Abstract
Background
Serum cystatin C (sCysC) and urinary cystatin C (uCysC) are potential biomarkers for early detection of chronic kidney disease (CKD) in cats. An in‐depth clinical validation is required.
Objectives
To evaluate CysC as a marker for CKD in cats and to compare assay performance of the turbidimetric assay (PETIA) with the previously validated nephelometric assay (PENIA).
Animals
Ninety cats were included: 49 CKD and 41 healthy cats.
Methods
Serum CysC and uCysC concentrations were prospectively evaluated in cats with CKD and healthy cats. Based on plasma exo‐iohexol clearance test (PexICT), sCysC was evaluated to distinguish normal, borderline, and low GFR. Sensitivity and specificity to detect PexICT < 1.7 mL/min/kg were calculated. Serum CysC results of PENIA and PETIA were correlated with GFR. Statistical analysis was performed using general linear modeling.
Results
Cats with CKD had significantly higher mean ± SD sCysC (1.4 ± 0.5 mg/L) (P < .001) and uCysC/urinary creatinine (uCr) (291 ± 411 mg/mol) (P < .001) compared to healthy cats (sCysC 1.0 ± 0.3 and uCysC/uCr 0.32 ± 0.97). UCysC was detected in 35/49 CKD cats. R 2 values between GFR and sCysC or sCr were 0.39 and 0.71, respectively (sCysC or sCr = μ + GFR + ε). Sensitivity and specificity were 22 and 100% for sCysC and 83 and 93% for sCr. Serum CysC could not distinguish healthy from CKD cats, nor normal from borderline or low GFR, in contrast with sCr.
Conclusion
Serum CysC is not a reliable marker of reduced GFR in cats and uCysC could not be detected in all CKD cats.
Keywords: Chronic kidney disease, Creatinine, Feline, Glomerular Filtration Rate
Abbreviations
- CBC
complete blood cell count
- CKD
chronic kidney disease
- Cr
creatinine
- CV
coefficient of variation
- CysC
cystatin C
- DSH/DLH
domestic shorthair and longhair cats
- GFR
glomerular filtration rate
- IRIS
International Renal Interest Society
- LOD
limit of detection
- PECCT
plasma exogenous creatinine clearance test
- PEC‐ICT
plasma exogenous creatinine‐iohexol clearance test
- PenICT
plasma endo‐iohexol clearance test
- PexICT
plasma exo‐iohexol clearance test
- PENIA
particle enhanced nephelometric assay
- PETIA
particle enhanced turbidimetric assay
- PICT
plasma iohexol clearance test
- RI
reference interval
- SBP
systolic blood pressure
- sCr
serum creatinine
- sCysC
serum cystatin C
- uCr
urinary creatinine
- uCysC
urinary cystatin C
- UPC
urinary protein:creatinine ratio
- USG
urine specific gravity
Chronic kidney disease (CKD) is common in geriatric cats, with a prevalence from 30% up to 60% in cats older than 10 years.1, 2, 3 Since CKD is an irreversible and progressive disease, early detection and treatment is of major importance, aiming to slow down disease progression and to improve quality of life and longevity.4, 5 Glomerular filtration rate (GFR) is considered the gold standard method to evaluate kidney function, but measurement is time‐consuming and is not routinely used. Therefore, the indirect GFR markers, serum creatinine (sCr), and urea, are routinely measured to estimate GFR. However, these markers are insensitive. It is widely reported but poorly documented that their serum concentration only increases when approximately 75% of the functional renal mass is lost.6 Moreover, they are both influenced by muscle mass, age, feeding status, sex, and intraindividual variation.7, 8, 9 All those disadvantages support the need for new indirect biomarkers that can be measured easily and reliably.
Cystatin C (CysC), a 13 kDa protein, is a proteinase inhibitor, produced in every nucleated cell at a constant rate, that is responsible for intracellular protein catabolism.10, 11 Most of the properties required for an ideal endogenous GFR marker apply for CysC.12 Compared to sCr, several human13, 14, 15, 16 and canine studies17, 18 have shown a better correlation of sCysC with GFR. In addition, urinary Cystatin C (uCysC) is a biomarker for tubular damage in humans19, 20 and dogs.21 In a pilot study, our group observed a significant difference in sCysC and uCysC concentration between CKD and healthy cats. We also validated the human particle enhanced nephelometric immunoassay (PENIA) for CysC measurement22 in cats and established a reference interval (RI) of 0.58–1.95 mg/L for sCysC. In addition, we demonstrated that there is no influence of breed, age, and sex on feline sCysC7 and that it is not mandatory to with‐hold food in cats prior to evaluation of feline sCysC.23 These findings make sCysC a promising marker to estimate GFR in feline medicine.
Three human CysC quantitation devices are currently available: ELISA,24 particle enhanced turbidimetric assay14 (PETIA) and particle enhanced nephelometric assay (PENIA).25 The latter 2 analytical methods are more suitable for clinical use, since an ELISA is more expensive, labour‐intensive, and time‐consuming.26 No commercial veterinary assays are currently available, which requires validation of human assays. The PENIA was validated previously by our group.22 The validation of the PETIA has been added as supporting information to this article.
