Abstract
Diurnal salivary cortisol profiles are valuable indicators of adrenocortical functioning in epidemiological research and clinical practice. However, normative reference values derived from a large number of participants and across a wide age range are still missing.
To fill this gap, data were compiled from 15 independently conducted field studies with a total of 104,623 salivary cortisol samples obtained from 18,698 unselected individuals (mean age: 48.3 years, age range: 0.5 to 98.5 years, 39% females). Besides providing a descriptive analysis of the complete dataset, we also performed mixed-effects growth curve modeling of diurnal salivary cortisol (i.e., 1 to 16 hours after awakening). Cortisol decreased significantly across the day and was influenced by both, age and sex. Intriguingly, we also found a pronounced impact of sampling season with elevated diurnal cortisol in spring and decreased levels in autumn. However, the majority of variance was accounted for by between-participant and between-study variance components. Based on these analyses, reference ranges (LC/MS-MS calibrated) for cortisol concentrations in saliva were derived for different times across the day, with more specific reference ranges generated for males and females in different age categories. This integrative summary provides important reference values on salivary cortisol to aid basic scientists and clinicians in interpreting deviations from the normal diurnal cycle.
Keywords: cortisol, saliva, reference value, circadian rhythm, lifespan
1. Introduction
Characterizing diurnal cortisol profiles is a hallmark of research into the regulation of the pituitary-adrenal axis and both, deviations in absolute hormone levels and in their circadian rhythm have been associated with many physiological diseases and psychiatric disorders (e.g. Carrion et al., 2002; Sephton et al., 2000). Accordingly, cortisol secretion has been viewed as an important mediator of downstream biological dysfunction and adverse health outcomes in the long term (see Chrousos, 2009, for review) that is responsive to a large variety of sociodemographic or lifestyle (i.e. environmental exposure) factors.
One approach to investigate the responsiveness to such factors is to compare the data of selected participants cohorts (e.g. high stress occupations) with reference values that have been derived from demographically similar but unselected participant samples (cf. Grimes and Schulz, 2002). The foundations for the successful applicability of such epidemiological designs have been laid during the past decades by (A) the ability to measure steroid hormones in saliva, providing a non-invasive and easy means to determine the biological active cortisol fraction while participants engaged in their normal daily activities (Riad-Fahmy et al., 1982, Hellhammer et al., 2009), and by (B) the accomplishment of many small- and large-scale studies, which sampled diurnal salivary cortisol from hundreds up to several thousands of participants (e.g. Whitehall II, Badrick et al., 2007; MIDUS, Love et al., 2010; TRIALS, Oldehinkel et al., 2015; see also Adam and Kumari, 2009). Despite the ensuing availability of reference values for diurnal salivary cortisol (Aardal and Holm, 1995; Hansen et al., 2003, Törnhage, 2002, Wust et al., 2000), many researchers are still reluctant to inform their designs by these data.
One reason for this is that many of the biochemical assays routinely used to quantify cortisol levels in saliva were originally developed for other biological matrices such as blood serum/plasma or urine. These assays were often adopted for saliva analysis without any further adjustments, which entails large between-assay variability and the overestimation of salivary cortisol at low concentrations as compared to unbiased mass spectrometric methods (Miller et al., 2013; Bae et al., 2016). These measurement peculiarities become particularly problematic for research designs that lack internal control groups and would therefore need to rely on reference values that have been derived using incompatible assays. Accordingly, the salivary cortisol reference values available to date (Aardal and Holm, 1995; Hansen et al., 2003, Törnhage, 2002, Wust et al., 2000) were derived using different immunoassays and can therefore hardly serve as universally applicable data sources for such purposes. Finally, these reference values are constrained to specific populations (e.g., children from 7 to 15 years of age; Törnhage, 2002) and sampling times (e.g., only the post-awakening period; Wust et al., 2000), or suffer from a limited precision due to small sample sizes (e.g., 7 ≤ N ≤ 37 participants per age/sex cohort; Aardal and Holm, 1995).
In order to overcome these issues, we generated an exhaustive dataset from which we derived unbiased, precise and detailed reference ranges. Salivary cortisol values were combined from 15 independent field studies conducted in a number of different countries. The resulting dataset is referred to by the acronym CIRCORT because it enables a holistic characterization of circadian cortisol changes in unselected populations. This dataset is comprised of cortisol data from 18,698 individuals across a broad age range (0.5 to 98.5 years), including 104,623 saliva specimens across 26,134 days. Apart from a first descriptive presentation of the CIRCORT dataset, the main aim of this article is to provide reference ranges for daytime salivary cortisol for both sexes across the lifespan, as well as to investigate a potential role of circannual changes.
2. Methods
2.1. Study populations
The CIRCORT database was a convenience sample comprised of (published and unpublished) population-based and specific target group field studies conducted predominantly in North America and Europe (see Table 1). One investigator (C.K.) contacted researchers involved in these field studies and obtained approval to utilize the data on salivary cortisol secretion. Inclusion criteria were the availability of information on awakening time, records of exact sampling times (i.e., via self-report or objective determination), and demographic information on participant age and sex. Further inclusion criteria were the provision of appropriate participant instructions to maximize protocol adherence (including avoidance of smoking, meals or brushing of teeth prior to sampling) and saliva collection with purpose-designed collection vials (e.g., Salivette or IBL tubes). As most of the submitted cortisol data were determined by the DELFIA-assay (Dressendörfer et al., 1992) at the University of Trier, or the IBL chemiluminescence assay (IBL International, Hamburg, Germany) at the University of Dresden, the CIRCORT database was restricted to studies using these two analytical methods. The schedule of collection across the day varied across studies from a maximum of four samples during the first hour after awakening to studies that sampled every two hours and then after stressful events. Table 1 provides more specific details on the sampling regimens for the included studies. About half of the samples in the database were obtained from 5 representative, population-based studies (e.g., British Birth Cohort, Rotterdam Study, MIDUS or KORA; see Table 1 for more information), while the remaining were either representative studies of specific professional settings (e.g., Whitehall II) or were convenience samples of defined populations (e.g. school children). Thus, the dataset included healthy participants as well as participants suffering from the various psychological disorders and physiological diseases that are an essential feature of such populations. The vast majority of participants were Europeans or Americans of European descent. All included studies were conducted in accordance with the Declaration of Helsinki and obtained written informed consent from all participants.
Table 1.
