Abstract
2-arachidonoylglycerol (2-AG) is the most abundant endogenous cannabinoid in the brain and an agonist at two cannabinoid receptors (CB1 and CB2). The synthesis, degradation and signaling of 2-AG have been investigated in detail but its relationship to other endogenous monoacylglycerols has not been fully explored. Three congeners that have been isolated from the CNS are 2-linoleoylglycerol (2-LG), 2-oleoylglycerol (2-OG), and 2-palmitoylglycerol (2-PG). These lipids do not orthosterically bind to cannabinoid receptors but are reported to potentiate the activity of 2-AG, possibly through inhibition of 2-AG degradation. This phenomenon has been dubbed the ‘entourage effect’ and has been proposed to regulate synaptic activity of 2-AG. To clarify the activity of these congeners of 2-AG we tested them in neuronal and cell-based signaling assays.
The signaling profile for these compounds is inconsistent with an entourage effect. None of the compounds inhibited neurotransmission via CB1 in autaptic neurons. Interestingly, each failed to potentiate 2-AG-mediated depolarization-induced suppression of excitation (DSE), behaving instead as antagonists. Examining other signaling pathways we found that 2-OG interferes with agonist-induced CB1 internalization while 2-PG modestly internalizes CB1 receptors. However in tests of pERK, cAMP and arrestin recruitment, none of the acylglycerols altered CB1 signaling.
Our results suggest 1) that these compounds do not serve as entourage compounds under the conditions examined, and 2) that they may instead serve as functional antagonists. Our results suggest that the relationship between 2-AG and its congeners is more nuanced than previously appreciated.
Keywords: entourage, cannabinoid, CB1, 2-arachidonoylglycerol, 2-oleoylglycerol, 2-linolenoylglycerol, and 2-palmitoyglycerol, 2-AG, 2-OG, 2-PG, 2-LG, acylglycerol, congener
Graphical abstract

INTRODUCTION
Though best known as the endogenous target for the psychoactive ingredient of marijuana and hashish [1], cannabinoid receptors are part of an important, widely distributed endogenous signaling system. This system consists of cannabinoid receptors (CB1, CB2, [2,3]) as well as lipid messengers and the enzymes that produce and metabolize them. The two chief candidate endogenous cannabinoids, or endocannabinoids, are 2-arachidonoyl glycerol (2-AG) [4] and arachidonoyl ethanolamide (anandamide, AEA, [5]), consisting of an arachidonic acid backbone and a head-group (glycerol and ethanolamine, respectively). Much is now known about the role and metabolism of 2-AG (reviewed in [6–8]) and to a lesser extent AEA, but it is sometimes overlooked that each eCB is a member of a larger lipid family. Closely related lipid species with fatty acids of different lengths and saturation are also present; in the case of 2-AG, 2-oleoylglycerol (2-OG), 2-linoleoylglycerol (2-LG) and 2-palmitoylglycerol (2-PG; Fig. 1). These lipids have been shown to also be present in brain homogenates [9,10] though at levels ~10x lower than those of 2-AG. But what is their role? If 2-AG and these congeners are metabolized by the same enzymes then the congeners may compete with 2-AG for breakdown; the net consequence being an enhancement of 2-AG levels and a prolongation of 2-AG signaling. Alternatively, they might act directly at the cannabinoid receptor, either competitively or cooperatively depending on their binding site and activation characteristics. Lastly, their presence may simply be incidental – they might be spatially segregated from eCBs and involved in processes unrelated to cannabinoid receptor signaling. For example, 2-OG and 2-PG may be endogenous ligands for GPR119 [11,12].
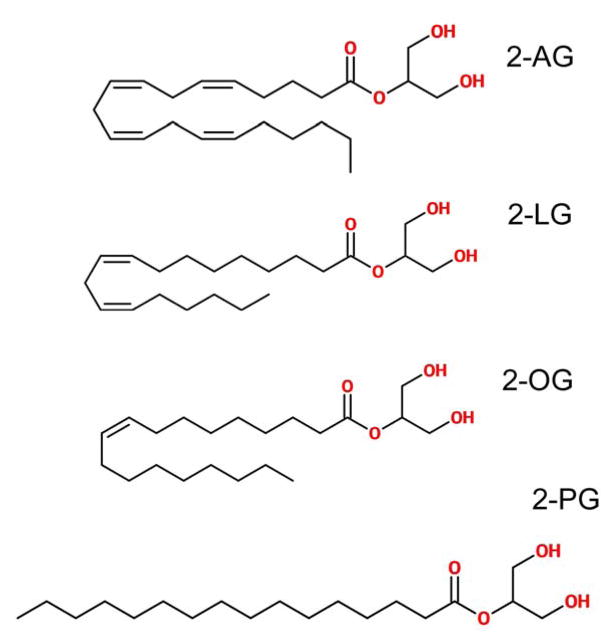
Figure 1.
Structures of 2-AG and its congeners 2-LG, 2-OG and 2-PG
Available evidence points to the first arrangement. A study of 2-LG and 2-PG reported that these compounds did not bind to the cannabinoid receptors or inhibit adenylyl cyclase activity via CB1 or CB2 [13]. But in combination, 2-LG and 2-PG potentiated 2-AG actions in several behavioral assays, and 2-LG inhibited 2-AG breakdown in a neuronal and hematopoietic cell lines [13]. In a separate study 2-PG and 2-LG enhanced a 2-AG inhibition of tumor necrosis factor alpha when applied with 2-AG [14].