The objectives of this study were 4‐fold. Firstly, sCysC and uCysC were compared between a large number of CKD and healthy cats. Secondly, the correlation of sCysC and sCr with GFR measured using plasma exogenous creatinine clearance test (PECCT), plasma endo‐iohexol clearance test (PenICT) and plasma exo‐iohexol clearance test (PexICT), was compared. Thirdly, the sensitivity and specificity of sCysC to detect decreased GFR were determined and compared with sCr. Fourthly, to determine which assay would be most suitable for clinical use, PENIA versus PETIA measurements of sCysC were each correlated with GFR estimated by PECCT, PenICT, and PexICT.
Materials and Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Local Ethical and Deontological Committee of the Faculty of Veterinary Medicine, Ghent University (EC2011_197). Informed consent was obtained from all owners whose animals participated in the study.
Animals
Adult CKD and healthy cats were included, regardless of breed and sex. In all cats, physical examination, complete blood count (CBC), serum biochemistry (BC), including total thyroxine (TT4) measurement in cats older than 6 years, and urinalysis were performed to assess the general health status. Diagnosis of CKD was made prior to inclusion. Cats were diagnosed with CKD based on the presence of compatible clinical and laboratory findings (i.e. renal azotemia (sCr and urea exceeding the RI we established in a previous study7 i.e. (0.73‒1.83 mg/dL; 64.5‒161.8 μmol/L) for sCr and (17.4‒ 35.6 mg/dL; 6.2‒12.7 mmol/L) for serum urea) and urine specific gravity (USG) <1.035). Cats with CKD were classified into four stages according to the IRIS guidelines.27 Cats with borderline sCr, but abnormal renal ultrasonographic findings at time of inclusion, with available follow‐up data confirming CKD, were also included. CKD cats with evidence of relevant concurrent systemic diseases based on their history, physical examination, CBC, BC, or urinalysis were excluded. Cats that were receiving calcium antagonists were excluded from inclusion in the study because of concerns that these medications would modify GFR. For patients that were receiving angiotensin converting enzyme (ACE)‐inhibitors, or angiotensin receptor blockers (ARB), treatment was discontinued at least 1 week prior to inclusion in the study. Renal diet and phosphorus binders did not have to be withdrawn.
Cats with lack of important abnormalities in history, physical examination, CBC, BC an urinalysis were defined as “healthy”. Criteria for normal urinalysis were: USG >1.035, inactive urine sediment, urinary protein:creatinine ratio (UPC) <0.4, and negative bacterial urine culture.28 Healthy cats receiving medication within 1 month prior to inclusion that could affect kidney health, such as nonsteroidal anti‐inflammatory drugs (NSAIDs) corticosteroids, antibiotics, β‐blocking agents, calcium antagonists, and ACE‐inhibitors or ARB, were excluded.
Procedures
A standard physical examination was performed in all cats including systolic blood pressure (SBP) measurement using the Doppler ultrasonic technique and a standardized procedure according to the ACVIM consensus guidelines.29 Cats were considered hypertensive if SBP >160 mmHg.29 Thyroid gland palpation was performed in cats older than 6 years, as previously described.30 All cats were fasted at least 10 hour before the sampling procedure. Five mL of blood was taken by jugular venipuncture using a 23 G needle. After centrifugation (5 minutes at 1931 × g), serum was analyzed the same day. The CBC1 and BC2 were performed in all cats. Ten mL of urine was taken by cystocentesis with a 22 G needle. The USG was determined using a manual refractometer3 . Urinalysis consisted of a urinary dipstick test,4 , 5 measurement of UPC,6 sediment analysis and bacterial culture.7 Urine was centrifuged (3 minutes at 355 × g) and the urinary sediment was analyzed within 30 minutes according to Paepe et al.31 The supernatant and remaining serum were divided in aliquots of 0.300 mL and stored at −72°C until batched analysis.
Glomerular filtration rate was measured by the combined plasma exogenous creatinine‐iohexol clearance test (PEC‐ICT), using a protocol previously described by van Hoek et al.32 Briefly, a 22 G catheter was placed in the cephalic vein. Creatinine8 was dissolved in 4 mL of 0.9% sodium chloride. First iohexol9 (64 mg/kg [0.1 mL/kg]), followed by Cr (40 mg/kg) and 3 mL of 0.9% sodium chloride was injected. Blood samples were taken by jugular venipuncture just before the injection and at 5, 15, 30, 60, 120, 180, 360, 480, 600 minutes after injection. The samples were placed in EDTA tubes, centrifuged (5 minutes at 1931 × g) and plasma was stored in aliquots of 0.300 mL at −72°C until analysis.
