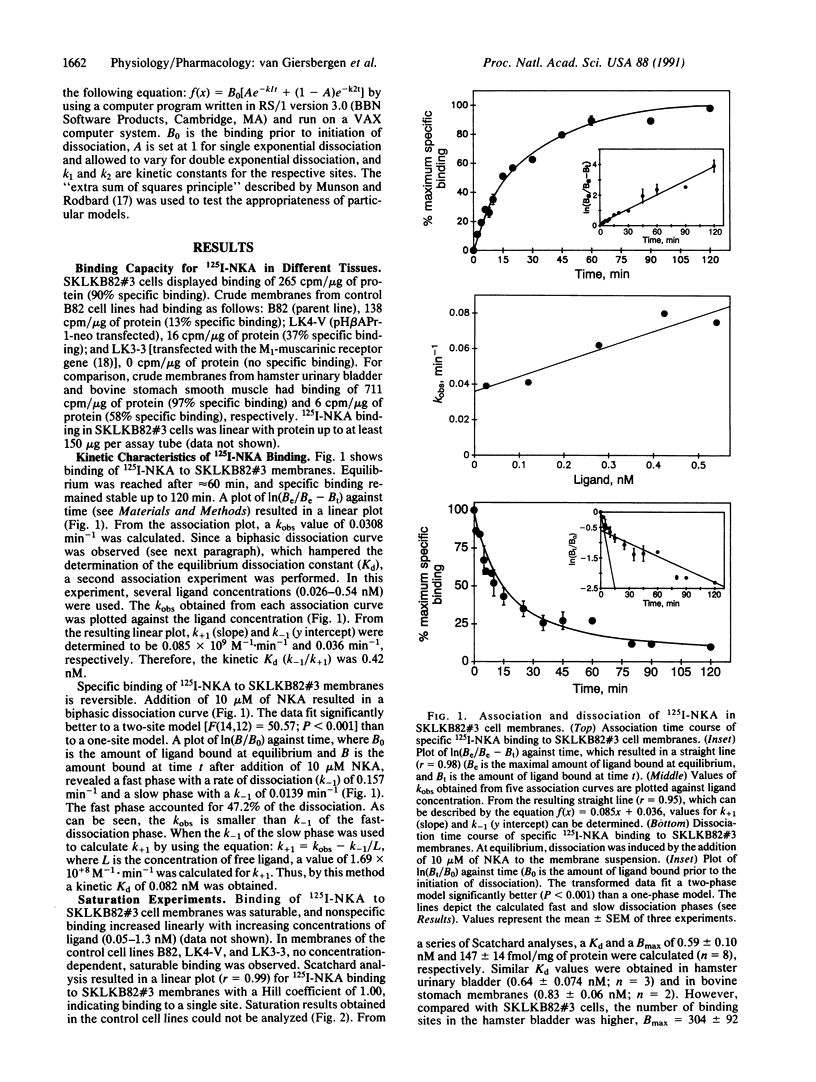
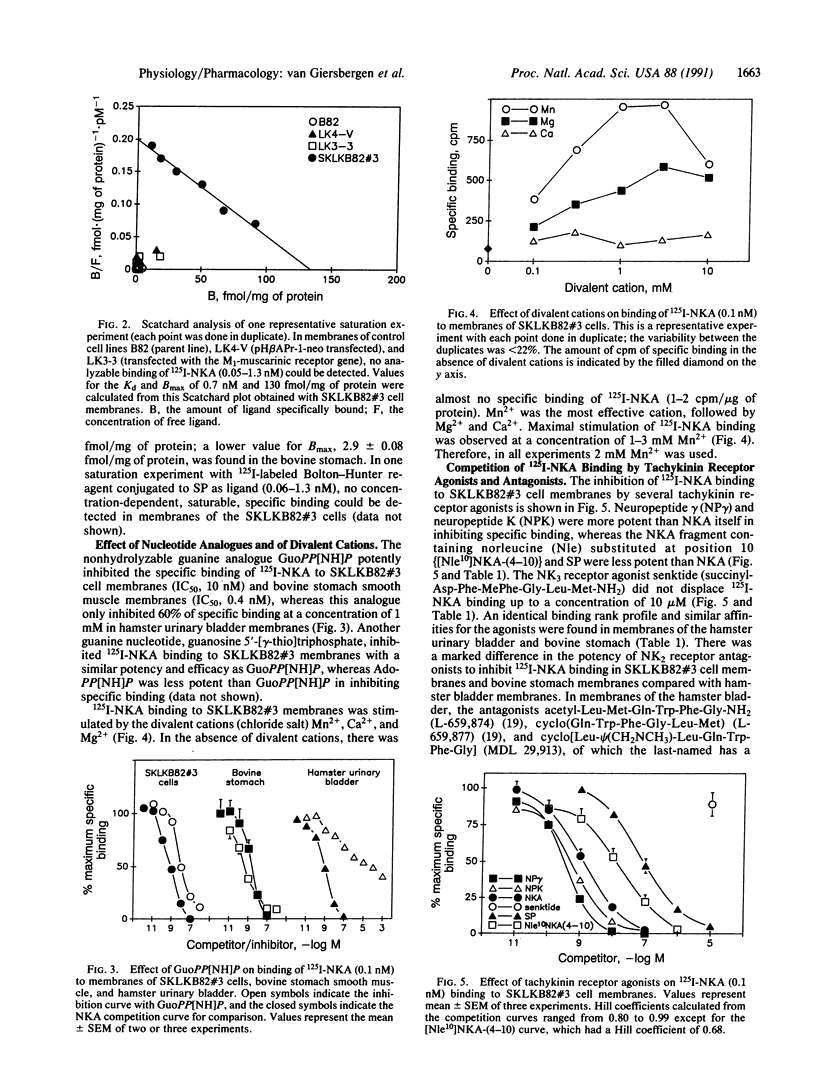
Abstract
Membranes isolated from a murine fibroblast B82 cell line (SKLKB82#3) transfected with the bovine stomach cDNA pSKR56S exhibited binding of [His(125I)1]neurokinin A (125I-NKA) to a single population of sites with a Bmax of 147 fmol/mg of protein and a Kd of 0.59 nM. Control cell lines had little or no specific binding. The ligand binding in SKLKB82#3 cells was reversible and was inhibited by peptides in the potency rank of neuropeptide gamma greater than neuropeptide K greater than neurokinin A greater than [10-norleucine]neurokinin A-(4-10) greater than substance P much greater than senktide (succinyl-Asp-Phe-MePhe-Gly-Leu-Met-NH2). Specific binding was enhanced by Mn2+, Mg2+, and Ca2+ and was inhibited by guanine nucleotide analogues. Thus, SKLKB82#3 cells have been transfected with NK2 receptors that have become associated with an endogenous guanine nucleotide-binding protein. In comparison with membranes from the hamster urinary bladder, a tissue enriched in NK2 receptors, NK2 receptor antagonists displayed markedly different potencies, either more or less potent, in inhibiting specific binding in membranes of the transfected cells. Furthermore, inhibition of 125I-NKA binding by nucleotide analogues was markedly different in SKLKB82#3 cells compared with hamster bladder tissue. The different binding profile in the cells is not due to an artefact introduced during cDNA transfection because a similar profile was also observed in bovine stomach membranes. These results may indicate the existence of two distinct NK2 receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaujouan J. C., Saffroy M., Petitet F., Torrens Y., Glowinski J. Neuropeptide K, scyliorhinin I and II: new tools in the tachykinin receptor field. Eur J Pharmacol. 1988 Jul 7;151(2):353–354. doi: 10.1016/0014-2999(88)90826-6. [DOI] [PubMed] [Google Scholar]
- Beaujouan J. C., Torrens Y., Viger A., Glowinski J. A new type of tachykinin binding site in the rat brain characterized by specific binding of a labeled eledoisin derivative. Mol Pharmacol. 1984 Sep;26(2):248–254. [PubMed] [Google Scholar]
- Bergström L., Beaujouan J. C., Torrens Y., Saffroy M., Glowinski J., Lavielle S., Chassaing G., Marquet A., D'Orleans-Juste P., Dion S. 3H-neurokinin A labels a specific tachykinin-binding site in the rat duodenal smooth muscle. Mol Pharmacol. 1987 Dec;32(6):764–771. [PubMed] [Google Scholar]