The presumed potentiation of 2-AG activity by these related lipids has been dubbed the ‘entourage effect’ and is viewed as a potential means of regulating synaptic activity, and other 2-AG actions, particularly if they are co-synthesized with 2-AG. However this work was primarily done with immortalized cell lines that do not fully recapitulate endogenous cannabinoid signaling and would benefit from further analysis using a neuronal model, particularly one that endogenously produces 2-AG. We have made use of autaptic hippocampal neurons, a model system that possesses the necessary machinery for depolarization-induced suppression of excitation (DSE [15]), a form of 2-AG- and CB1-dependent retrograde signaling found in many CNS circuits (reviewed in Kano 2009). We have extensively characterized the underpinnings of endocannabinoid signaling in these neurons [16–18]. Autaptic neurons have the additional virtue of synapsing only onto themselves, thereby making for an architecturally simple model. In contrast to conventional neuronal cultures, which involve a complex network of interconnected neurons, autaptic neurons are free of secondary inputs from neighboring neurons. Importantly for our purposes we have shown that the recovery time of DSE is determined by the complement of enzymes available to break down 2-AG [19]. As a consequence, deletion, addition, or pharmacological blockade of enzymes involved in 2-AG metabolism alters the rapidity of 2-AG breakdown and the recovery time course of DSE. Entourage compounds, based on their presumed inhibition of 2-AG degradation, are predicted to elicit greater inhibition for a given depolarization, substantially slow DSE recovery, and generally enhance the net inhibition induced by DSE. In addition to our examination of 2-AG congeners in neurons, we have also explored the effects of these congeners on CB1 signaling via several additional pathways using cell lines expressing rodent CB1 receptors.
We report here our findings that 2-OG, 2-LG and 2-PG behave in a manner inconsistent with a role as entourage compounds in these diverse models of 2-AG signaling.
METHODS
Animals and cell culture
All animal care and experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. Mouse hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as previously described [20,21]. Briefly, neurons were obtained from animals (at postnatal day 0–2, killed via rapid decapitation without anesthesia) and plated onto a feeder layer of hippocampal astrocytes that had been laid down previously [22]. Cultures were grown in high-glucose (20 mM) minimum essential media containing 10% horse serum, without mitotic inhibitors and used for recordings after 8 days in culture and for no more than 3 h after removal from culture medium [15]. All electrophysiological experiments were performed exclusively on excitatory neurons. All tests were made on neurons from at least two different preparations.
Electrophysiology
When a single neuron is grown on a small island of permissive substrate, it forms synapses – or ‘autapses’ – onto itself. All experiments were performed on isolated autaptic neurons. Whole-cell, voltage-clamp recordings from autaptic neurons were carried out at room temperature using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA, USA). The extracellular solution contained (mM) NaCl 119, KCl 5, CaCl2 2, MgCl2 1, glucose 30 and HEPES 20. Continuous flow of solution through the bath chamber (2 mL·min-1) ensured rapid drug application and clearance. Drugs were typically prepared as a stock then diluted into extracellular solution at their final concentration and used on the same day. Recording pipettes of 1.8–3 MΩ were filled with solution containing (mM): potassium gluconate 121.5, KCl 17.5, NaCl 9, MgCl2 1, HEPES 10, EGTA 0.2, MgATP 2 and LiGTP 0.5. Access resistance was monitored and only cells with a stable access resistance were included for data analysis.
Materials
2-AG and 2-LG were obtained from Cayman Chemical (Ann Arbor, MI). 2-PG was obtained from Tocris Biosciences (Bristol, UK). 2-OG was obtained from Avanti Polar Lipids (Alabaster, AL, US). These drugs were stored at −80°C and diluted shortly before use.
Cell culture
Stable cell lines were generated as described previously (HEK-rCB1) [23]. The amino terminal HA (hemagglutinin) tag was introduced to facilitate receptor localization. Trafficking, adenylyl cyclase and pERK1/2 assays were performed with these cell lines. Cells were grown and maintained in Dulbecco’s modified Eagle media with 10% fetal bovine serum and penicillin/streptomycin (Invitrogen/GIBCO, Carlsbad, CA) at 37°C in 5% CO2. CHO cell lines used for arrestin assays are described below.