Characteristics of the 15 studies contributing to the complete CIRCORT dataset.
| Study ID |
Population | Country | No. of participants (days per participant) |
Age in years (mean, range) |
% Male |
Mean time of awakening |
Samples (n) |
Assay | Sampling scheme | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Children | USA | 111 (1.9) | 3.8 (3.2–4.5) | 45% | 07:36 | 804 | DELFIA | AW30, 11, 16, BT | – |
| 2 | Children | USA | 170 (1.9) | 4.3 (0.5–7) | 52% | 07:57 | 1.222 | DELFIA | AW, 10, 16, BT | – |
| 3 | School children | Netherlands | 96 (1.9) | 9.1 (8.9–11.4) | 53% | 07:01 | 830 | IBL | AW, AW15, AW30, AW45, 12:00 |
– |
| 4 | Children. population sample | Netherlands | 1.758 (1.0) | 11.1 (10–12) | 49% | 07:00 | 5.119 | IBL | AW, AW30, 20:00 | TRAILS (Rosmalen et al., 2005) |
| 5 | School children | Netherlands | 160 (1.8) | 12 (12–12) | 49% | 07:27 | 1.022 | DELFIA | AW, AW15, AW30, AW45, 12:00, 20:00 |
NTR (Bartels et al., 2003) |
| 6 | Adolescents | Netherlands | 326 (2.9) | 17.1 (16.1–18.3) | 27% | 06:49 | 5.256 | IBL | AW, AW30, 9:30, 15:00, 19:00, 22:00 |
NTR (Kupper et al., 2005) |
| 7 | Adolescents | Netherlands | 159 (1.8) | 18.2 (17.7–19.0) | 45% | 07:45 | 1.383 | IBL | AW, AW15, AW30, AW45, 12:00 |
– |
| 8 | Students | Switzerland | 152 (3.9) | 27.2 (22–48) | 66% | 07:01 | 2.666 | DELFIA | AW, AW30, 17:00, 18:00, 18:30 |
– |
| 9 | Adults. industrial employees | Germany | 731 (3.7) | 41.7 (16–63) | 90% | 05:57 | 17.774 | IBL | AW, AW30, 8:00, 11:00, 15:00, 20:00, S15, S45 |
– |
| 10 | Adults. birth cohort | UK | 6.081 (1.0) | 45.2 (44.3–47.6) | 48% | 07:21 | 11.857 | IBL | AW45, 11:15 | British Birth Cohort (Power et al., 2008) |
| 11 | Adults. industrial employees | Germany | 539 (1.9) | 45.4 (20–71) | 88% | 05:22 | 3.276 | IBL | 11:00, 13:00, 18:00, 22:00, S15 / AW, AW30, 8:00 |
– |
| 12 | Adults. nationwide | USA | 1.138 (3.9) | 57.6 (33–84) | 45% | 06:39 | 16.980 | IBL | AW, AW30, 12:00, BT | MIDUS (Karlamangla et al., 2013) |
| 13 | Adults | Germany | 1.128 (1.0) | 60.1 (49–72) | 48% | 07:42 | 4.244 | IBL | AW, AW30, 12:00, BT | KORA (Lederbogen et al., 2010) |
| 14 | Adults. civil servants | UK | 4.148 (1.0) | 61.1 (50.5–73.9) | 73% | 06:43 | 24.454 | IBL | AW, AW30, 9:30, 15:00, 19:00, 22:45 |
Whitehall II (Badrick et al., 2007) |
| 15 | Retirees | Netherlands | 2.001 (1.0) | 75.0 (65.2–98.5) | 43% | 07:33 | 7.766 | IBL | AW, AW30, 17:00, BT | Rotterdam Study (Hofman et al., 2007) |
| Total | 18.698 (1.4) | 48.3 (8.9–98.5) | 61% | 06:47 | 104.623 | |||||
Note. Abbreviations in the sampling scheme column: AW = awakening sample. AW15-AW45 = 15. 30 or 45 min after awakening. BT = bedtime. S15 = 15 after stressor onset. S45 = 45 minutes after stressor onset. BS = beginning of working shift. numbers indicate the intended sampling time.
2.2. Data management
All study data were merged into a single large dataset (with long format; see Lopez-Duran et al., 2014). For each participant, we calculated the time between the self-reported awakening time and the provided sampling times as absolute hours in decimals (e.g., 20:45 = 20.75 h). Cortisol concentrations were converted to the same scale corresponding approximately to values in nmol/l as calibrated by LC-MS/MS following our previous recommendations (Miller et al., 2013). Notably, this conversion procedure was informed by assay data from the same laboratories that conducted the biochemical saliva analyses for each of the contributing studies.
The initial data of 107,955 samples were refined by excluding samples from study days with missing information on the awakening time (n = 418), samples with missing collection times (n = 22), days when awakening occurred later than 12.00 h (n = 170 samples), and days with collection times prior to awakening (n = 2,709 samples). Thus, 3.07% of the samples were removed by data cleaning. Furthermore, we excluded samples with implausibly high concentrations above 60 nmol/l (n = 13, 0.01%; see also Miller et al., 2013), whereas cortisol concentrations below a lower limit of quantification LOQ = 0.15 nmol/l (n = 325, 0.30%) were imputed by random samples from a uniform distribution U(0.05, 0.15).
2.3. Statistical analysis
Data preparation and analyses were carried out using the packages plyr (Wickham, 2011) and lme4 (Bates et al., 2014) with R 3.2.2 statistical software (R Core Team, 2015). Two separate types of analyses were performed: The descriptive analysis served to present the complete CIRCORT dataset, whereas the regression analyses served to derive reference values for diurnal cortisol from a subset of these data.
2.3.1. Descriptive analysis
The cortisol concentrations scaled in nmol/l (LC/MS-MS) of the complete CIRCORT dataset served as the primary outcome variable. The descriptive analyses of diurnal cortisol profiles are based on the cortisol distribution characteristics (i.e. the median, the quartiles, and the 10th and 90th percentiles) during the following time periods: Post-awakening time periods were 0 to 15 min, 15 to 30 min, 30 to 45 min, and 45 min to 1 hour after awakening. Thereafter, diurnal time periods were calculated as hourly periods from 1 until 12 hours post-awakening and as two-hourly periods until 20 hours after awakening.