- Bergström L., Torrens Y., Saffroy M., Beaujouan J. C., Lavielle S., Chassaing G., Morgat J. L., Glowinski J., Marquet A. [3H]neurokinin B and 125I-Bolton Hunter eledoisin label identical tachykinin binding sites in the rat brain. J Neurochem. 1987 Jan;48(1):125–133. doi: 10.1111/j.1471-4159.1987.tb13136.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Pruss R. M., Krstenansky J. L., Robinson P. J., Stauderman K. A. A tachykinin peptide receptor joins an elite club. Trends Pharmacol Sci. 1988 Jan;9(1):3–5. doi: 10.1016/0165-6147(88)90228-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Shatzer S. A. Agonist and antagonist binding to tachykinin peptide NK-2 receptors. Life Sci. 1988;42(26):2701–2708. doi: 10.1016/0024-3205(88)90246-9. [DOI] [PubMed] [Google Scholar]
- Burcher E., Buck S. H., Lovenberg W., O'Donohue T. L. Characterization and autoradiographic localization of multiple tachykinin binding sites in gastrointestinal tract and bladder. J Pharmacol Exp Ther. 1986 Mar;236(3):819–831. [PubMed] [Google Scholar]
- Burcher E., Buck S. H. Multiple tachykinin binding sites in hamster, rat and guinea-pig urinary bladder. Eur J Pharmacol. 1986 Sep 9;128(3):165–177. doi: 10.1016/0014-2999(86)90763-6. [DOI] [PubMed] [Google Scholar]
- Burcher E. The study of tachykinin receptors. Clin Exp Pharmacol Physiol. 1989 Jun;16(6):539–543. doi: 10.1111/j.1440-1681.1989.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Guard S., Watson S. P., Maggio J. E., Too H. P., Watling K. J. Pharmacological analysis of [3H]-senktide binding to NK3 tachykinin receptors in guinea-pig ileum longitudinal muscle-myenteric plexus and cerebral cortex membranes. Br J Pharmacol. 1990 Apr;99(4):767–773. doi: 10.1111/j.1476-5381.1990.tb13004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Takahashi T., Kuno M., Nakayama K., Masu Y., Nakanishi S. Expression of two different tachykinin receptors in Xenopus oocytes by exogenous mRNAs. J Neurosci. 1987 Oct;7(10):3265–3273. doi: 10.1523/JNEUROSCI.07-10-03265.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Krause J. E. Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science. 1990 Feb 23;247(4945):958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- Kage R., McGregor G. P., Thim L., Conlon J. M. Neuropeptide-gamma: a peptide isolated from rabbit intestine that is derived from gamma-preprotachykinin. J Neurochem. 1988 May;50(5):1412–1417. doi: 10.1111/j.1471-4159.1988.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Lai J., Mei L., Roeske W. R., Chung F. Z., Yamamura H. I., Venter J. C. The cloned murine M1 muscarinic receptor is associated with the hydrolysis of phosphatidylinositols in transfected murine B82 cells. Life Sci. 1988;42(24):2489–2502. doi: 10.1016/0024-3205(88)90348-7. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Campbell N. J., Williams B. J., Iversen L. L. Multiple tachykinin binding sites in peripheral tissues and in brain. Eur J Pharmacol. 1986 Nov 4;130(3):209–217. doi: 10.1016/0014-2999(86)90270-0. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Javitch J. A., Snyder S. H. 3h-substance P binding to salivary gland membranes. Regulation by guanyl nucleotides and divalent cations. Mol Pharmacol. 1983 May;23(3):563–569. [PubMed] [Google Scholar]