On-cell Western for receptor internalization
HA-CB1 human embryonic kindey (HEK) cells were grown to 95% confluence in DMEM + 10% FBS + 0.5% Pen/Strep. Cells were washed once with Hepes buffered saline (HBS)/bovine serum albumen (BSA) (BSA @ 0.08 mg/ml) 200μL per well. Drugs were applied at the indicated concentrations to cells, after which they were incubated for 1h at 37°C. Cells were then fixed with 4% PFA for 20 minutes and washed 4 times (200μl per well) with tris buffered saline (TBS). Blocking buffer (Odyssey Blocking buffer, LI-COR, Inc. Lincoln, NE) was applied at 100μl per well for 1h at room temperature. Anti-HA antibody, (monoclonal, 1:500, Covance, Princeton, NJ) diluted in 50:50 Odyssey Blocking Buffer and PBS, was then applied for one hour at room temperature. Following this, the plate was washed 4 times (200 μl per well) with TBS. Secondary antibody diluted (anti-mouse 680 antibody 1:800, LI-COR, Inc. Lincoln, NE) in 50:50 blocking buffer and PBS, was then applied for one hour at room temperature. Following this, the plate was washed 4 times (200μl per well) with TBS. The plate was imaged using an Odyssey scanner (700 channel, 5.0 intensity, LI-COR, Inc. Lincoln, NE).
pERK1/2 assays
Cells were seeded on poly-D-lysine coated 96-well plates (75,000 cells/well) and grown overnight at 37°C, 5% CO2, humidified air. The following day, media was replaced by HBS/BSA (0.2 mg/ml) and cells were challenged with drugs/compounds for the indicated time. After drug incubation, plates were emptied and quickly fixed with ice cold 4% PFA for 20 mins followed by ice-cold methanol with the plate maintained at −20°C for 15 min. Plates were then washed with TBS/0.1% Triton X-100 for 25 mins (5 × 5 min washes). The wash solution was then replaced by Odyssey blocking buffer and incubated further for 90 min with gentle shaking at room temperature. Blocking solution was then removed and replaced with blocking solution containing anti-phospho-ERK1/2 antibody (1:150)(Cell Signaling Technology®, Danvers, MA) and was shaken overnight at 4°C. The next day, plates were washed with TBS containing 0.05% Tween-20 for 25 min (5 × 5 min washes). Secondary antibody, donkey anti-rabbit conjugated with IR800 dye (Rockland, Limerick, PA), prepared in blocking solution, was added and gently shaken for 1 hour at room temperature. The plates were then again washed 5 times with TBS/0.05% Tween-20 solution. The plates were patted dry and scanned using LI-COR Odyssey scanner. Receptor internalization and pERK1/2 activation (expressed in %) were calculated by dividing average integrated intensities of the drug treated wells by average integrated intensities of vehicle-treated wells. All assays were performed in triplicate, unless otherwise mentioned.
Cyclase assays
Cyclase assays were optimized using Perkin Elmer’s LANCE® ultra cAMP kit (Catalog # TRF0262, Perkin Elmer, Boston, MA) as per the manufacturer’s instruction. All assays were performed at room temperature using 384-optiplates (Catalog# 6007299). Briefly, cells were resuspended in 1X stimulation buffer (1X Hank’s Balanced Salt Solution (HBSS), 5 mM HEPES, 0.5 mM IBMX, 0.1% BSA, pH 7.4, made fresh on the day of experiment). Cells were incubated for 1 hour at 37 °C, 5% CO2 and humidified air and then transferred to a 384-optiplate, followed by stimulation with drugs/compounds made in DMSO/ethanol as appropriate, for 5 mins. Cells were then lysed by addition of 10 μl Eu-cAMP tracer working solution (4X, made fresh in 1X lysis buffer supplied with the kit) and 10 μl Ulight™ anti-cAMP working solution (4X, made fresh in 1X lysis buffer) and further incubated for 1 hour at room temperature. Plates were then read with the TR FRET mode on an Enspire plate reader (Perkin Elmer, Boston, MA).
Arrestin recruitment assays
Arrestin recruitment assays were performed using the PathHunter® CHO-K1 CNR1 (CHO-mCB1) (catalog # 93-0959C2) (DiscoveRx, Fremont, CA). The assay principle is based on enzyme fragment complementation technology. In this engineered cell line, a deletion mutant of β galactosidase (β gal) (EA, or enzyme acceptor fragment) is fused with arrestin and a smaller fragment of the enzyme (ProLink™) is fused to the C terminal domain of the cannabinoid receptor. The activation of the cannabinoid receptor leads to arrestin recruitment and formation of an active β gal enzyme, which then acts on substrate to emit light that can be measured on a luminescence plate reader. Cells were thawed, grown and maintained in PathHunter AssayComplete™ (catalog # 92-0018GF2) media.
All assays were performed in poly-D-lysine coated 96 well plates (Corning Costar® 3596). About 20000 cells/well were plated and grown overnight at 37 °C, 5% CO2 in humidified air. The next day, media was replaced with 90 μl of HBS/BSA (BSA, 0.2 mg/ml) and cells were challenged with 10 μl of drugs/compounds (10X concentration) in HBS/BSA and incubated for 90 mins at 37 °C, 5% CO2, humidified air. Reactions were terminated by addition of Pathhunter™ detection reagent (DiscoveRx) and the plate was further incubated for 60 mins at room temperature. Complementation reactions were monitored for chemiluminiscence using an Enspire multiplate reader.
Lipid Extraction and HPLC/MS/MS analysis
Mice were sacrificed at mid-day via cervical dislocation, the brain removed, the hippocampus dissected on an ice-cold dissecting plate, and tissue was flash frozen in liquid nitrogen, then stored at −80 °C. Partial purification of endogenous lipids from the hippocampus tissue on C18 solid phase extraction columns was performed as previously described [24].