2.3.2. Regression analysis and derivation of reference values
In order to tabulate the reference ranges for diurnal cortisol that accounted for the asymmetry regarding different covariates, we adopted and extended a mixed-effects growth curve model of diurnal cortisol from Hruschka et al. (2005). The concentration-dependency of measurement error was accounted for by using log-transformed cortisol as dependent variable (Miller and Plessow, 2013). The reference model is manifested by formulae 1–3, where the cortisol concentration at the ith measurement occasion, on the dth day, from the pth participant, of the sth study is regressed on time since awakening (Time and Time2, scaled in 6 hours) and awakening time (TimeAw, centered at 07.00 h of day).
| 1) |
| 2) |
| 3) |
Mathematically, this model specification implies a multiplicative impact of the predictors. Thus, the back-transformed intercept exp(β0) reflects an estimate of participants’ median post-awakening cortisol level scaled in nmol/l, whereas the slope term exp(β1 + β2) represents the scaling factor for exp(β0) to estimate the median amount of cortisol after a time period of 6 hours has elapsed. To account for random variation in β0 and β1, variance components being attributable to between-study (ξ0s), between-participant (ξ0p and ξ1p), and between-day (ξ0d) differences were also estimated. Notably, we refrained from modeling the cortisol awakening response (CAR) because an accurate assessment of this distinct aspect of diurnal cortisol secretion requires additional means of data control not undertaken in most of the included studies (i.e., objective verification of awakening and sampling times; Stalder et al., 2016). Hence, we excluded all samples and cortisol values obtained within 30 minutes after awakening (see Hruschka et al., 2005) from the regression analyses.
Proceeding from this baseline model, we evaluated if the potential predictors – sex (0 = female, 1 = male) and age – incrementally explained variance by using likelihood ratio tests. Age was stratified and reverse Helmert coded (i.e. each age stratum is contrasted to the average to the preceding strata). The first two strata included all participants younger than 5 years, and those aged 5–10 years, respectively. Thereafter, age was stratified by 10-year intervals until 80 years. A final stratum included all participants who were older than 80 years (up to 98 years). Moreover, the effect of sampling at different times of year, season (spring = March – May, summer = June – August, autumn = September – November, winter = December – February), was also investigated for all studies that provided the required information (ID: 4, 7, 9–11,13). Proceeding from these models, we tabulated reference ranges for the diurnal time periods (listed in section 2.3.1.) in males and females across all age strata. This method for constructing reference ranges essentially corresponds to a discretized, multilevel version of the polynomial regression approach proposed by Royston (1991).
3. Results
3.1. Descriptive analyses of post-awakening and diurnal cortisol profiles
The complete CIRCORT dataset was comprised of 104,638 samples obtained from 18,698 individuals during 26,134 days. Study sizes varied between 830 samples in a two-day study on 96 children and 24,454 samples from 4,148 participants in the Whitehall II study. On average, participants provided 4.0 (± 1.66) samples per observation day. Cortisol data across multiple days (range 2 – 4) were obtained from 3,403 participants (two days: 1,127; three days: 519; four days: 1,757). 39.0% of all samples were collected from female participants. The mean age of participants was 48.27 years (±19.22), ranging from 0.5 to 98.5 years. Awakening time varied considerably around a median of 06.92 h of day (IQR: 06.08 h – 07.50 h), with 90% of awakenings occurring between 05.00 h and 09.00 h. Notably, the German participants reported the earliest mean awakening time at 06.12 h of day, whereas the Dutch participants reported a comparably late awakening at 07.23 h. Awakening times of the other countries was reported to be in between these two extremes (USA – 06.78 h, United Kingdom – 06.93 h, Switzerland – 07.02 h). Table 1 lists these sample characteristics for each of the 15 studies.
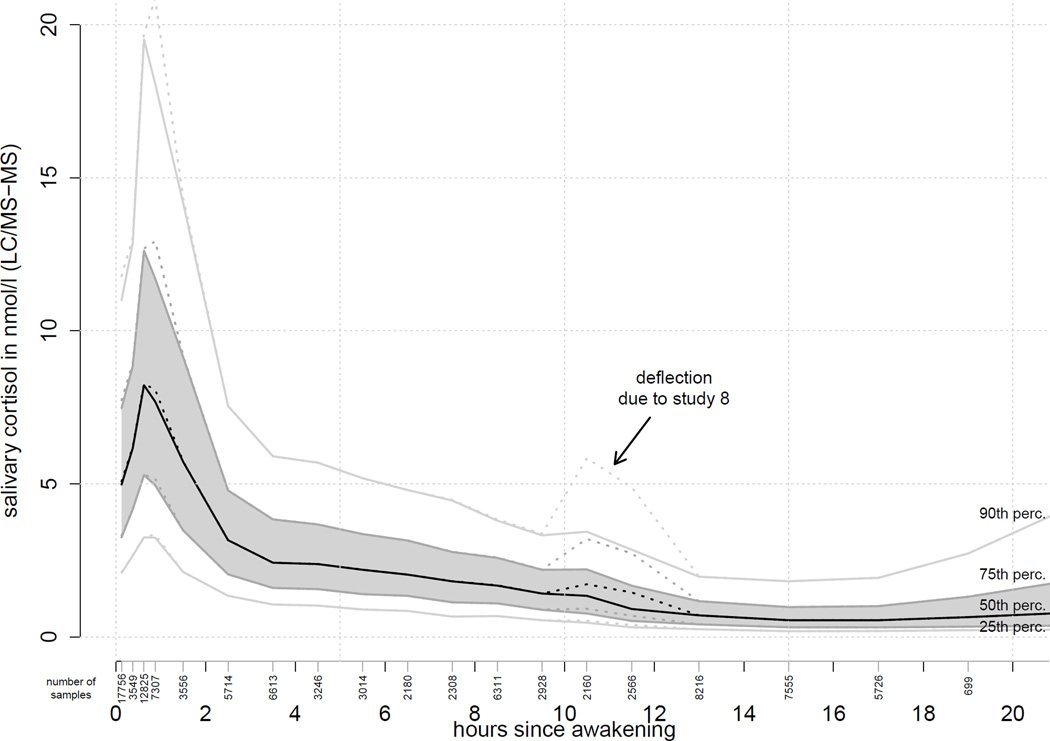
Figure 1 shows the median cortisol, quartiles and 90th percentile up to 20 hours after awakening. The CIRCORT dataset documented a clear diurnal rhythm, with a pronounced cortisol increase in the first 0.5 h of the post-awakening period (see Figure 1). Subsequently, the cortisol concentrations decreased throughout the day, but tended to rise again as the circadian phase (24 hours) was completed. The concentration nadir was approximately found at approximately 16.00 h of day. When all data were combined, there was an indication of a transient increase at 10–12 hours after awakening. However, more in-depth analyses revealed that this inflection was driven by a single study (ID: 8), and is therefore unlikely to be a general feature of diurnal cortisol profiles.