- MacDonald M. R., Takeda J., Rice C. M., Krause J. E. Multiple tachykinins are produced and secreted upon post-translational processing of the three substance P precursor proteins, alpha-, beta-, and gamma-preprotachykinin. Expression of the preprotachykinins in AtT-20 cells infected with vaccinia virus recombinants. J Biol Chem. 1989 Sep 15;264(26):15578–15592. [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Giuliani S., Rovero P., Dion S., Regoli D., Giachetti A., Meli A. Competitive antagonists discriminate between NK2 tachykinin receptor subtypes. Br J Pharmacol. 1990 Jul;100(3):589–592. doi: 10.1111/j.1476-5381.1990.tb15851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Dion S., D'Orléans-Juste P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38(1):1–15. doi: 10.1159/000138512. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Smith K. E., Hoss W. P. Guanine nucleotides regulate [3H]substance P binding in rat small intestine. Regul Pept. 1985 Aug;11(4):275–285. doi: 10.1016/0167-0115(85)90200-9. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Krause J. E. Neuropeptide K potently stimulates salivary gland secretion and potentiates substance P-induced salivation. Proc Natl Acad Sci U S A. 1989 Jan;86(1):392–396. doi: 10.1073/pnas.86.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Krause J. E. gamma-Preprotachykinin-(72-92)-peptide amide potentiates substance P-induced salivation. Eur J Pharmacol. 1989 Feb 28;161(2-3):267–271. doi: 10.1016/0014-2999(89)90858-3. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Nakata Y., Segawa T. Substance P receptors in bovine brain membranes are coupled to GTP inhibitory binding protein. Eur J Pharmacol. 1986 Jun 5;125(1):157–158. doi: 10.1016/0014-2999(86)90097-x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Lundberg J. M., Jörnvall H., Mutt V. Neuropeptide K: isolation, structure and biological activities of a novel brain tachykinin. Biochem Biophys Res Commun. 1985 Apr 30;128(2):947–953. doi: 10.1016/0006-291x(85)90138-x. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Sasai Y., Tanaka K., Fujiwara T., Tsuchida K., Shigemoto R., Kakizuka A., Ohkubo H., Nakanishi S. Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem. 1989 Oct 25;264(30):17649–17652. [PubMed] [Google Scholar]