Statistical analysis
For electrophysiology analyses, depolarization response curves were obtained to determine pharmacological properties of endogenous 2-AG signaling by depolarizing neurons for progressively longer durations (50 msec, 100 msec, 300 msec, 500 msec, 1 sec, 3 sec and 10 sec). The data are fitted with by nonlinear regression, allowing calculation of an ED50, the effective dose or duration of depolarization at which a 50% inhibition is achieved. Statistical significance between the DSE “depolarization-response” curves was taken as non-overlapping 95% confidence intervals.
Plate assays were done in triplicate and tested for statistical significance using a 1 way ANOVA with Bonferroni post hoc test. All HPLC/MS/MS parameters and analyses for 2-AG, 2-LG, and 2-OG were as previously described [24,25].
RESULTS
2-OG, 2-LG and 2-PG act as antagonists rather than entourage compounds in autaptic hippocampal neurons
We first tested whether acylglycerols alter 2-AG- and CB1-mediated signaling in autaptic hippocampal neurons. As noted above these neurons express a well-characterized 2-AG-based cannabinoid signaling system. We first recorded from cells to obtain baseline EPSCs, and then treated the cells for five minutes with a given acylglycerol at 5μM. This concentration was chosen because we have previously found that maximal inhibition of signaling in these neurons corresponds to 5μM 2-AG [15]. As shown in Figure 2A1, 2-OG did not inhibit EPSCs, indicating that 2-OG does not directly inhibit neurotransmission via CB1 (Fig. 2A1, relative EPSC charge (2-OG 5μM): 1.05 ± 0.02, n=5).
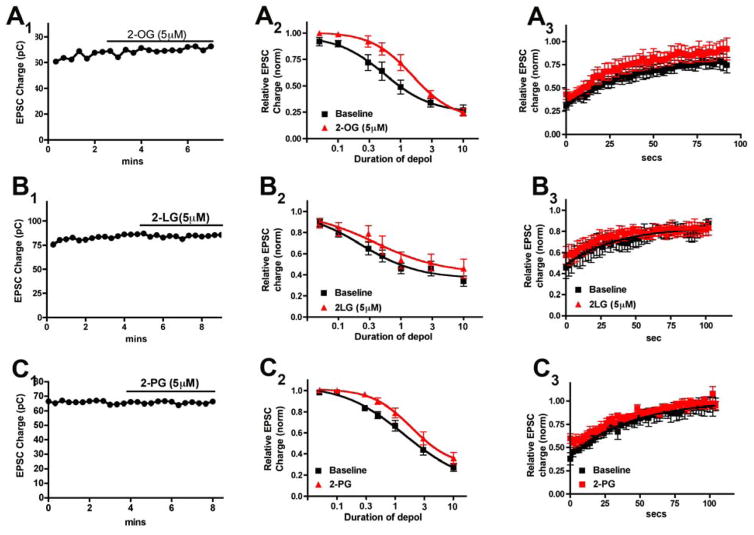
Figure 2. 2-OG, 2-LG and 2-PG act as an antagonists rather than entourage compounds in autaptic hippocampal neurons.
A1, B1, C1) Sample time courses show that EPSCs are unaltered by 5 μM of each of the 2-acylglycerols. A2, B2, C2) Depolarization-response curve shows the DSE response profile before (black squares) or with (red triangles) 5 μM 2-OG, 2-LG or 2-PG. In each case the curve is shifted to the right rather than to the left. In each case the 95% CI for the ED50 is non-overlapping indicating a statistically significant shift to the right. A3, B3, C3) Summary recovery/decay time courses for 3 second depolarization showing that recovery time course is not slowed by treatment with any of the acylglycerols.
Depolarization of excitatory autaptic hippocampal neurons elicits a form of retrograde inhibition termed depolarization induced suppression of excitation (DSE [15]). This can be quantified by subjecting the neuron to a series of successively longer depolarizations (50ms, 100ms, 300ms, 500ms, 1sec, 3 sec, 10 sec) resulting in progressively greater inhibition of neurotransmission [26]. This yields a “depolarization-response curve” that permits the characterization of some pharmacological properties of cannabinoid signaling, including the calculation of an effective-dose (depolarization) 50 (ED50), corresponding in this case to the duration of depolarization that results in 50% of the maximal response. An entourage compound would be expected to shift a depolarization response curve to the left by prolonging the lifetime of synaptic 2-AG. Instead for 2-OG we observed a clear rightward shift in the curve (Fig. 2A2; ED50 before drug (duration of depolarization (95% CI)): 560ms (0.53–0.59sec); after drug: 1.5sec (1.51–1.59); n=5; 95% CI non-overlapping).
