Abstract
Ovarian cancer is the most common cause of gynecological cancer death in United States women. Efforts to improve progression free survival (PFS) and quality of life (QoL) after treatment for ovarian cancer are necessary. Observational studies suggest that lifestyle behaviors, including diet and physical activity, are associated with lower mortality in this population. The Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES) NRG 0225 study is a randomized, controlled trial designed to test the hypothesis that a 24 month lifestyle intervention will significantly increase PFS after oncological therapy for stage II-IV ovarian cancer. Women are randomized 1:1 to a high vegetable and fiber, low-fat diet with daily physical activity goals or an attention control group. Secondary outcomes to be evaluated include QoL and gastrointestinal health. Moreover an a priori lifestyle adherence score will be used to evaluate relationships between adoption of the diet and activity goals and PFS. Blood specimens are collected at baseline, 6, 12 and 24 months for analysis of dietary adherence (carotenoids) in addition to mechanistic biomarkers (lipids, insulin, telomere length). Women are enrolled at NRG clinic sites nationally and the telephone based lifestyle intervention is delivered from The University of Arizona call center by trained health coaches. A study specific multi-modal telephone, email, and SMS behavior change software platform is utilized for information delivery, coaching and data capture. When completed, LIVES will be the largest behavior-based lifestyle intervention trial conducted among ovarian cancer survivors.
Keywords: Ovarian cancer, Diet, Physical activity, Clinical trial, Cancer survival, Behavior
1. Introduction
Ovarian cancer is diagnosed in approximately 21,980 women annually in the United States. Survival after a diagnosis of ovarian cancer is poor. An estimated 44% of women are expected to be alive after 5 years, 36% after 10 years [1,2]. Risk factors for ovarian cancer are largely unmodifiable [3–5]. Efforts to identify modifiable risk factors that may reduce the risk for disease progression remain limited with diet and physical activity leading the list of potential targets for behavior change.
However, evidence supporting the role of diet as a modifier of ovarian cancer risk or survival remains limited. An analysis from the Women’s Health Initiative (WHI) prospective, low fat dietary modification trial of 48,835 post-menopausal women suggested that long term adoption of a low fat diet was associated with a significant 40% reduction in ovarian cancer risk [5]. A systematic review suggested that total, animal and dairy fat were most consistently associated with higher risk [6]. Pre-diagnosis diet (n = 341 ovarian cancer patients) of higher versus lower fruits and vegetable intake was associated with a 39% greater survival time an effect largely driven by consumption of green, yellow and cruciferous vegetables [7]. One study (n = 636 cases) showed 27% lower mortality in women reporting a high diet quality score – a score that reflected greater vegetable, fruit, and fiber and lower fat, and animal fat intake [8].
The role of physical activity in ovarian cancer is not clearly understood. Mechanistic support for this hypothesis relates to improvements in immune function [9] and reductions in estrogen [10,11], body fat [12, 13], and insulin [14]. An analysis of pre-diagnosis physical activity among postmenopausal women suggested that higher moderate-to-vigorous physical activity was associated with a 26% lower risk for mortality [15].
These data support our efforts to develop a randomized, controlled trial, the Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES) NRG 0225 study, to prospectively test the effect of diet in combination with physical activity on increasing progression-free survival (PFS) in women previously treated for ovarian cancer. The large recruitment base and research infrastructure of the NRG Oncology Cooperative Group affords a unique opportunity to efficiently test our hypotheses relating dietary intake and physical activity to ovarian cancer PFS. The LIVES trial is designed to fill the gap in current knowledge in relation to optimal diet and activity behaviors after ovarian cancer treatment. The longer-term goal of this research is to contribute to the available evidence and in turn support the development of clinical guidance related to diet and physical activity behavior following treatment for ovarian cancer.
2. Research design and methods
2.1. Hypothesis and study objectives
The hypothesis for this trial is that PFS will increase among women previously treated for Stage II–IV ovarian, fallopian tube, or primary peritoneal cancer who are randomly assigned to a lifestyle intervention that includes a low fat, high vegetable and fruit diet and daily physical activity for a period of 24 months.
Several a priori secondary objectives are also being evaluated including assessment of quality of life (QoL), bowel health and physical function. Exploratory objectives to evaluate change in circulating levels of plasma carotenoids between treatment arms and to examine patient compliance with the healthy lifestyle intervention in order to assess what patient-specific factors inform on compliance to the lifestyle intervention and whether PFS is better among compliant individuals are also included in the protocol for evaluation.
2.2. Trial design
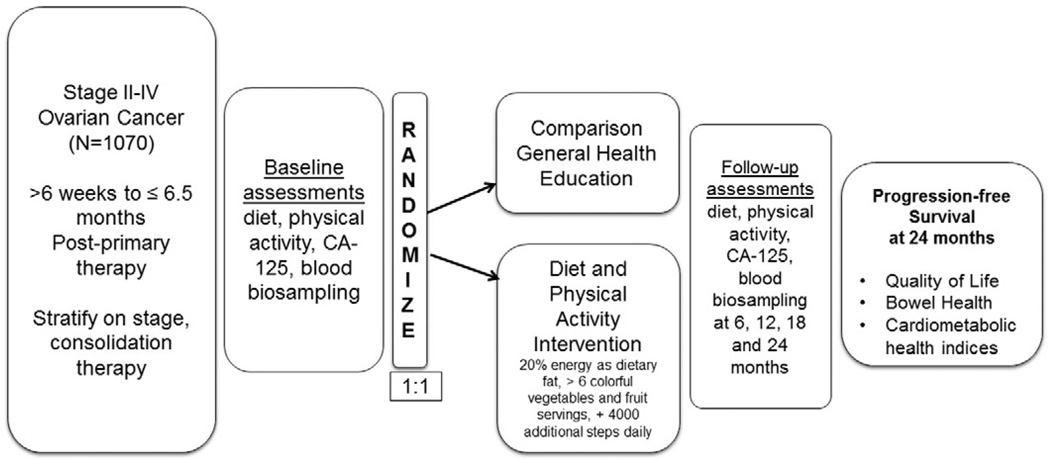
The LIVES study is a Phase III, randomized clinical trial focused on lifestyle behavior change including a combined lifestyle intervention focused on diet and physical activity behaviors. The intervention is a telephone-based coaching program grounded in Bandura’s Social Cognitive Theory (SCT) [16]. The design is illustrated in Fig. 1. In total, 1200 women will be randomized from NRG clinical sites across the U.S. and Canada with a target of 1070 evaluable participants.
Fig. 1.
Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIVES) study design.
The decision to intervene for a period of 24 months was based on evidence that progression-free survival was estimated at 22.5 months at the time the study was designed, having ample time to change and maintain change in diet and physical activity behaviors, informed by prior lifestyle intervention trials [17,18] and the realization of a 5-year grant cycle for recruitment and follow-up. After completing the two-year protocol intervention, participants are being followed for another 5 years to gather information on disease progression and survival as well as quality of life. The study data will be considered mature when 857 PFS events are documented.
