google Scholar]