An entourage compound should also slow the DSE recovery time course by decreasing the degradation of 2-AG. We have examined the contributions of various candidate enzymes to the recovery time course of DSE and DSI (in inhibitory neurons) and found that the recovery is directly determined by the enzymatic complement in a given cell [19]. In autaptic neurons DSE is slower than the fastest form of DSI because the former occurs via MAGL alone while the latter occurs via a combination of MAGL and COX2, resulting in a more rapid clearance of 2-AG during DSI. Competition for breakdown at MAGL could therefore reasonably be expected to slow the DSE recovery time course. However, we did not observe such an effect for 2-OG as seen in the example of a three-second depolarization in Figure 2A3 (t1/2 decay baseline (95% CI): 25.9 sec (18–45); After 2-OG: 23.2 sec (15–45)). We conducted the same series of experiments for 2-LG and 2-PG (Figs 2B and 2C) (t1/2 decay baseline (95% CI): 24.9 sec (15–74); After 2-LG: 16.9 sec (11–47)); (t1/2 decay baseline (95% CI): 30.6 sec (22–49); After 2-PG: 35.6 sec (27–52))). As expected, neither drug acted on its own to inhibit neurotransmission (Figs 2B1, 2C1, Relative EPSC charge (2-LG, 5μM): 0.97 ± 0.03, n=10; (2-PG, 5μM): 1.01 ± 0.01, n=5). But like 2-OG, neither drug shifted the depolarization response to the left. Instead both 2-LG and 2-PG shifted the curves to the right but not as much as 2-OG (Fig. 2B2; ED50 before 2-LG (duration of depolarization (95% CI)): 237ms (207–270); after drug: 385ms (287–510); n=5; 95% CI non-overlapping; Fig. 2C2; ED50 before 2-PG (duration of depolarization (95% CI)): 1.44 sec (1.21–1.70); after drug: 1.92sec (1.85–1.99); n=5; 95% CI non-overlapping). Thus, all three “entourage” compounds appeared to function as mild antagonists of 2-AG action at CB1 receptors.
2-OG interferes with CP55940-induced CB1 internalization
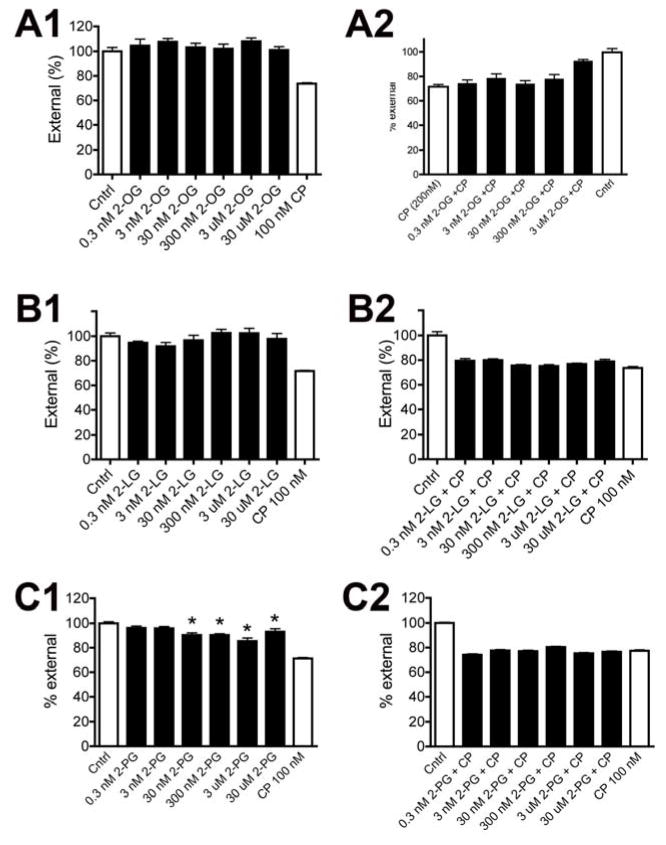
The above results suggest that 2-OG might antagonize signaling at CB1 receptors. To follow up on this possibility we examined the response profile of CB1 internalization following treatment with 2-OG in rCB1-expressing HEK293 cells using the on-cell western technique [27]. Consistent with earlier studies and the results shown in Figure 2, we found that the CB1 agonist CP55940 (100nM) internalized the CB1 receptor, but that 2-OG did not, even at concentrations as high as 30μM (Fig. 3A1; p>0.05, 1 way ANOVA with Bonferroni posthoc test). We then examined whether 2-OG would antagonize CP55940-induced internalization. We tested a range of concentrations, finding that the highest concentration (30μM) attenuated internalization (Fig. 3A2). We repeated these experiments for 2-LG (Fig. 3B; p>0.05 1 way ANOVA with Bonferroni posthoc test) finding that it neither induced internalization nor affected CP55940-induced internalization. Lastly we examined 2-PG, finding that 2-PG modestly internalized CB1 on its own at concentrations as low as 300nM and did not antagonize CP55940-induced internalization (Fig. 3C).
Figure 3. 2-OG prevents internalization of the CB1 receptor by the CB1 agonist CP55940 while 2-PG directly internalizes CB1.
A1) On-cell Westerns of CB1-expressing HEK cells shows that 2-OG does not induce internalization while CB1 agonist CP55940 (CP) does. A2) The highest concentration of 2-OG tested (30uM) prevents internalization of CB1 by CP. B1) 2-LG also did not cause modest internalization of CB1. B2) 2-LG did not interfere with CP-induced internalization. C1) In contrast to the other compounds, 2-PG did cause internalization of the CB1 receptor. C2) 2-PG did not however interfere with CP-induced internalization. *, p<0.05 1-way ANOVA with Bonferroni post hoc test vs. control.