2.3. Eligibility and exclusion criteria
Several eligibility criteria are included in the study design in order to promote some degree of homogeneity in study participants, expand inclusion beyond women who are already actively engaged in healthy lifestyle behaviors and to control for effect modification to a degree that also supports timely recruitment. Eligible patients must be 18 years of age or older and have a histological diagnosis of epithelial ovarian cancer, fallopian tube or primary peritoneal carcinoma, clinical stage II, III or IV at diagnosis. Patients must have completed all primary chemotherapy and consolidation therapy (if administered) at least 6 weeks, and no > 6–1/2 months, prior to enrollment and must be in remission. Patients must have achieved a documented complete response to treatment based on normal CA-125 and CT scan (i.e. there must be no clinical evidence of persistent or recurrent disease based on CA-125 and CT scan) and have a Gynecological Oncology Group (GOG) Performance Grade of 0–2. Patients must be free of chronic disease that would preclude randomization into a lifestyle intervention trial including serious psychiatric illness. Ineligible patients include women with a history of other invasive malignancies within the last 5 years, with the exception of non-melanoma skin cancer. In addition, women diagnosed with chronic disease/illness precluding their participation (i.e., diabetics receiving insulin, myocardial infarction or unstable angina within previous six months, chronic hepatitis, rheumatoid disease, renal or hepatic disease/dysfunction) are not eligible to participate. Patients with a life expectancy of <1 year or with a Body Mass Index (BMI) <20 kg/m2 as well as those following a restricted diet (Vegan, prescribed weight loss, etc.) or who engage in any endurance related physical activity (marathons, triathlons) within the previous 12 months are not eligible for study participation.
2.4. Recruitment and consent
This treatment protocol is approved by the Division of Cancer Prevention at the National Cancer Institute and then monitored by the University of Arizona (UA) Human Subjects Institutional Review Board (IRB) for centralized delivery of study intervention and by the IRB for each recruitment site where in patients are consented for trial participation. Recruitment for participants in the LIVES study occurs at the local NRG clinics using standard dissemination methods of the cooperative group as well as through marketing efforts targeting the cancer survivor population at large. The promotion of this study in survivor networks is a key component of the recruitment strategy. These efforts include web-based marketing through Facing Our Risk of Cancer Empowered (FORCE), National Ovarian Cancer Coalition (NOCC), and Up the Volume. Participants in the LIVES trial are entered through the NRG clinics. All NRG clinics seeking to be registered into the NRG centralized system; participants may not enter the trial at clinical treatment sites not registered with NRG. NRG clinic personnel must complete required study training either on-line or during training session made available at the bi-annual NRG cooperative group meetings. The training program has standardized content that was specifically developed for administration of GOG 0225 at the clinic level. Content is largely driven by the protocol content. Training includes a review of the literature supporting the trial, study hypotheses and aims, forms review, standard operating procedures for patient administration and processing of forms, clinic-based measures and time points for collection and detailed information regarding contacts for problem-solving. In addition, the training includes an on-line video of the waist circumference measurement procedure. Upon completion of the training, study personnel must demonstrate competency in protocol content with a score of >90% on a standardized, online protocol examination prior to enrolling eligible participants in the trial. Once qualified as a recruitment site, clinics develop internal approaches to identify participants for eligibility screening, implement the screening checklist and offer the trial to all eligible patients. If interested, eligible patients complete the consent process and are registered into the trial through the centralized NRG OPEN database. Only trained personnel are consenting patients for participation, per protocol.
2.5. Randomization
After completing eligibility screen, consent process and all baseline assessments, eligible, consented women are equally assigned to the intervention or control arms using a stratified randomized block design. Randomization is performed electronically with stratification by stage of disease at diagnosis and presence of prior consolidation therapy. The assigned study regimen is revealed to the patient after registration is complete.
2.6. Characteristics of study participants
As of June 1, 2016, 781 women have been randomized into the trial representing over 65% of the recruitment goal. Retention is over 90%, above estimates used to set trial statistical power. Enrollment represents participants from 187 clinics and 44 states. Enrollment was opened at NRG (GOG) clinical sites in June 2012. Characteristics of the study sample through 6/1/16 are described in Table 1.
Table 1.
Baseline characteristics of n = 781 LIVES trial participants (through 6/1/16).
| Characteristic | Number (n) and percent (%) |
|---|---|
| Age | |
| 21–40 y | 21 (2.7%) |
| 41–50 y | 124(15.9%) |
| 51–60 y | 247 (31.6%) |
| 61–70 y | 285 (36.5%) |
| 71–80 y | 101 (12.9%) |
| >80 y | 3 (0.4%) |
| Education | |
| <High school | 22 (2.8%) |
| High school | 116(14.9%) |
| Some college | 220 (28.2%) |
| College degree | 423 (54.2%) |
| Race/ethnicity | |
| Non-Hispanic White | 679 (86.9%) |
| Non-Hispanic Black | 37 (4.7%) |
| Asian (non-Hispanic) | 13 (1.7%) |
| Native American | 9 (1.2%) |
| Hispanic | 38 (4.9%) |
| [Missing] | 5 (0.6%) |
| Body mass Index (BMI) | |
| <20 | 9 (1.2%) |
| 20–24.9 kg/m2 | 277 (35.5%) |
| 25–29.9 kg/m2 | 250 (32.0%) |
| 30–34.9 kg/m2 | 145 (18.6%) |
| > 35 kg/m2 | 99 (12.7%) |
| [Missing] | 1 (0.1%) |
| Smoking status (self-report) | |
| Current | 79(10.1%) |
| Past | 209 (26.8%) |
| Never | 492 (63.0%) |
| [Missing] | 1 (0.2%) |
| Stage of disease (medical record) | |
| I | 0 |
| II | 121(15.5%) |
| III | 554 (70.9%) |
| IV | 106(13.6%) |
| Consolidation therapy (medical record) | |
| Yes | 191 (24.5%) |
| No | 590 (75.5%) |
| History of chronic disease (self-report) | |
| Cardiovascular disease | 286 (36.6%) |
| Diabetes (non-insulin treated) | 47 (6.0%) |
| Hypertension | 276 (35.3%) |
2.7. Telephone counseling
Treatment for both the intervention and attention control group study participants are delivered in both English and Spanish by trained health coaches through a multi-modal software application developed by Arizona Research Labs at the UA. Participants receive communication to support lifestyle behavior goals from the study in a variety of methods including telephone calls, Short Message Service (SMS) messaging, email, forums and mail (newsletters, cards). These communications are treatment specific and forums are user-group assignment specific by invitation only in order to maintain trial fidelity.