Figure 1.
Cortisol levels from the complete CIRCORT dataset, showing the median and the 10th, 25th, 75th and 90th percentiles. Dashed lines show an atypical increment at 11 hours after awakening, which was due to a single study (#8) and not representative. Vertically oriented numbers below the x-axis denote the amount of samples informing each time stratum.
3.2. Circa- and infradian variability of diurnal cortisol profiles
After exclusion of all cortisol data which were obtained during the first 30 min after awakening, the dataset was comprised of N = 83,306 samples. Table 2 lists the results of the mixed-effects growth curve models analyzing the diurnal cortisol profiles with respect to the potential impact of age and sex, while accounting for study-, participant- and daytime-specific variance. Proceeding from a median post-awakening concentration of 8.1 nmol/l (LC/MS-MS), salivary cortisol decreased throughout the day to ~24.4% after 6 hours had elapsed since awakening, and to ~9.6% after 12 hours had elapsed since awakening. As indicated by the quadratic effect of time (Χ2(1)= 4026.3, p < .001), salivary cortisol decreased slower in the late circadian phase, and tended to increase again thereafter. Later awakening times were consistently associated with lower cortisol levels (9.3% ± 0.3% decrease per hour of later awakening; Χ2(1)= 912.2, p < .001). Moreover, higher cortisol was associated with a more accentuated cortisol decrease across the day (r = −0.39). In all models, the majority of variance was attributed to between-study variability, which is not surprising as each study recruited participants with different demographic characteristics. The conclusions were not affected by the in- or exclusion of study 8 (cf. Figure 1), which resulted in a reduction of the study-specific variance component by 63.5%1 when omitted. In sum, the fixed and random effects of the baseline model explained R2 = 62.7% of the total cortisol variance.
Table 2.
Mixed effects growth curve models of diurnal salivary cortisol
| Model | (1) Time + Awakening |
(2) … + Age + Sex |
(3) … + Season |
|---|---|---|---|
| No. of samples / studies | 83.306 / 15 | 83.306 / 15 | 28.667 / 6 |
| Predictors | |||
| Intercept (β0) | 2.091 (0.105) | 2.099 (0.095) | 2.140 (0.214) |
| Time since awakening (β1, per 6 hours) | −1.647 (0.011) | −1.648 (0.011) | −1.440 (0.017) |
| Time since awakening2 (β2) | 0.238 (0.004) | 0.238 (0.004) | 0.174 (0.006) |
| Time of awakening (β3, per hour after 7:00) | −0.098 (0.003) | −0.099 (0.003) | −0.105 (0.006) |
| Male sex (β0) | 0.031 (0.009) | 0.047 (0.017) | |
| Age > 5 years | −0.056 (0.039) | – | |
| Age > 10 years | 0.131 (0.073) | – | |
| Age > 20 years | 0.080 (0.038) | 0.048 (0.044) | |
| Age > 30 years | 0.029 (0.023) | −0.012 (0.017) | |
| Age > 40 years | 0.015 (0.015) | −0.009 (0.010) | |
| Age > 50 years | 0.022 (0.011) | 0.001 (0.007) | |
| Age > 60 years | 0.021 (0.008) | 0.007 (0.007) | |
| Age > 70 years | 0.025 (0.007) | 0.004 (0.007) | |
| Age > 80 years | 0.029 (0.006) | 0.014 (0.006) | |
| Season: Spring | Reference | ||
| Season: Summer | −0.052 (0.023) | ||
| Season: Autumn | −0.111 (0.025) | ||
| Season: Winter | −0.007 (0.025) | ||
| Variance components | |||
| Days (ξd) | 0.034 | 0.034 | 0.034 |
| Participants (ξ0p + ξ1p) | 0.130 + 0.063 | 0.130 + 0.063 | 0.190 + 0.064 |
| Studies (ξs) | 0.163 | 0.130 | 0.248 |
| Residuals (εidps) | 0.463 | 0.463 | 0.436 |
Note. As regressions were performed on log-scaled cortisol concentrations, all of the above listed predictors multiplicatively impact cortisol on its natural nmol/l scale. Bold font indicates fixed effects, that fall below a significance threshold of α = 0.05. Values in parenthesis indicate standard errors.
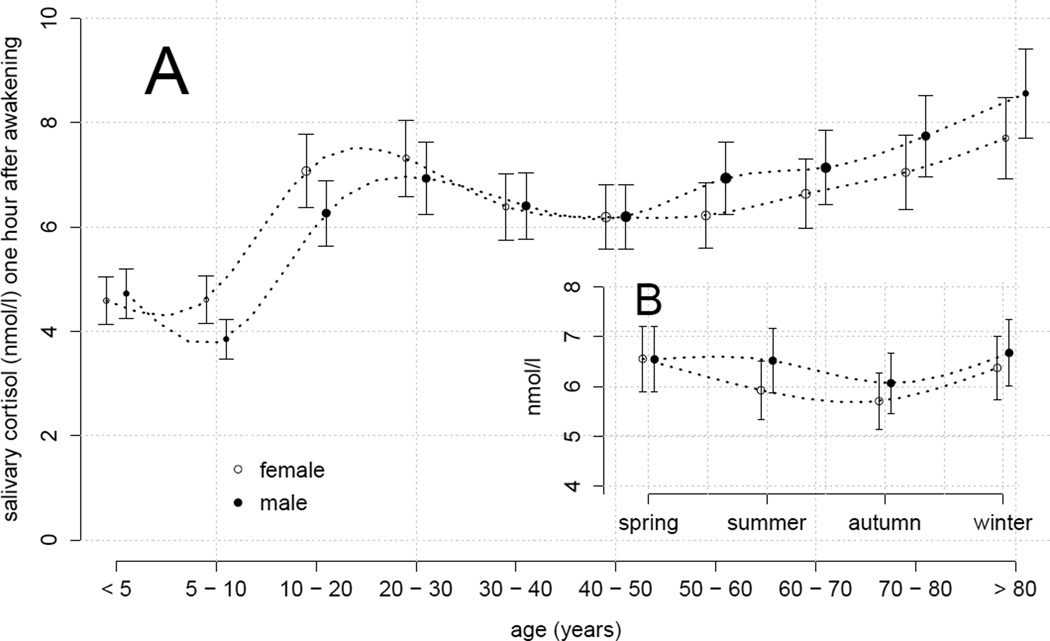
Participant age accounted for a significant amount of the variation in diurnal cortisol levels (χ2(9) = 86.8, p < .001). Saliva cortisol concentrations in infants were significantly lower as compared to adults (i.e., ~71% of the lifespan average cortisol levels)2. After the transition to adolescence, cortisol levels remained relatively stable until late adulthood, but significantly increased thereafter during retirement age (approximately 2% per decade, resulting in ~18% higher cortisol in 70–80 year olds as compared to the lifespan average). In addition, males were found to display about 103% (±1%) of the diurnal salivary cortisol in women (χ2(1) = 12.3, p < .001). Although this isolated effect of sex was relatively small, a consideration of the interactions between sex and the age strata revealed some interesting differential changes across the life span (χ2(11) = 72.4, p < .001). Specifically, 5–20 year old males had slightly lower diurnal salivary cortisol than females of the same age. Moreover, we discovered a significant dissociation between males and females during late adulthood (i.e., >50 years) with females showing the above reported cortisol increase due to ageing with a delay of 10 years as compared to males (see Table 2 and Figure 2A).