The acylglycerols do not potentiate arrestin recruitment
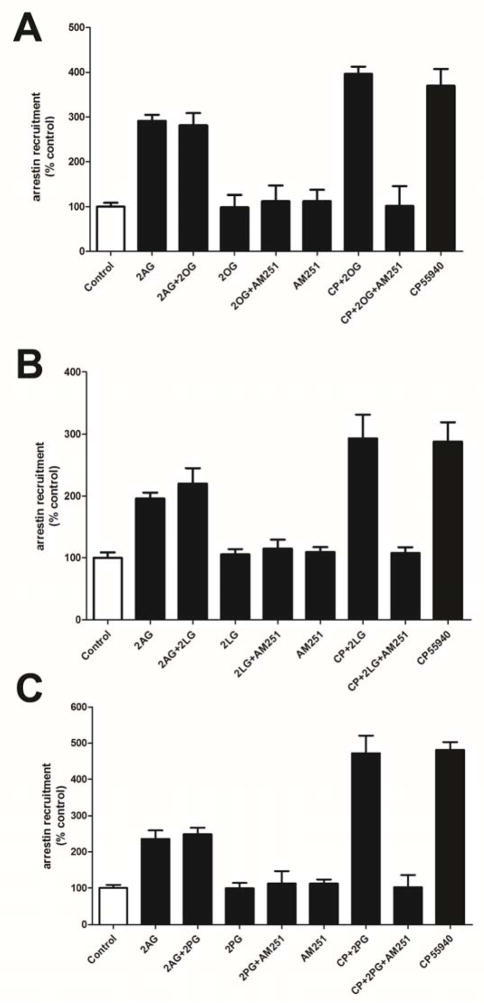
Using a CB1-expressing CHO cell line we tested whether any of the acylglycerols induced arrestin recruitment on their own or whether they altered the recruitment by agonists CP55940 and 2-AG. As expected we found that the compounds did not induce arrestin recruitment on their own (Fig. 4). Additionally none of the compounds altered the arrestin recruitment induced by the synthetic CB1 agonist CP55940 or the endocannabinoid, 2-AG (Fig. 4).
Figure 4. The acylglycerols do not enhance 2-AG or CP55940 arrestin recruitment.
In CB1-expressing CHO cells, at 5 μM the 2-AG congeners 2-OG (A), 2-LG (B) and 2-PG (C) each do not induce arrestin recruitment on their own, nor do they potentiate arrestin recruitment by CB1 agonists CP55940 or 2-AG. 1-way ANOVA with Bonferroni post hoc test.
The acylglycerols do not alter CB1 inhibition of adenylyl cyclase or activation of pERK
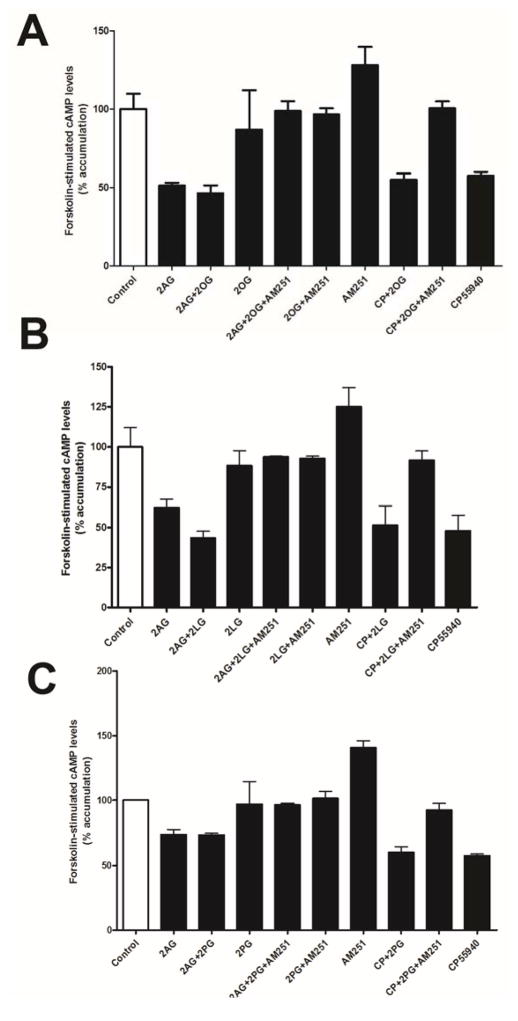
In separate experiments we examined the inhibition of forskolin-stimulated cAMP accumulation in HEK293 cells stably expressing rCB1 receptors. This inhibition is thought to occur via Gα subunits after activation of a GPCR and may be differentially activated [27,28]. None of the acylglycerols tested directly inhibited forskolin-stimulated cAMP accumulation and they also had no apparent effect on either 2-AG or CP55940 inhibition of cAMP accumulation (Fig. 5; p>0.05, 1 way ANOVA with Bonferroni posthoc test).
Figure 5. 2-OG, 2-LG and 2-PG do not affect 2-AG or CP55940 inhibition of adenylyl cyclase.