All health coaches complete general training on the protocol, ovarian specific cancer knowledge, palliative care, effective and acceptable phone communication over a period of 2 weeks. The training is delivered by a multi-disciplinary team that includes the Principal Investigator, study coordinator, medical oncologist, a study survivor advocate and a study oncology nurse. This introductory training period is followed by a 4-week intensive training in health coaching. Most importantly, health coaches for the intervention group complete extensive and focused training in Motivational Interviewing. This training applies cognitively based behavior change strategies with Motivational Interviewing approaches that include goal setting, self-monitoring, feedback and reinforcement to support self-efficacy. As part of the standardized training protocol all coaches participate in mock recorded coaching sessions. Only the intervention coaches have their recorded calls scored using Motivational Interviewing Treatment Integrity (MITI) guidelines and continue to do so until they reach the beginning proficiency level [19], All coaches must pass a final mock call to the study Principal Investigator before they are progressed to coaching LIVES participants. When necessary additional training is provided, on occasion (25 to date) coaches are passed over for employment if unable to meet quality assurance metrics for performance. Ongoing evaluation and re-training of coaches is performed on a monthly basis and informs on employment status. The evaluation includes review by the study coordinator of a random sample of 10% of calls completed during the first week and 3% each month thereafter as well as MITI scoring of calls conducted by the intervention group coaches. A MITI score of 20 is considered acceptable performance. If performance issues are identified (call etiquette, protocol adherence, and low MITI scores for intervention coaches) additional training is provided and a re-assessment of performance conducted. If performance does not reach protocol standards the employee is dismissed. This treatment protocol is approved and monitored by the UA Human Subjects Institutional Review Board.
2.8. Behavioral theory
A theory based behavioral approach, grounded in Bandura’s SCT [16, 20] with integration of the theory of planned behavior and locus of control models was developed as a framework for the intervention arm of LIVES [21]. Self-monitoring, tailoring and repetitive messaging is used in addition to the telephone based coaching to assist participants in achieving optimal behavior change. The coaching approach integrates what is known as “narrative therapy” for person-centered, meaningful dialogue to promote behavior change [22].
Social cognitive theory posits an individual with given information, attitude, beliefs and needs functioning in a given social and physical environment will engage in behavior that will have a consequent outcome. The perceived self-efficacy of each participant influences the acquisition of new behaviors, inhibition of existing behaviors and the disinhibition of behaviors. In addition, self-efficacy influences the amount of time and effort an individual is willing to put towards changing a behavior. Finally, self-efficacy impacts psychosocial elements to include anxiety, distress and thought patterns around behavior change.
Locus of control [23] suggests an individual’s health is controlled by their beliefs. These beliefs can be an effective motivator when evaluated on a person by person basis. The theory of planned behavior [24] theorizes behavior can be predicted through an individual’s attitude and the subjective norm of a particular behavior. Because no two individual are the same, a patient centered approach allows the coach to help guide participants through their individual journey in meeting the lifestyle goals. On each call, participants are coached using these frameworks to guide exploration of barriers and facilitators for specific behaviors resulting in tailored behavior change. LIVES participants set one to two small, achievable goals on each coaching call and efficacy for achieving each goal is assessed on every call.
2.9. Diet and physical activity goals
Earlier epidemiological evidence guided the intervention goals which support healthy dietary patterns and moderate physical activity to promote a reduction in cancer risk [25]. The combination of diet and physical activity was selected to ensure the most robust effect on the underlying biological mechanisms by which these lifestyle behaviors may improve PFS. Specifically, the goals include a plant-based diet focused on four servings of vegetables per day with an emphasis on cruciferous [26], dark green and orange colored vegetables [27], two servings of fruit per day with one being citrus [28,29], 30 g of fiber [30], and 20% of total calories obtained from fat [6,7,30–33]. Standardized portions define serving size with a serving of vegetables equivalent to 3/4 cup 100% vegetable juice, 1 cup raw leafy greens or 1 /2 cup cooked or raw vegetables. Fruit servings are defined as 1 medium (tennis ball sized), 1/2 cup raw cut up or 1/4 cup dried fruit. To maximize exposure to bioactive compounds white potatoes, fruit juices that are not 100% juice and corn are not counted towards daily vegetable and fruit goals. Participants are supported to consume vegetables and fiber beyond study goals as desired; fat intake below 15% total energy is not advised in order to assure adequacy of fatty acid intake. Fruit intake above 2 servings daily is not advised given conflicting observational evidence suggesting higher intake may be associated with higher risk for ovarian cancer [6].
The physical activity goals were selected based on literature supporting walking as the primary activity of interest to ovarian cancer survivors [34], evidence of a protective role for physical activity in ovarian cancer [35] and emerging research suggesting sedentary time may be as relevant as low total activity in increasing ovarian cancer risk [36–38]. Participants are supported to increase steps by an average of 4000 steps per day and longer term to meet or exceed the recommended 10,000 steps daily. Steps can be achieved through walking or women may convert other activities (e.g., yoga or weight lifting) to steps using estimates provided in the study manual to meet their activity goals.
Strategies to achieve and maintain study activity goals start with self-monitoring. Participants wear pedometers and record daily steps in lifestyle journals throughout the intervention to promote behavior change [39,40], assess progress towards study goals and assist in coaching. Lifestyle journals are returned to the study bi-monthly for review and activity is reviewed during coaching calls for the intervention group participants and captured in real-time using the study designed web-based platform. To add variety and interest important to adopting a more active lifestyle participants are encouraged to participate in individual or group-based aerobic activity 2 times/week for 10–30 min, depending on individual tolerance, and to integrate strength and flexibility training weekly. These approaches meet the guidelines outlined by the American College of Sports Medicine (ACSM) and American Cancer Society (ACS) [12,41] to increase moderate physical activity among cancer survivors. In addition, participants are advised and provided educational materials to reduce sedentary time an approach that can be effective in individuals with lower activity levels in order to build self-efficacy towards larger changes in physical activity over time.
2.10. Participant contact
Contact for patients participating in LIVES is a combination of in person assessment visits with local NRG clinic personnel as well as telephone-based and electronic contact from the UA coaching call center. In-person clinic visits occur every 3 months and include measurement of the patient’s weight, height (annually) and waist circumference at the baseline, 6, 12 and 24 month visits. Questionnaires are completed and blood samples are collected during clinic visits (Table 2). The minimum frequency of contact is standardized for telephone contact. Women assigned to the intervention receive 33 scheduled telephone calls over 24 months and women assigned to the attention control condition receive 22 scheduled telephone calls over the same time period. Counseling calls are made more frequently in the first 6 months then tapered downward in frequency and number over the 24-month trial period (Table 2). Telephone contact may be tailored in frequency at the request of individual participants to either promote optimal behavior support or reduce participant burden that may contribute to recidivism. However, the minimum goal of 33 and 22 calls for the intervention and control groups, respectively, is adhered to by rescheduling or shortening call length at the participant’s convenience. An example of tailoring might be email and/or SMS text contact only while a participant is on vacation or added email correspondence if additional education is needed in the initial phases of the trial. Alternately, women may opt for extension of the weekly telephone contact schedule for an additional 2 weeks before reducing down to every other week calls/contact.
Table 2.