Figure 2.
Effect plots based on mixed-effects modeling of the CIRCORT dataset, which show the predicted median cortisol levels (±10%) at 1 hour after awakening. The point size represents the relative amount of data in the respective factor level. (A) Median salivary cortisol 1 hour after awakening (nmol/l, LC-MS/MS) for males and females across age groups. (B) Sex-dependent impact of season on median salivary cortisol 1 hour after awakening as predicted in 30–40 year olds.
The CIRCORT dataset also permitted an analysis of the possible influence of time of year. To investigate seasonal changes in cortisol, we fitted the mixed-effects model without sex by age interactions to a data subset comprising all 6 studies that provided information on the collection dates (N = 28,667; see methods section). Intriguingly, we found evidence of significant seasonal changes (χ2(3) = 23.3, p < .001) with cortisol decreasing from spring to autumn by 10.5% (± 2.2%) and then increasing again. Moreover, a trend for a sex by season interaction indicated ~10% higher diurnal salivary cortisol in males (as compared to females) during summer time (see Figure 2B). However, the corresponding likelihood ratio test failed to reach the statistical significance threshold (χ2(3) = 5.6, p = .13).
3.3. Age- and sex-specific reference values for diurnal cortisol
To generate reference values for unselected males and females across the life span, we determined the 5th, 50th (i.e., the median), and 95th percentiles for saliva cortisol concentrations at different times of day. They are based on the fully parameterized model (including the age, sex, and their interactions as covariates) with awakening time fixed at 07.00 h. The median and concentration ranges for diurnal cortisol are shown in Table 3.
Table 3.
Percentiles of diurnal salivary cortisol concentrations at various hours after awakening at 7:00.
| Age | Sex | 1 hour | 3.5 hours | 6 hours | 8.5 hours | 11 hours | 13.5 hours | 16 hours | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | 5th | 50th | 95th | ||
| < 5 | F | 1.3 | 4.7 | 16.7 | 0.7 | 2.6 | 9.1 | 0.4 | 1.5 | 5.4 | 0.3 | 1.0 | 3.5 | 0.2 | 0.7 | 2.4 | 0.1 | 0.5 | 1.9 | 0.1 | 0.4 | 1.6 |
| < 5 | M | 1.4 | 4.8 | 17.2 | 0.7 | 2.6 | 9.4 | 0.4 | 1.6 | 5.5 | 0.3 | 1.0 | 3.6 | 0.2 | 0.7 | 2.5 | 0.2 | 0.5 | 1.9 | 0.1 | 0.5 | 1.6 |
| 5 – 10 | F | 1.3 | 4.7 | 16.8 | 0.7 | 2.6 | 9.1 | 0.4 | 1.5 | 5.4 | 0.3 | 1.0 | 3.5 | 0.2 | 0.7 | 2.4 | 0.1 | 0.5 | 1.9 | 0.1 | 0.4 | 1.6 |
| 5 – 10 | M | 1.1 | 3.9 | 14.0 | 0.6 | 2.1 | 7.6 | 0.4 | 1.3 | 4.5 | 0.2 | 0.8 | 2.9 | 0.2 | 0.6 | 2.0 | 0.1 | 0.4 | 1.6 | 0.1 | 0.4 | 1.3 |
| 11 – 20 | F | 2.0 | 7.2 | 25.8 | 1.1 | 3.9 | 14.0 | 0.7 | 2.3 | 8.3 | 0.4 | 1.5 | 5.3 | 0.3 | 1.1 | 3.8 | 0.2 | 0.8 | 2.9 | 0.2 | 0.7 | 2.4 |
| 11 – 20 | M | 1.8 | 6.4 | 22.8 | 1.0 | 3.5 | 12.4 | 0.6 | 2.1 | 7.3 | 0.4 | 1.3 | 4.7 | 0.3 | 0.9 | 3.3 | 0.2 | 0.7 | 2.6 | 0.2 | 0.6 | 2.1 |
| 21 – 30 | F | 2.1 | 7.5 | 26.7 | 1.1 | 4.1 | 14.5 | 0.7 | 2.4 | 8.6 | 0.4 | 1.6 | 5.5 | 0.3 | 1.1 | 3.9 | 0.2 | 0.8 | 3.0 | 0.2 | 0.7 | 2.5 |
| 21 – 30 | M | 2.0 | 7.1 | 25.3 | 1.1 | 3.9 | 13.7 | 0.6 | 2.3 | 8.1 | 0.4 | 1.5 | 5.2 | 0.3 | 1.0 | 3.7 | 0.2 | 0.8 | 2.8 | 0.2 | 0.7 | 2.4 |
| 31 – 40 | F | 1.8 | 6.5 | 23.3 | 1.0 | 3.6 | 12.6 | 0.6 | 2.1 | 7.5 | 0.4 | 1.4 | 4.8 | 0.3 | 1.0 | 3.4 | 0.2 | 0.7 | 2.6 | 0.2 | 0.6 | 2.2 |
| 31 – 40 | M | 1.8 | 6.6 | 23.3 | 1.0 | 3.6 | 12.7 | 0.6 | 2.1 | 7.5 | 0.4 | 1.4 | 4.8 | 0.3 | 1.0 | 3.4 | 0.2 | 0.7 | 2.6 | 0.2 | 0.6 | 2.2 |
| 41 – 50 | F | 1.8 | 6.3 | 22.5 | 1.0 | 3.4 | 12.3 | 0.6 | 2.0 | 7.3 | 0.4 | 1.3 | 4.7 | 0.3 | 0.9 | 3.3 | 0.2 | 0.7 | 2.5 | 0.2 | 0.6 | 2.1 |
| 41 – 50 | M | 1.8 | 6.3 | 22.6 | 1.0 | 3.4 | 12.3 | 0.6 | 2.0 | 7.3 | 0.4 | 1.3 | 4.7 | 0.3 | 0.9 | 3.3 | 0.2 | 0.7 | 2.5 | 0.2 | 0.6 | 2.1 |
| 51 – 60 | F | 1.8 | 6.4 | 22.7 | 1.0 | 3.5 | 12.3 | 0.6 | 2.0 | 7.3 | 0.4 | 1.3 | 4.7 | 0.3 | 0.9 | 3.3 | 0.2 | 0.7 | 2.5 | 0.2 | 0.6 | 2.1 |
| 51 – 60 | M | 2.0 | 7.1 | 25.3 | 1.1 | 3.9 | 13.7 | 0.6 | 2.3 | 8.1 | 0.4 | 1.5 | 5.2 | 0.3 | 1.0 | 3.7 | 0.2 | 0.8 | 2.8 | 0.2 | 0.7 | 2.4 |
| 61 – 70 | F | 1.9 | 6.8 | 24.2 | 1.0 | 3.7 | 13.1 | 0.6 | 2.2 | 7.8 | 0.4 | 1.4 | 5.0 | 0.3 | 1.0 | 3.5 | 0.2 | 0.8 | 2.7 | 0.2 | 0.6 | 2.3 |
| 61 – 70 | M | 2.1 | 7.3 | 26.0 | 1.1 | 4.0 | 14.1 | 0.7 | 2.3 | 8.4 | 0.4 | 1.5 | 5.4 | 0.3 | 1.1 | 3.8 | 0.2 | 0.8 | 2.9 | 0.2 | 0.7 | 2.4 |
| 71 – 80 | F | 2.0 | 7.2 | 25.7 | 1.1 | 3.9 | 13.9 | 0.7 | 2.3 | 8.3 | 0.4 | 1.5 | 5.3 | 0.3 | 1.1 | 3.7 | 0.2 | 0.8 | 2.9 | 0.2 | 0.7 | 2.4 |