A-C) 2-OG, 2-LG and 2-PG do not affect 2-AG or CP55940 inhibition of cAMP production. No significant differences were found by 1-way ANOVA with Bonferroni post hoc test.
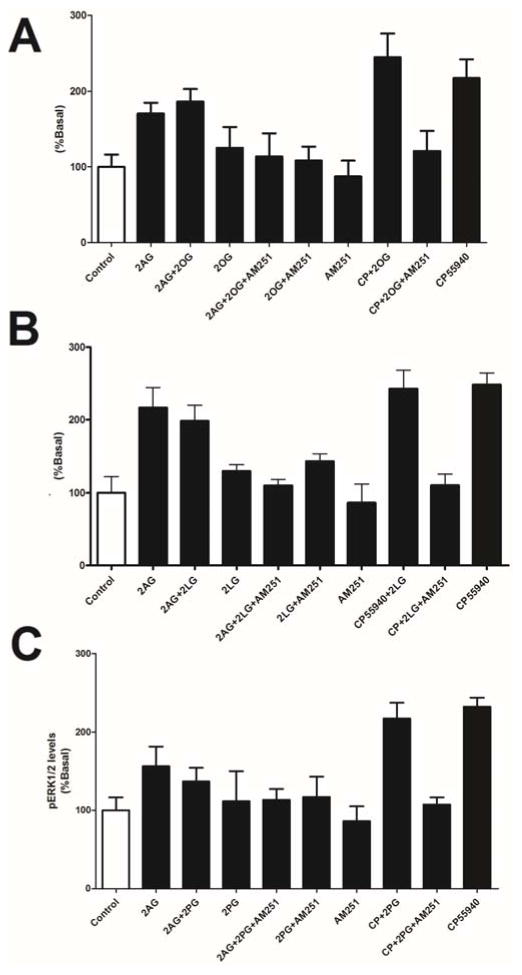
Similarly none of the compounds activated pERK on their own and each failed to alter activation of pERK by 2-AG or CP55940 (Fig. 6; p>0.05, 1 way ANOVA with Bonferroni posthoc test).
Figure 6. 2-LG, 2-PG and 2-OG do not alter pERK1/2 activation by 2-AG or CP55940.
A) 2-OG does not activate pERK1/2 and does not alter pERK1/2 signaling by 2-AG and CP55940. B&C) 2-LG and 2-PG also do not alter pERK1/2 signaling.
2-LG and 2-OG are present at levels comparable to those of 2-AG in the hippocampus
One premise of the entourage hypothesis is that the congeners of 2-AG are present in sufficient quantities to compete with 2-AG for degradation by 2-AG metabolizing enzymes. We therefore examined 2-LG and 2-OG levels in mouse hippocampus. As shown in Table 1, 2-LG was detected but its levels were found to be an order of magnitude lower than those of 2-AG. 2-OG however was found at more than twice the levels of 2-AG.
Table 1.
Levels of acylglycerols 2-AG, 2-LG and 2-OG in murine hippocampus.
| Acylglycerol | Levels (nmoles/g ± SEM) |
|---|---|
| 2-AG | 6.60 ± 0.89 |
| 2-LG | 0.50 ± 0.07 |
| 2-OG | 17.63 ± 4.72 |
DISCUSSION
Our chief finding is that in a neuronal model of endogenous 2-AG- and CB1-mediated synaptic plasticity the acylglycerols 2-OG, 2-LG and 2-PG do not behave in a manner consistent with entourage compounds. Instead all compounds, but most notably 2-OG, acted as antagonists. In other assays of CB1 internalization, cAMP inhibition, pERK activation and arrestin recruitment, these acylglycerols also failed to act as entourage compounds.
The acylglycerols exhibit some functional selectivity depending on the signaling pathway examined. For example, 2-PG behaved as a very modest antagonist in DSE, but as a low efficacy agonist in receptor internalization. Conversely, 2-OG showed the strongest antagonistic effect in neurons, but only weakly antagonized CB1 internalization in CB1-expressing HEK cells.
Because 2-AG was the first member of its class to be assigned a functional role at cannabinoid receptors, it has been tempting to view related lipids through a 2-AG-centric lens and to assume that they subserve 2-AG’s role in signaling. In the absence of clear evidence for separate function for the congeners of 2-AG, this has a certain intuitive appeal and is certainly a possibility that must be explored and tested. The autaptic preparation is well suited to study this question because the 2-AG dependency of DSE has been well characterized and the decay of DSE is determined by MAGL activity. Blockade of MAGL in this system under the same recording conditions used for these experiments clearly and substantially slows the decay time course of DSE, meaning that if there had been a change in the rate of metabolism of 2-AG we should have detected it. Our results therefore strongly argue that in the case of MAGL these 2-acyl glycerols do not engage this enzyme to alter the rate of 2-AG degradation in a functionally important manner. The antagonism we have observed appears to be limited to the inhibition of calcium channels in neurons and to a more limited extent to internalization of the receptor.