LIVES study-related assessments and interventions by treatment group.
| On-going study activity | Intervention group | Attention control group |
|---|---|---|
| Enrollment Questionnaire |
Baseline | Baseline |
| Questionnaires (RAND-36/GSRS-IBS, Food Frequency Questionnaire, Activity Questionnaire, Pittsburgh Sleep Quality Index) |
Baseline, 6, 12 and 24 months |
Baseline, 6, 12 and 24 months |
| Anthropometrics: height, weight, waist circumference |
Baseline, and every 3 months |
Baseline and every 3 months |
| Blood sampling | Baseline, 6, 12 and 24 months |
Baseline, 6, 12 and 24 months |
| Study contact | Lifestyle coaching <physical activity + diet goals > 0–24 months, 33 contacts by telephone (2/week for 4 weeks, 1/week for 2 weeks, 1/month for 12 months, and then every other month until study completion); 156 SMS messages; prn email |
General health education (22 contacts by telephone (1/week for 4 weeks, 1/month for 12 months, and then every other month until study completion); 156 SMS messages; prn email) |
| Retention efforts | Quarterly incentive and newsletter |
Quarterly incentive and newsletter |
| Actigraph Accelerometry for 7 days |
Sub-sample at baseline, 6,12 and 24 months (n = 250) |
Sub-sample at baseline, 6,12 and 24 months (n = 250) |
In addition to coach-participant communications and contact, the success of LIVES to retain participants is, to some extent, dependent upon clear communications and building of rapport between the individual NRG clinic site coordinators and the UA coaching call center. Communication is vital to reduce study attrition and clinic staff reach to individual participants is commonly employed when the coaches are unable to contact a participant. For more general study communications such as protocol updates or operations advisement conference calls are held monthly between the UA and NRG clinic sites. Clinics are expected to attend calls at least quarterly, to date the majority attending all monthly calls. Additionally, in person study updates are provided for clinic staff at the semi-annual NRG meetings.
3. Study outcomes and assessments
The primary outcome for the LIVES trial is PFS, defined as the number of months between study enrollment and documentation of disease progression or death from any cause, whichever is observed first, understanding that all participants must have documented complete remission for enrollment into NRG (GOG) 0225. Patients who are alive with no documentation of disease at the time of analysis will be censored at the date of last disease assessment. Disease progression is defined as increasing clinical, radiological or histological evidence of disease since study entry. Progression can also be indicated by 2 serum CA-125 measurements ≥2 times the upper limits of normal (ULN), or twice the nadir value in absence of normalization, performed at least 1 week apart. In the event of increasing symptoms and no elevation of CA-125, a CT scan or MRI with contrast is performed to evaluate progression.
Secondary outcomes designated in the protocol include change in QoL by treatment arm over 24 months as assessed by RAND-36 [42] with particular interest in physical functioning, pain, vitality/energy, social functioning, general health and role limitations due to physical and mental health, and emotional problems. The RAND-36 is administered by clinic-based, designated/trained study personnel at baseline and every 3 months throughout the trial. Additionally, the effect of treatment assignment on bowel function is assessed using the validated instrument, Gastrointestinal Symptom Rating Scale - Irritable Bowel Syndrome (GSRS-IBS) [43]. Overall Survival (OS) also is a secondary outcome. OS is defined as the time from entry to death due to any cause. Patients who are alive at the time of analysis will be censored on their date of last contact (regardless of whether or not this contact is on a subsequent protocol).
3.1. Study assessments
The study protocol includes several assessments beyond those collected as primary and secondary outcomes including self-report and objective measures. Table 2 lists the specific measures collected along with the protocol-specified time points for data collection. The baseline questionnaire ascertains information on study variables that have been postulated to act as confounders or effect modifiers on diet, activity and/or PFS that may influence the final analysis. These include, but are not limited to weight history, smoking/tobacco use, cancer therapies, reproductive history, use of select medications and medical history.
3.2. Self-report questionnaires of health behaviors and quality of life
In addition to the baseline health questionnaire that is collected in a modified form during follow up, the study participants also complete study-designated questionnaires to track the targeted health behaviors (diet and physical activity). Diet is assessed by the validated, self-report, 153-item Arizona Food Frequency Questionnaire [44] that was administered in a similar trial previously conducted in breast cancer survivors [45]. Physical activity is assessed by self-report at baseline, 6, 12 and 24 months using the validated Arizona Activity Frequency Questionnaire [46] which includes the updated Metabolic Equivalents for activities [47].
Other self-reported data collection includes the Pittsburgh Sleep Quality Index (PSQI), a 19-item questionnaire used to quantify sleep duration, disturbance, latency, efficiency, quality, daytime dysfunction and sleep medication use [48]. This validated questionnaire was added to the protocol early on as advancing evidence suggested a link between sleep and cancer as well as a potential influence on diet, activity and bodyweight. This is the one questionnaire collected by telephone by trained personnel at the Behavioral Measurements and Interventions Shared Resource of the UA Cancer Center. Additionally, during regularly scheduled clinic visits, each study participant completes standardized questionnaires related to general QoL (as measured by the General Health subscale of RAND-36 [42]), physical and bowel functioning (as measured by the Physical Functioning subscale of RAND-36 [42]) and the Gastrointestinal Symptom Rating Scale (GSRS-IBS) [43], measures expected to be modified differentially between the study groups.
3.3. Objective physical activity assessment
Self-report physical activity has shown modest, but not ideal, correlations with objective measures [49]. In an effort to improve on self-reported assessments as is suggested for cancer survivorship research [50, 51], this study applies accelerometry as an objective measurement of activity. Accelerometry affords an opportunity to quantify activity in relation to frequency, intensity and sedentary time [52]. As of July 30, 2015, all prospective trial enrollees (an estimated 500 participants; 250 intervention and 250 control women) are requested to wear accelerometers (Actigraph GT9X) on their waist for 7 consecutive days including time spent sleeping at baseline, 6, 12 and 24 months. Accelerometers are mailed to participants along with written instructions and an instructional video developed for use by LIVES trial participants. Valid wear time is 10 h/day for at least 4 of the 7 days of assessment [53]. Re-wears are implemented only if the equipment is lost or malfunctions; however, on-going monitoring of accelerometer wear allows the study coordinator to reach out to participants to problem solve and promote wear daily. Accelerometer readings will be assessed using 60-second epochs and established Freedson cut-points to define intensity domains: light (<1952 counts per minute (cpm)), moderate (1952–5724 cpm), and vigorous (>5725 cpm) activity [54], Number of minutes spent in MVPA in bouts of ≥10 min [55,56] and sedentary time (<100 cpm) [57] also will be applied for data analysis [54, 58,59] assessing differences within and between treatment groups over time. Non-wear will be defined by intervals of at least 60 min of zero activity counts [55]. Data collection by accelerometry corresponds temporally with biosample collections and self-reported measures of physical activity.