| 71 – 80 | M | 2.2 | 7.9 | 28.2 | 1.2 | 4.3 | 15.3 | 0.7 | 2.5 | 9.1 | 0.5 | 1.6 | 5.9 | 0.3 | 1.2 | 4.1 | 0.2 | 0.9 | 3.2 | 0.2 | 0.7 | 2.6 |
| > 80 | F | 2.2 | 7.9 | 28.1 | 1.2 | 4.3 | 15.2 | 0.7 | 2.5 | 9.0 | 0.5 | 1.6 | 5.8 | 0.3 | 1.1 | 4.1 | 0.2 | 0.9 | 3.1 | 0.2 | 0.7 | 2.6 |
| > 80 | M | 2.5 | 8.8 | 31.2 | 1.3 | 4.8 | 16.9 | 0.8 | 2.8 | 10.0 | 0.5 | 1.8 | 6.5 | 0.4 | 1.3 | 4.5 | 0.3 | 1.0 | 3.5 | 0.2 | 0.8 | 2.9 |
Note. All concentrations are scaled in nmol/l (LC/MS-MS calibrated). Abbreviations in the sex column: F = female (grey shaded cells). M = male.
4. Discussion
The CIRCORT dataset was constructed to generate reference ranges for diurnal salivary cortisol levels in humans and to determine the impact of various demographic variables on these data. Combining data from 15 large-scale studies from Europe and the United States of America, we here report saliva cortisol concentrations (LC/MS-MS calibrated) for both sexes across the whole lifespan.
To the best of our knowledge, this is the largest such dataset from unselected populations. Our analyses confirmed the expected daytime changes of salivary cortisol levels with an immediate increase after wake-up (see also Stalder et al., 2016) and a subsequent decline in mean levels throughout the day, leading to a concentration nadir around bedtime (~16 hours after awakening). Further, we provide support for the conclusion that sex and age are important predictors of adrenocortical activity. Specifically, age was associated with a gradual increase of diurnal cortisol levels during adolescence and beyond the age of 50 years, which is consistent with previous studies (e.g. Ross et al., 2014, Van Cauter et al., 1996, Wang et al., 2014). In addition to this general finding, our analyses further provide evidence that females show an earlier increase of diurnal cortisol levels than males during adolescence (cf. Platjes et al., 2013, Shirtcliff et al., 2012). Notably, Törnhage (2002) also reported such an increase during adolescence, which was clearly attributable to the onset of puberty. In old age by contrast, males showed an earlier increase of diurnal cortisol levels than females.
Intriguingly, we also found evidence for an annual rhythm in adrenocortical activity, with the seasonal influence superimposed upon the daily cortisol rhythm. Specifically, saliva cortisol levels peaked in spring and winter (i.e. the acrophase), whereas sampling during summer and autumn seemed to result in lowered cortisol levels. Although the presence of a circannual rhythm in cortisol levels concurs with other behavioral data (e.g., birth rates or incidence rates of psychosomatic pathologies) and ecological concepts of homeostatic change driven by environmental parameters (Foster and Roenneberg, 2008, Romero et al., 2009), many previous studies on seasonal cortisol changes documented substantial variability, with acrophases during winter (Agrimonti et al., 1982 [blood], Del Ponte et al., 1984 [blood], Hansen et al., 2001 [urine], Reinberg et al., 1978 [blood]), spring (Persson et al., 2008 [saliva]), and early summer (Hadlow et al., 2014 [blood], Matchok et al., 2007 [blood]). While part of this variability may be attributable to a sex-by-season interaction (which was nominally present, but did not reach the threshold for claiming statistical significance; see Figure 2B) and/or different characteristics of the collected specimens, a recently published Australian study (Hadlow et al., 2014) argued that the time of sunrise may be the most crucial moderator (or in this context zeitgeber) of systematic shifts in the cortisol acrophase. In this study, a one-hour-increase in the time of sunrise was accompanied by an increase of median cortisol by approximately 5% resulting in a difference between cortisol peak and nadir of 8.6% at 30°S (which is indeed slightly below the ~10% we report for a latitude of 50°N). Based on those findings, we hypothesize that the absolute latitude of the conducting study and the amplitude of annual cortisol changes should be highly correlated and countries proximate to the equator would find a much weaker rhythm. However, the CIRCORT dataset did not permit further investigation of this hypothesis because only Dutch, German and British studies provided information on the month of sampling. Given the likely impact of such environmental variability on adrenocortical functioning, other non-monitored variables such as the altitude of the major habitat should also be considered as potential moderators in future studies.