Our results do not rule out other functional roles for these compounds. As noted above 2-OG and 2-PG have been proposed to be GPR119 agonists. Indeed, depending on the activation properties of 2-AG and its congeners at GPR119, 2-AG might in principle serve as an entourage compound for 2-OG. It is possible that a great excess of a given congener might exert an effect on 2-AG metabolism and signaling but as noted previously, 5 μM is the concentration of 2-AG that induces maximal inhibition in autaptic neurons. And as we have shown here, in the case of 2-LG and 2-OG in the hippocampus, neither is in great excess of 2-AG. However this low-micromolar concentration, though physiological, is relatively high for an acylglycerol and it is difficult to envision concentrations for compounds such as 2-OG in excess of this.
Our results do not bear directly on potential entourage effects of anandamide and its congeners, but they do highlight the importance of systematically testing these interactions.
Because 2-AG based cannabinoid signaling was described first, one is tempted to view the related lipids as orbiting a cannabinoid sun. The embodiment of this notion – the entourage hypothesis - yields specific testable predictions that we have investigated using five models, including a 2-AG-based neuronal model of endogenous cannabinoid signaling. We find that in no case do the lipids 2-OG, 2-PG, or 2-LG act as entourage compounds for 2-AG. If these lipids are wrested out of the orbit of 2-AG, it remains to be learned what physiological roles - if any - these lipids have in the CNS.
Acknowledgments
This work was supported by grants from the National Institutes of Health: DA01322 (KM), DA021696 (KM) and EY24625 (AS).
Abbreviations
- DSE
depolarization induced suppression of excitation
- 2-AG
2-arachidonoyl glycerol
- 2-PG
2-palmitoyl glycerol
- 2-OG
2-oleoyl glycerol
- 2-LG
2-linoleoyl glycerol
- EPSC
excitatory postsynaptic current
- pERK
phosphorylated extracellular signaling related kinase
- eCB
endocannabinoid
- AEA
arachidonoyl ethanolamine
- HEK
human embryonic kidney
- HBS
HEPES buffered saline
- BSA
bovine serum albumen
- TBS
tris buffered saline
- DMEM
Dulbecco’s minimal essential medium
- MAGL
monoacylglycerol lipase
- CHO
chinese hamster ovary
Footnotes
CONFLICT OF INTEREST
The authors declare that they do not have a conflict of interest relating to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 2.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cdna. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 4.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Murataeva N, Straiker A, Mackie K. Parsing the players: 2-ag synthesis and degradation in the cns. Br J Pharmacol. 2013 doi: 10.1111/bph.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urquhart P, Nicolaou A, Woodward DF. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochim Biophys Acta. 2015;1851:366–376. doi: 10.1016/j.bbalip.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P, Makriyannis A. Quantitative method for the profiling of the endocannabinoid metabolome by lc-atmospheric pressure chemical ionization-ms. Analytical chemistry. 2007;79:5582–5593. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]
- 11.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of gpr119 receptor activity with endocannabinoid-like lipids. American journal of physiology. 2012;303:E1469–1478. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 12.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-oleoyl glycerol is a gpr119 agonist and signals glp-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 14.Gallily R, Breuer A, Mechoulam R. 2-arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice. Eur J Pharmacol. 2000;406:R5–7. doi: 10.1016/s0014-2999(00)00653-1. [DOI] [PubMed] [Google Scholar]
- 15.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol. 2007;578:773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol. 2009;76:1220–1227. doi: 10.1124/mol.109.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straiker A, Wager-Miller J, Hu SS, Blankman JL, Cravatt BF, Mackie K. Cox-2 and fatty acid amide hydrolase can regulate the time course of depolarization-induced suppression of excitation. Br J Pharmacol. 2011;164:1672–1683. doi: 10.1111/j.1476-5381.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straiker A, Mackie K. Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience. 2009;163:11. doi: 10.1016/j.neuroscience.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: Evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levison SW, McCarthy KD. Characterization and partial purification of aim: A plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J Neurochem. 1991;57:782–794. doi: 10.1111/j.1471-4159.1991.tb08220.x. [DOI] [PubMed] [Google Scholar]
- 23.Daigle TL, Kearn CS, Mackie K. Rapid cb1 cannabinoid receptor desensitization defines the time course of erk1/2 map kinase signaling. Neuropharmacology. 2008;54:36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raboune S, Stuart JM, Leishman E, Takacs SM, Rhodes B, Basnet A, Jameyfield E, McHugh D, Widlanski T, Bradshaw HB. Novel endogenous n-acyl amides activate trpv1-4 receptors, bv-2 microglia, and are regulated in brain in an acute model of inflammation. Front Cell Neurosci. 2014;8:195. doi: 10.3389/fncel.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tortoriello G, Rhodes BP, Takacs SM, Stuart JM, Basnet A, Raboune S, Widlanski TS, Doherty P, Harkany T, Bradshaw HB. Targeted lipidomics in drosophila melanogaster identifies novel 2-monoacylglycerols and n-acyl amides. PLoS ONE. 2013;8:e67865. doi: 10.1371/journal.pone.0067865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straiker A, Wager-Miller J, Hutchens J, Mackie K. Differential signaling in human cannabinoid cb(1) receptors and their splice variants in autaptic hippocampal neurons. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atwood BK, Wager-Miller J, Haskins C, Straiker A, Mackie K. Functional selectivity in cb(2) cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of cb(2) ligands. Mol Pharmacol. 2012;81:250–263. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]