3.4. Bio-samples
Blood sampling for the LIVES trial is performed at baseline, 6, 12 and 24 months on all participants consenting to complete; an estimated 90% of enrolled women to date. These bio-samples (serum and plasma) are designated for use in evaluating plasma carotenoids as an indicator of fruit and vegetable intake [60], as well several mechanistic biomarkers known to be either modified by diet and/or physical activity interventions (insulin, glucose, lipids) [14,61] and/or biomarkers for which evidence exists to suggest the biomarkers may have prognostic value for ovarian cancer (IL-6, omentin) [62–65]. In addition, in a random sub-sample of women DNA is being isolated from whole blood to assess telomere length and change in telomere length by treatment group over time. All samples are to be collected in the fasting state, processed according to study protocols and shipped to the NRG centralized biobank for long-term storage using standardized procedures.
3.5. Anthropometrics
Each participant completes physical measurements in the study clinic at baseline and every 3 months. These measures are collected by trained clinic personnel using standardized methodologies [66]. These assessments include height in centimeters and weight in kilograms as measured using standardized, calibrated beam scales. Waist circumference is also measured to the nearest centimeters, under clothing, using the study-provided Gulick II measurement tape and following standardized methods for which all study personnel complete a video-directed training on the correct procedure.
3.6. Compliance score
A priori compliance score was developed for application in this lifestyle behavior trial based on prior evidence that 1) compliance to diet and physical activity interventions can vary widely for individuals enrolled in cancer survivor intervention trials and 2) compliance is likely to influence the effect size achieved between treatment group and ultimately could impact if the intervention modifies PFS as hypothesized. The compliance score is calculated based on self-report measures of intake (fat, fruit, vegetables) and physical activity (mean daily steps); each relative to the specific behavioral goals of the intervention.
4. Data analysis plan
The primary analysis for the treatment effect on PFS will be based on a Log rank test stratified by stage of disease (II and III vs. IV) and consolidation therapy (yes or no). The primary analysis will be supported by the intent to treat (ITT) patient set, including all patients enrolled on the study and their randomized treatment assignment. Multivariable Cox regression models will be used to estimate the hazard ratio and confidence interval while adjusting for baseline covariates. Similar methods will be used to explore possible time dependency of the intervention effect, and moderating factors that impact the efficacy of the intervention.
Additional exploratory analyses will consider the cumulative incidence functions for the components of the PFS endpoint across treatment arms, the effect of the intervention on changes in self-reported diet and exercise, and how these changes relate to more objective indicators of life style changes (including accelerometer readings and visceral adiposity measurements). The effects of these intervening factors on PFS can be considered using Path Analysis methods, and described using multivariable models appropriate to the endpoint.
4.1. Sample size
The NRG (GOG) has extensive clinical trial experience with women who are eligible for the LIVES study. The study was designed to have 80% power to detect a PFS hazard ratio of 0.80 or less in the intervention group (ref: control), using a Log rank test with 2-sided significance threshold of 0.05. The final analysis will be completed when 857 [67] PFS events are observed. The event target accounts for an annual loss to follow-up rate of 1%, and noncompliance rates of 5% in each group. Median PFS in the control group was estimated to be 18 months using data from previous clinical trials in similar patient populations. Assuming exponentially distributed PFS times, a 0.80 hazard ratio corresponds to a median PFS of 22.5 months in the intervention group, a clinically meaningful delay of 4.5 months.
One interim analysis for efficacy will be performed using an O’Brien-Fleming [68] group sequential boundary function, after approximately 50% of the expected events have occurred. If the null hypothesis of no intervention effect can be rejected at the 0.003 level, early termination of the study will be considered.
Participants are expected to accrue to the study for 5 years. After completion of the protocol intervention, participants are followed for an additional five years to collect disease progression and survival information. A total of 1200 participants will be accrued, of which 1070 are expected to be available for the final analysis.
5. Discussion
The LIVES trial will be the largest lifestyle intervention trial ever undertaken in ovarian cancer survivors. It builds on current more generalized clinical recommendations for diet and physical activity after a diagnosis of cancer [69] testing the role of lifestyle behavior change in modifying PFS for a highly recalcitrant disease. In order to test these approaches in a timely manner the trial is being conducted in the setting of a large cooperative group. To assure protocol fidelity, particularly in relation to the intervention, all behavior modification is delivered centrally by trained health coaches using a predominantly telephone communication approach, but one that is complemented with email and SMS. The approach, should it prove to be effective in increasing PFS in ovarian cancer survivors, is economically scalable for wider application in the survivorship setting. Notably, the trial design and protocol has been recently expanded with supplemental funding from the. Notably the trial design and protocol have been recently expanded with supplemental funding from the National Cancer Institute to include disease-relevant biomarkers. These biomarkers include metabolic (glucose and insulin), inflammatory (interleukin-6 and C-reactive protein) and a novel marker, omentin, which has been previously shown to correlate to central adiposity and ovarian cancer risk in an animal model [65]. These biomarkers will inform on the underlying mechanisms of diet and activity bioactivity in relation to disease progression and were selected based upon evidence suggesting a relationship with cancer survival and/or health effects demonstrated in relation to diet and physical activity exposures.
Similar to other large scale multi-site behavior trials, LIVES is not without challenges and limitations. Perhaps most limiting is the potential for selection bias when relatively healthy patients choose to enroll in the LIVES trial. This bias referred to as the “healthy volunteer effect”, is described in the literature [70], and has been demonstrated in trials of female cancer survivors participating in other lifestyle interventions [71]. In an effort to reduce selection bias, approved NRG clinics are encouraged to solicit all eligible patients and monthly conference calls are conducted with the Principal Investigator and study coordinator, providing an opportunity to engage and troubleshoot with clinics to identify barriers to enrollment.
Beyond the usual barriers of adopting a healthier lifestyle, ovarian cancer survivors present with a unique set of treatment related symptoms that pose additional challenges for the participant and coach to work through together. Treatment for ovarian cancer is rigorous, including extensive surgery with a minimum of 6 cycles of taxane-based chemotherapy [72]. Women frequently report chemotherapy induced neuropathy in their hands and feet, making it difficult to engage in physical activity. The incorporation of more activity is further complicated by the experience of chemo-related fatigue, as it is a frequently reported symptom. Many women undergo bowel resection and are left with temporary (reversible) or permanent ostomy. Further, resections often leave the gastrointestinal tract with scar tissue leading to irregular bowels and at times may result in obstruction. In fact one of the most common and detrimental complications of ovarian cancer treatment is bowel obstruction [73]. These side effects necessitate the role of the coach and highlight the importance of individual tailoring to support the patient in order to facilitate optimal behavior change.
Cooperative groups, such as NRG, are advantageous for conducting intervention trials in rarer cancers, such as ovarian cancer allowing for rapid and diverse enrollment into clinical trials. However, protocols take time to approve and funding is generally limited to only the clinic site for patient related study activities. This leaves researchers to identify funds for delivery of the actual intervention. The initial presentation of the study design for LIVES began in 2007, with the inaugural participant enrolled in September 2012. Preliminary funding for the delivery of the intervention originated from advocacy groups including Up the Volume, the West Valley Ovarian Cancer Alliance and the National Ovarian Cancer Coalition. The Biomarker, Imaging and Quality of Life Studies Funding Program (BIQSFP) through the National Cancer Institute provided monies for initial bio-specimen collection kits as well as carotenoid analysis. Future collaborative group efforts should focus on the sustainability of funding efforts to ensure the goals of the study are achieved.