Regarding the combined impact of all analyzed covariates, predominately age and season were able to account for substantial between-study variance. Nonetheless, a large portion of residual variance in the CIRCORT dataset also highlights the importance of considering the complex ultradian dynamics of adrenocortical activity that are characterized by continuous cortisol elimination and intermittent phases of cortisol secretion (Spiga et al., 2014), and limit the stability (and therefore predictive value) of salivary cortisol measures derived from studies with only a limited number of samples (see also Doane et al., 2015).
Despite the large sample size of the CIRCORT dataset, the similarity of sample collection strategies among all included studies, and that all samples were analyzed in the same two laboratories (i.e. Trier or Dresden, Germany) there are still some limits on the generalizability of these findings. First, we decided to refrain from modeling cortisol during the first 30 minutes after awakening, as the majority of studies did not verify the exact time of awakening and it is known that even small sampling shifts can result in biased estimates of the CAR (Stalder et al., 2016). Accordingly, the present investigation did not provide any information on the extent of cortisol secretion in response to awakening (but see Wust et al., 2000). In order to achieve this aim, future studies should rigorously verify the self-reported awakening times (e.g., by actigraphy) and sampling times using electronic monitoring devices (e.g., MEMS® caps). Second, we converted salivary cortisol concentrations to adjust for measurement bias due to antibody cross-reactivity and steric effects of further saliva matrix components (Miller et al., 2013; Bae et al., 2016). Therefore, the provided reference ranges only apply to salivary cortisol as measured by a compatible LC/MS-MS protocol and would need to be adjusted whenever cortisol data from other assays are compared with our values and ranges. Finally, the individual studies addressed very different participant populations with limited overlap. Thus the relative variance portions bound by the between-study and between-participant components, respectively, should be interpreted with caution. To our mind, the best interpretation is to view the between-study component as a combined variance that predominantly arises from between-participant differences. In consequence, the between-participant component should probably be regarded as an estimate of the variance within a population of similar demographic and social characteristics.
In sum, the present article introduced the CIRCORT database from which we derived detailed sex-specific reference values for diurnal salivary cortisol across the whole lifespan. While the dataset can be used in future studies to further investigate cortisol aggregate measures and the specific properties of cortisol distributions in phases of varying adrenocortical activity, the provided references values are particularly valuable for epidemiological studies that seek to investigate cortisol differences in special populations. Moreover, these values may become useful for diagnostic screening procedures in clinical settings (e.g., for Cushing’s or Addison’s disease; Putignano et al., 2003, Restituto et al., 2008), whenever mass spectrometric assays are used to determine cortisol concentration (see also Taylor et al., 2015). Finally our data highlight that circannual changes may be considered as covariate to increase the statistical power of study designs and to minimize the chance of effect confounds.
Acknowledgments
We thank all reviewers, contributing scientists, and assistants for enabling the completion of this project.
Role of the funding source
The studies contributing to the CIRCORT dataset were funded by grant awards to the individual researchers. The analyses were supported by an unrestricted grant of the Medical Faculty of Mannheim, Heidelberg University, by the Support-the-best grant awarded to CK. TRAILS has been financially supported by various grants from the Netherlands Organization for Scientific Research, and by the participating research centers. The MIDUS project was supported by an award from the US National Institute on Aging to CR (P01 AG20166). Data collection for the 1958 cohort was funded by the Medical Research Council (grant G0000934). The authors are grateful to the Centre for Longitudinal Studies (CLS), UCL Institute of Education for the use of these data and to the UK Data Service for making them available. However, neither CLS nor the UK Data Service bear any responsibility for the analysis or interpretation of these data. This study was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Footnotes
Further sensitivity analyses with country as additional predictor confirmed this result by showing no systematic differences for the Dutch, German, English, and U.S. American (−0.13 ≤ β ≤ 0.02, all p’s ≥ .5), but for the Swiss samples (β = 1.24, z = 4.67, p < .001).
This effect was mostly informed by two American studies with limited sample sizes. In an attempt to validate this finding, we reanalyzed the salivary cortisol data from German infants reported by Stalder et al. (2013). After conversion to LC/MS-MS scale, these data were comparable to the concentrations reported in this article.
Contributors
CK and JEF conceptualized and designed the present article. DMA, EB, MB, DIB, CLC, MCJD, BD, JEF, MRG, MK, FL, AJO, CP, JGR, CDR, HAT, and SEW implemented and supervised the data collection and data management of the contributing studies. RM, MJ, and SVS further preprocessed and analyzed the compiled dataset. RM and TS drafted the first version of the article. All authors reviewed and revised the article for intellectual content, and approved its final version.
Contributors
The authors have no conflict of interest to declare.