6. Conclusion
This trial will inform on the role of diet and physical activity in ovarian cancer PFS. This study is among only a few lifestyle behavioral intervention trials to date targeting this highly lethal disease. The comprehensive measurement of intervention fidelity in relation to coaching protocols and participant changes in diet and physical activity behaviors strengthens the study design and the final interpretation of the lifestyle effect on outcomes. The inclusion of disease-specific and QoL measurements assures a comprehensive assessment of health-related effects of lifestyle after ovarian cancer treatment. Further, the collection of biological specimens in LIVES will allow for exploration into plausible mechanistic underpinnings between lifestyle, symptoms and progression of disease. LIVES represents a novel, high impact and pivotal trial to build evidence-based recommendations for modifying PFS and QoL for women completing ovarian cancer therapy.
Acknowledgments
We would like to acknowledge the support of NRG/GOG staff including Sam DiBernardo, Linda Gedeon, Marion Piedmonte, and Heather Lankes, the Gynecologic Oncology Group under which this trial was initiated. We also recognize the support and contributions of the LIVES coaches, the Behavioral Measurement Intervention Shared Resource at the University of Arizona Cancer Center (P30 CA023074) as well as Up the Volume, West Valley Ovarian Cancer Alliance, and the National Ovarian Cancer Coalition for financial donations in support of implementation of the standardized coaching program.
Footnotes
Clinical Trials Identifiers: NCT00719303; Grant number: R01CA186700-01A1.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), Gynecologic Oncology Group Statistical Office (CA37517), NRG Oncology, 1 U10CA180822 (NRG Oncology SDMC) and U10CA180868 (NRG Oncology Operations). The following Gynecologic Oncology institutions participated in this study: Roswell Park Cancer Institute, University of Alabama, Duke University Medical Center, Abington Memorial Hospital, Walter Reed National Military Medical Center, Wayne State University, University of Minnesota Medical Center-Fairview, Northwestern University, University of California at Los Angeles Health System, Fred Hutchinson Cancer Research Center, University of North Carolina at Chapel Hill, University of Iowa Hospitals and Clinics, Indiana University Hospital, University of California Medical Center at Irvine-Orange Campus, Rush University Medical Center, University of New Mexico, Cleveland Clinic Foundation, Washington University School of Medicine, Ohio State University Comprehensive Cancer Center, Women’s Cancer Center for Nevada, University of Oldahoma Health Sciences Center, University of Virginia, Yale University, University of Wisconsin Hospital and Clinics, Women and Infants Hospital, Central Connecticut, Georgia Center for Oncology Research and Education, Gynecologic Oncology of West Michigan, St. Joseph’s Hospital and Medical Center, Carolinas Medical Center/Levine Cancer Institute, and Medical College of Wisconsin. Additional support for centralized coaching services and diet and physical activity assessment is provided by the Behavioral Measurements and Interventions Shared Resource the University of Arizona Cancer Center (P30 CA023074) and biomarker assessments are funded under 1R01CA186700-01A1 (Thomson and Basen-Engquist) including staffing support by the Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institution, MD Anderson Cancer Center. Additional biomarker funding support provided under the National Cancer Institute Biomarker, Imaging, and Quality of Life Studies Funding Program (BIQSFP).
Contributor Information
Cynthia A. Thomson, Email: cthomson@email.arizona.edu.
Tracy E. Crane, Email: tecrane@email.arizona.edu.
Austin Miller, Email: amiller@gogstats.org.
David O. Garcia, Email: davidogarcia@email.arizona.edu.
Karen Basen-Engquist, Email: kbasenen@mdanderson.org.
David S. Alberts, Email: dalberts@uaccarizona.edu.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR, Seamon LG. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012;120(3):612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Cuellar-Partida G, Painter JN, Nyholt DR, Morris AP, Fasching PA, Hein A, Burghaus S, Beckmann MW, Lambrechts D, Van Nieuwenhuysen E, Vergote I, Vanderstichele A, Doherty JA, Rossing MA, Wicklund KG, Chang-Claude J, Eilber U, Rudolph A, Wang-Gohrke S, Goodman MT, Bogdanova N, Dork T, Durst M, Hillemanns P, Runnebaum IB, Antonenkova N, Butzow R, Leminen A, Nevanlinna H, Pelttari LM, Edwards RP, Kelley JL, Modugno F, Moysich KB, Ness RB, Cannioto R, Hogdall E, Jensen A, Giles GG, Bruinsma F, Kjaer SK, Hildebrandt MA, Liang D, Lu KH, Wu X, Bisogna M, Dao F, Levine DA, Cramer DW, Terry KL, Tworoger SS, Missmer S, Bjorge L, Salvesen HB, Kopperud RK, Bischof K, Aben KK, Kiemeney LA, Massuger LF, Brooks-Wilson A, Olson SH, McGuire V, Rothstein JH, Sieh W, Whittemore AS, Cook LS, Le ND, Gilks CB, Gronwald J, Jakubowska A, Lubinski J, Gawelko J, Song H, Tyrer JP, Wentzensen N, Brinton L, Trabert B, Lissowska J, McLaughlin JR, Narod SA, Phelan C, Anton-Culver H, Ziogas A, Eccles D, Gayther SA, Gentry-Maharaj A, Menon U, Ramus SJ, Wu AH, Dansonka-Mieszkowska A, Kupryjanczyk J, Timorek A, Szafron L, Cunningham JM, Fridley BL, Winham SJ, Bandera EV, Poole EM, Morgan TK, Risch HA, Goode EL, Schildkraut JM, Webb PM, Pearce CL, Berchuck A, Pharoah PD, Montgomery GW, Zondervan KT, Chenevix-Trench G, McGregor S Australian Ovarian Cancer, C. International Endogene. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum. Mol. Genet. 2015 doi: 10.1093/hmg/ddv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toss A, Tomasello C, Razzaboni E, Contu G, Grandi G, Cagnacci A, Schilder RJ, Cortesi L. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. BioMed Res. Int. 2015;2015:341723. doi: 10.1155/2015/341723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sueblinvong T, Carney ME. Current understanding of risk factors for ovarian cancer. Curr. Treat. Options in Oncol. 2009;10(1–2):67–81. doi: 10.1007/s11864-009-0108-2. [DOI] [PubMed] [Google Scholar]
- 6.Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA. Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol. Biomark. Prev. 2014;23(2):255–273. doi: 10.1158/1055-9965.EPI-13-0515. a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolecek TA, McCarthy BJ, Joslin CE, Peterson CE, Kim S, Freels SA, Davis FG. Prediagnosis food patterns are associated with length of survival from epithelial ovarian cancer. J. Am. Diet. Assoc. 2010;110(3):369–382. doi: 10.1016/j.jada.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Thomson CA, Wertheim TECBC, Neuhouser ML, Li W, Snetselaar LG, Basen-Engquist KM, Zhou Y, Irwin ML. Diet quality and survival after ovarian cancer: results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruijsen-Jaarsma M, Revesz D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc. Immunol. Rev. 2013;19:120–143. [PubMed] [Google Scholar]
- 10.McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, Chubak J, Stanczyk FZ, Bowen D, Irwin ML, Rudolph RE, Potter JD, Schwartz RS. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2004;13(7):1099–1105. a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. [PubMed] [Google Scholar]
- 11.Bertone ER, Willett WC, Rosner BA, Hunter DJ, Fuchs CS, Speizer FE, Colditz GA, Hankinson SE, Nurses’ Health S. Prospective study of recreational physical activity and ovarian cancer. Journal of the National Cancer Institute. 2001;93(12):942–948. doi: 10.1093/jnci/93.12.942. [DOI] [PubMed] [Google Scholar]
- 12.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 13.Rieck G, Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2006;20(2):227–251. doi: 10.1016/j.bpobgyn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 14.McTiernan A. Intervention studies in exercise and cancer prevention. Med. Sci. Sports Exerc. 2003;35(11):1841–1845. doi: 10.1249/01.MSS.0000093749.90499.63. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Chlebowski R, LaMonte MJ, Bea JW, Qi L, Wallace R, Lavasani S, Walsh BW, Anderson G, Vitolins M. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: Results from the Women’s Health Initiative. Gynecol. Oncol. 2014;133(1):4–10. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandura A. Social Foundations of Thought and Action: a Social Cognitive Theory. Prentice-Hall, Inc.; 1986. [Google Scholar]
- 17.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, Haan M, Hollenbach KA, Jones L, Marshall JR, Ritenbaugh C, Stefanick ML, Thomson C, Wasserman L, Natarajan L, Thomas RG, Gilpin EA. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control. Clin. Trials. 2002;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski R, Blackburn G, Elashoff R, Thomson C, Goodman M, Shapiro A, Giuliano A, Karanja N, Hoy M, Nixon D. Dietary fat reduction in postmenopausal women with primary breast cancer: phase III Women’s Intervention Nutrition Study (WINS) Proc. Annu. Meet. Am. Soc. Clin. Oncol. 2005;10 [Google Scholar]
- 19.Moyers T, Martin T, Manuel J, Miller W, Ernst D. Substance Abuse and Addictions. Albuquerque: University of New Mexico; 2010. Revised Global Scales: Motivational Interviewing Treatment Integrity 3.1. 1 (MITI 3.1. 1)Unpublished Manuscript Center on Alcoholism. [Google Scholar]
- 20.Bandura A, McClelland DC. Social learning theory. 1977 [Google Scholar]
- 21.Gil KM, von Gruenigen VE. Physical Activity and Cancer. Springer; 2010. Physical activity and gynecologic cancer survivorship; pp. 305–315. [DOI] [PubMed] [Google Scholar]
- 22.Midtgaard J. Theoretical and practical outline of the Copenhagen PACT narrative-based exercise counselling manual to promote physical activity in post-therapy cancer survivors. Acta Oncol. 2013;52(2):303–309. doi: 10.3109/0284186X.2012.742206. [DOI] [PubMed] [Google Scholar]
- 23.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol. Monogr. Gen. Appl. 1966;80(1):1. [PubMed] [Google Scholar]
- 24.Ajzen I. The theory of planned behavior. Org. Behav. Hum. Process. 1991;50:179–211. [Google Scholar]
- 25.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J. Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 26.Nagle CM, Purdie DM, Webb PM, Green A, Harvey PW, Bain CJ. Dietary influences on survival after ovarian cancer. Int. J. Cancer. 2003;106(2):264–269. doi: 10.1002/ijc.11204. [DOI] [PubMed] [Google Scholar]
- 27.Paxton RJ, Garcia-Prieto C, Berglund M, Hernandez M, Hajek RA, Handy B, Brown J, Jones LA. A randomized parallel-group dietary study for stages II–IV ovarian cancer survivors. Gynecol. Oncol. 2012;124(3):410–416. doi: 10.1016/j.ygyno.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Q, Miller EG, Ahmad H, Tang L, Patil BS. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr. Cancer. 2001;40(2):180–184. doi: 10.1207/S15327914NC402_15. [DOI] [PubMed] [Google Scholar]
- 29.el SAA, Zhu Q, Barakat BM, Wani G, Zhao Q, El-Mahdy MA, Wani AA. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through down-regulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009;69(23):8910–8917. doi: 10.1158/0008-5472.CAN-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edefonti V, Decarli A, La Vecchia C, Bosetti C, Randi G, Franceschi S, Dal Maso L, Ferraroni M. Nutrient dietary patterns and the risk of breast and ovarian cancers. Int. J. Cancer. 2008;122(3):609–613. doi: 10.1002/ijc.23064. [DOI] [PubMed] [Google Scholar]
- 31.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, Pettinger M, Lane DS, Lessin L, Yasmeen S, Singh B, Khandekar J, Shikany JM, Satterfield S, Chlebowski RT. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2007;99(20):1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolahdooz F, Ibiebele TI, van der Pols JC, Webb PM. Dietary patterns and ovarian cancer risk. Am. J. Clin. Nutr. 2009;89(1):297–304. doi: 10.3945/ajcn.2008.26575. [DOI] [PubMed] [Google Scholar]
- 33.Edefonti V, Randi G, Decarli A, La Vecchia C, Bosetti C, Franceschi S, Dal Maso L, Ferraroni M. Clustering dietary habits and the risk of breast and ovarian cancers. Ann. Oncol: Off. J. Eur. Soc. Med. Oncol./ESMO. 2009;20(3):581–590. doi: 10.1093/annonc/mdn594. [DOI] [PubMed] [Google Scholar]
- 34.Stevinson C, Capstick V, Schepansky A, Tonkin K, Vallance JK, Ladha AB, Steed H, Faught W, Courneya KS. Physical activity preferences of ovarian cancer survivors. Psychooncology. 2009;18(4):422–428. doi: 10.1002/pon.1396. [DOI] [PubMed] [Google Scholar]
- 35.Cust AE. Physical activity and gynecologic cancer prevention. Recent Results Cancer Res. 2011;186:159–185. doi: 10.1007/978-3-642-04231-7_7. [DOI] [PubMed] [Google Scholar]
- 36.Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928–1935. doi: 10.1002/cncr.28028. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Xie X, Lee AH, Binns CW. Sedentary behaviours and epithelial ovarian cancer risk. Cancer Causes Control. 2004;15(1):83–89. doi: 10.1023/B:CACO.0000016633.47025.2a. [DOI] [PubMed] [Google Scholar]