References
- Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Clinical Chemistry and Laboratory Medicine. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Agrimonti F, Angeli A, Frairia R, Fazzari A, Tamagnone C, Fornaro D, Ceresa F. Circannual rhythmicities of cortisol levels in the peripheral plasma of health subjects. Chronobiologia. 1982;9:107–114. [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. The Journal of Clinical Endocrinology & Metabolism. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bae Y-J, Gaudl A, Jaeger S, Stadelmann S, Hiemisch A, Wieland K, Willenberg A, Schaab M, von Klitzing K, Thiery J, Döhnert M, Kratzsch J. Immunoassay or LC/MS-MS for the measurement of salivary cortisol in children? Clinical Chemistry and Laboratory Medicine. 2016;54:811–822. doi: 10.1515/cclm-2015-0412. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv:1406.5823. 2014 [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Del Ponte A, Guagnano MT, Sensi S. Time-related behaviour of endocrine secretion: circannual variations of FT3, cortisol, HGH and serum basal insulin in healthy subjects. Chronobiology International. 1984;1:297–300. doi: 10.3109/07420528409063910. [DOI] [PubMed] [Google Scholar]
- Doane LD, Chen FR, Sladek MR, Van Lenten SA, Granger DA. Latent trait cortisol (LTC) levels: Reliability, validity, and stability. Psychoneuroendocrinology. 2015;55:21–35. doi: 10.1016/j.psyneuen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Current Biology. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. The Lancet. 2002;359:341–345. doi: 10.1016/S0140-6736(02)07500-1. [DOI] [PubMed] [Google Scholar]
- Hadlow NC, Brown S, Wardrop R, Henley D. The effects of season, daylight saving and time of sunrise on serum cortisol in a large population. Chronobiology International. 2014;31:243–251. doi: 10.3109/07420528.2013.844162. [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Garde AH, Christensen JM, Eller NH, Netterstrøm B. Evaluation of a radioimmunoassay and establishment of a reference interval for salivary cortisol in healthy subjects in Denmark. Scandinavian Journal of Clinical and Laboratory Investigation. 2003;63:303–310. doi: 10.1080/00365510310001942. [DOI] [PubMed] [Google Scholar]
- Hansen ÅM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica Chimica Acta. 2001;309:25–35. doi: 10.1016/s0009-8981(01)00493-4. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hofman A, Breteler MMB, van Duijn CM, Krestin GP, Pols HA, Stricker BHC, Tiemeier H, Uitterlinden AG, Vingerling JR, Witteman JCM. The Rotterdam Study: objectives and design update. European Journal of Epidemiology. 2007;22:819–829. doi: 10.1007/s10654-007-9199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between-and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences—findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38:2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper N, de Geus EJ, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kühner C, Kirschbaum C, Meisinger C, Lammich J, Holle R, Krumm B, von Lengerke T, Wichmann HE, Deuschle M, Ladwig KH. Salivary cortisol in a middle-aged community sample: results from 990 men and women of the KORA-F3 Augsburg study. European Journal of Endocrinology. 2010;163:443–451. doi: 10.1530/EJE-10-0491. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress. 2014;17:285–295. doi: 10.3109/10253890.2014.915517. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiology International. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F. Transformation techniques for cross-sectional and longitudinal endocrine data: Application to salivary cortisol concentrations. Psychoneuroendocrinology. 2013;38:941–946. doi: 10.1016/j.psyneuen.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F, Rauh M, Gröschl M, Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013;38:50–57. doi: 10.1016/j.psyneuen.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Rosmalen JG, Buitelaar JK, Hoek HW, Ormel J, Raven D, Reijneveld SA, Veenstra R, Verhulst FC, Vollebergh WA, Hartman CA. Cohort profile update: the Tracking Adolescents’ Individual Lives Survey (TRAILS) International Journal of Epidemiology. 2015;1:76–76n. doi: 10.1093/ije/dyu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R, Garde AH, Hansen ÅM, Österberg K, Larsson B, Ørbæk P, Karlson B. Seasonal variation in human salivary cortisol concentration. Chronobiology International. 2008;25:923–937. doi: 10.1080/07420520802553648. [DOI] [PubMed] [Google Scholar]
- Platje E, Vermeiren RR, Branje SJ, Doreleijers TA, Meeus WH, Koot HM, Frijns T, van Lier PAC, Jansen LMC. Long-term stability of the cortisol awakening response over adolescence. Psychoneuroendocrinology. 2013;38:271–280. doi: 10.1016/j.psyneuen.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Power C, Li L, Hertzman C. Cognitive development and cortisol patterns in mid-life: findings from a British birth cohort. Psychoneuroendocrinology. 2008;33:530–539. doi: 10.1016/j.psyneuen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Putignano P, Toja P, Dubini A, Giraldi FP, Corsello SM, Cavagnini F. Midnight salivary cortisol versus urinary free and midnight serum cortisol as screening tests for Cushing’s syndrome. The Journal of Clinical Endocrinology & Metabolism. 2003;88:4153–4157. doi: 10.1210/jc.2003-030312. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Version 3.2.2. 2015 Retrieved from http://r-project.org.
- Reinberg A, Lagoguey M, Cesselin F, Touitou Y, Legrand JC, Delassalle A, Antreassian J, Lagoguey A. Circadian and circannual rhythms in plasma hormones and other variables of five healthy young human males. Acta Endocrinologica. 1978;88:417–427. doi: 10.1530/acta.0.0880417. [DOI] [PubMed] [Google Scholar]
- Restituto P, Galofré JC, Gil MJ, Mugueta C, Santos S, Monreal JI, Varo N. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clinical Biochemistry. 2008;41:688–692. doi: 10.1016/j.clinbiochem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Riad-Fahmy D, Read GF, Walker RF, Griffiths K. Steroids in saliva for assessing endocrine function. Endocrine Reviews. 1982;3:367–395. doi: 10.1210/edrv-3-4-367. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE. The reactive scope model – a new model integrating homeostasis, allostasis, and stress. Hormones and Behavior. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Constructing time-specific reference ranges. Statistics in Medicine. 1991;10:675–690. doi: 10.1002/sim.4780100502. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, Lightman SL. HPA Axis-Rhythms. Comprehensive Physiology. 2014;4:1273–1298. doi: 10.1002/cphy.c140003. [DOI] [PubMed] [Google Scholar]
- Stalder T, Bäumler D, Miller R, Alexander N, Kliegel M, Kirschbaum C. The cortisol awakening response in infants: Ontogeny and associations with development-related variables. Psychoneuroendocrinology. 2013;38:552–559. doi: 10.1016/j.psyneuen.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH, Miller R, Wetherell MA, Lupien SJ, Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Keevil B, Huhtaniemi IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. European Journal of Endocrinology. 2015;173:D1–D12. doi: 10.1530/EJE-15-0338. [DOI] [PubMed] [Google Scholar]
- Törnhage CJ. Reference values for morning salivary cortisol concentrations in healthy school-aged children. Journal of Pediatric Endocrinology and Metabolism. 2002;15:197–204. doi: 10.1515/jpem.2002.15.2.197. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. The Journal of Clinical Endocrinology & Metabolism. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- Wang X, Sánchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman T, Diez Roux AV. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–320. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. The split-apply-combine strategy for data analysis. Journal of Statistical Software. 2011;40:1–29. [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise & Health. 2000;2:79–88. [PubMed] [Google Scholar]