- 38.Zheng W, Shu XO, McLaughlin JK, Chow WH, Gao YT, Blot WJ. Occupational physical activity and the incidence of cancer of the breast, corpus uteri, and ovary in Shanghai. Cancer. 1993;71(11):3620–3624. doi: 10.1002/1097-0142(19930601)71:11<3620::aid-cncr2820711125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 39.Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25(17):2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 40.Harris T, Kerry SM, Victor CR, Shah SM, Iliffe S, Ussher M, Ekelund U, Fox-Rushby J, Whincup P, David L, Brewin D, Ibison J, DeWilde S, Limb E, Anokye N, Furness C, Howard E, Dale R, Cook DG. PACE-UP (pedometer and consultation evaluation–UP)–a pedometer-based walking intervention with and without practice nurse support in primary care patients aged 45–75 years: study protocol for a randomised controlled trial. Trials. 2013;14:418. doi: 10.1186/1745-6215-14-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. M. American College of Sports, American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 42.Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 43.Wiklund I, Fullerton S, Hawkey C, Jones R, Longstreth G, Mayer E, Peacock R, Wilson I, Naesdal J. An irritable bowel syndrome-specific symptom questionnaire: development and validation. 2009 doi: 10.1080/00365520310004209. [DOI] [PubMed] [Google Scholar]
- 44.Martínez ME, Marshall JR, Graver E, Whitacre RC, Woolf K, Ritenbaugh C, Alberts DS. Reliability and validity of a self-administered food frequency questionnaire in a chemoprevention trial of adenoma recurrence. Cancer Epidemiol. Biomark. Prev. 1999;8(10):941–946. [PubMed] [Google Scholar]
- 45.Thomson CA, Giuliano A, Rock CL, Ritenbaugh CK, Flatt SW, Faerber S, Newman V, Caan B, Graver E, Hartz V. Measuring dietary change in a diet intervention trial: comparing food frequency questionnaire and dietary recalls. Am. J. Epidemiol. 2003;157(8):754–762. doi: 10.1093/aje/kwg025. [DOI] [PubMed] [Google Scholar]
- 46.Neuhouser ML, Di C, Tinker LF, Thomson C, Sternfeld B, Mossavar-Rahmani Y, Stefanick ML, Sims S, Curb JD, Lamonte M, Seguin R, Johnson KC, Prentice RL. Physical activity assessment: biomarkers and self-report of activity-related energy expenditure in the WHI. Am. J. Epidemiol. 2013;177(6):576–585. doi: 10.1093/aje/kws269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 48.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Clark BK, Winkler E, Healy GN, Gardiner PG, Dunstan DW, Owen N, Reeves MM. Adults’ past-day recall of sedentary time: reliability, validity, and responsiveness. Med. Sci. Sports Exerc. 2013;45(6):1198–1207. doi: 10.1249/MSS.0b013e3182837f57. [DOI] [PubMed] [Google Scholar]
- 50.Rogers LQ. Objective monitoring of physical activity after a cancer diagnosis: challenges and opportunities for enhancing cancer control. Phys. Ther. Rev. 2010;15(3):224–237. doi: 10.1179/174328810X12814016178872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broderick JM, Ryan J, O’Donnell DM, Hussey J. A guide to assessing physical activity using accelerometry in cancer patients. Support. Care Cancer: Off. J. Multinatl. Assoc. Support. Care Cancer. 2014 doi: 10.1007/s00520-013-2102-2. [DOI] [PubMed] [Google Scholar]
- 52.Shirona EJ, Cook NR, Manson JE, Buring JE, Rimm EB, Lee IM. Comparison of self-reported and accelerometer-assessed physical activity in older women. PLOS One. 2015;10(12):e0145950. doi: 10.1371/journal.pone.0145950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tudor-Locke C. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev. Chronic Dis. 2012;9 doi: 10.5888/pcd9.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedson PS, Melanson EL, Sirard JR. Calibration of the Computer Science and Applications, Inc.-.accelerometer. Med. Sci. Sports Exerc. 1997;30(5) doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 56.Jovanovic J, Baum G, Hughes DC, McFalls KN, Carmack Taylor C, Basen-Engquist K. Use of actigraphs and self reports to measure bouts and intensity of exercise in cancer survivors. Med. Sci. Sports Exerc. 2007;39(5):S62. [Google Scholar]
- 57.Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA, Inc. accelerometer hip and wrist sites. Med. Sci. Sports Exerc. 2000;32(Suppl):S450–S456. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]
- 59.Hendelman D, Miller K, Bagget C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med. Sci. Sports Exerc. 2000;32(Suppl):S442–S449. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 60.Rock CL, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Newman VA, Hollenbach KA, Jones L, Caan BJ, Pierce JP. Plasma carotenoids and recurrence-free survival in women with a history of breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23(27):6631–6638. doi: 10.1200/JCO.2005.19.505. [DOI] [PubMed] [Google Scholar]
- 61.Torng PL, Lee YC, Huang CY, Ye JH, Lin YS, Chu YW, Huang SC, Cohen P, Wu CW, Lin CT. Insulin-like growth factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis suppressor in ovarian endometrioid carcinoma. Oncogene. 2008;27(15):2137–2147. doi: 10.1038/sj.onc.1210864. [DOI] [PubMed] [Google Scholar]
- 62.Dijkgraaf EM, Welters MJ, Nortier JW, van der Burg SH, Kroep JR. Interleukin-6/ interleukin-6 receptor pathway as a new therapy target in epithelial ovarian cancer. Curr. Pharm. Des. 2012;18(25):3816–3827. doi: 10.2174/138161212802002797. [DOI] [PubMed] [Google Scholar]
- 63.Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML, Wei LH. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71(2):424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 64.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012;38(7):904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, Woodward W, Schmandt R, Broaddus R, Lu K, Kolonin MG. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18(3):771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frisasncho AR. Anthropometric Standards an Interactive Nutritional Reference of Body Size and Body Composition for Children and Adults. Ann Arbor, MI: The University of Michigan Press; 2008. [Google Scholar]
- 67.Rubinstein LV, Gail MH, Santner TJ. Planning the duration of a comparative clinical trial with loss to follow-up and a period of continued observation. J. Chronic Dis. 1981;34(9–10):469–479. doi: 10.1016/0021-9681(81)90007-2. [DOI] [PubMed] [Google Scholar]
- 68.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 69.Nutrition and physical activity guidelines for cancer survivors. CA: a cancer journal for clinicians. 2012;62(4):275–276. doi: 10.3322/caac.21146. [DOI] [PubMed] [Google Scholar]
- 70.Thomson CA, Harris RB, Craft NE, Hakim IA. A cross-sectional analysis demonstrated the healthy volunteer effect in smokers. J. Clin. Epidemiol. 2005;58(4):378–382. doi: 10.1016/j.jclinepi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 71.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, Faerber S, Gold EB, Hajek RA, Hollenbach K, Jones LA, Karanja N, Madlensky L, Marshall J, Newman VA, Ritenbaugh C, Thomson CA, Wasserman L, Stefanick ML. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374(9698):1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 73.Tunca JC, Buchler DA, Mack EA, Ruzicka FF, Crowley JJ, Carr WF. The management of ovarian-cancer-caused bowel obstruction. Gynecol. Oncol. 1981;12(2):186–192. doi: 10.1016/0090-8258(81)90148-7. [DOI] [PubMed] [Google Scholar]