Assays
Serum CysC and uCysC were analyzed with PENIA10 using the nephelometer,11 previously validated for cats,22 and with PETIAl12 using the Cobas auto‐analyzer.13 The validation report of the PETIA for CysC measurement in cats has been added as Data S1. Urinary CysC was expressed as a ratio to the urinary Cr (uCr) concentration, to compensate for differences in urine flow rates.33 Plasma Cr was analyzed with an enzymatic assay14 and plasma concentrations of the stereo‐isomers endo‐and exo‐iohexol were determined by high performance liquid chromatography with ultraviolet UV detection, both validated by van Hoek et al.32 Serum Cr measured in BC2 was analyzed with a modified Jaffé assay, validated at a veterinary commercial laboratory.15
Pharmacokinetics for GFR determination
All analyses were performed using WinNonlin.16 The data were subjected to noncompartmental analysis, as described by Watson et al.34 The area under the plasma concentration versus time curve (AUC) was calculated using the trapezoidal rule with extrapolation to infinity. The ratio of endo‐and exo‐iohexol was determined per batch of iohexol and the administered dose of each stereoisomer was calculated. The plasma clearance of Cr, endo‐ and exo‐iohexol was determined by dividing the dose administered by AUC and indexed to bodyweight (mL/min/kg).
Statistical analysis
Statistical analyses were performed using statistical software17 and at the 0.05 significance level. The renal parameters SBP, sCr, serum urea, sCysC, USG, UPC, and uCysC/uCr approached normal distribution and the Student's t‐test was used to test for significant differences between the healthy cats and cats with CKD. The effect of status (CKD, healthy) on sCysC and uCysC, the effect of the IRIS stage on sCysC and uCysC, the relationship between uCysC/uCr and UPC and the comparison between sCysC measured with PENIA versus PETIA were tested by ANOVA. In case of a significant effect, pairwise comparisons were performed with the Tukey's test. If the uCysC concentration was < limit of detection (LOD), it was arbitrarly fixed to 0.
The correlation between GFR and either sCysC or sCr were determined with ANOVA using general linear model (GLM): Marker = μ + GFR + ε with marker being sCr, sCysCPENIA, sCysCPETIA, 1/sCr, 1/sCysCPENIA, 1/sCysCPETIA; GFR was GFR‐Cr, GFR‐exo or GFR‐endo; ε, error term of the model. For each marker, the R 2 of the regression was determined.
We also studied if sCysC could distinguish cats with normal, borderline, or low GFR, based on PexICT, compared with sCr. The cut‐off concentrations for normal GFR were defined as GFR ≥1.7 mL/min/kg, for borderline GFR as GFR 1.2–1.7 mL/min/kg and for low GFR as GFR <1.2 mL/min/kg, as determined previously by our group.35
Sensitivity and specificity of sCr and of sCysC to detect decreased GFR (PexICT < 1.7 mL/min/kg) were calculated. “Positive test” was defined as sCr >1.83 mg/dL (161.8 μmol/L) and sCysC >1.95 mg/L. “Negative test” was defined as sCr <1.83 mg/dL (161.8 μmol/L) and sCysC <1.95 mg/L. These concentrations were based on the reference intervals (RI's) we established in a previous study.7 The nonparametric receiving operating curve (ROC) for sCysC and sCr were additionally configured.
Results
Study Population
In total, 90 cats were recruited (age range: 1.1 years to 19 years), namely 49 CKD and 41 healthy cats.
For the 49 CKD cats, breed distribution was: 1 Siamese, 1 Oriental Shorthair, 1 Persian, 1 Maine Coon, 1 Burmese, 2 Ragdolls, 2 Birmans, 4 British Shorthair cats, and 36 domestic shorthair and longhair cats (DSH/DLH). Four cats were female intact, 19 female neutered, and 26 male neutered. Mean ± SD age was 10 ± 4.7 years and mean ± SD body weight was 4.1 ± 1.2 kg. One cat had IRIS stage 1 nonproteinuric CKD and was diagnosed based on ultrasonographic findings, low USG and borderline sCr. The cat had IRIS stage 2 CKD and IRIS stage 3 CKD, 5 months and 2 years after inclusion respectively. Twenty cats had IRIS stage 2 CKD, of which 5 were proteinuric (UPC > 0.4), 5 borderline proteinuric (UPC [0.2–0.4]), and 10 did not have proteinuria. Thirteen cats had IRIS stage 3 CKD. Six of those cats were proteinuric, 3 were borderline proteinuric, and the other 4 cats did not have proteinuria. Fifteen cats had IRIS stage IV CKD, of which 11 were proteinuric and 4 borderline proteinuric. One cat with severe proteinuria (UPC = 4.22) also had glucosuria without hyperglycemia. Three CKD cats were treated with ACE‐inhibitors but treatment was ceased 1 month prior to inclusion. One CKD cat was treated with ARB, and treatment was stopped 2 weeks prior to inclusion.
For the 41 healthy cats, breed distribution was: 1 Birman cat, 1 Persian cat, 2 Ragdolls, 2 British Shorthair, and 35 domestic short‐or longhair (DSH/DLH) cats. Two cats were female intact, 25 female neutered, and 14 male neutered. Mean ± SD age was 9.9 ± 3.5 years and mean ± SD body weight was 4.4 ± 1.2 kg.
Systolic blood pressure measurement was performed in 45 CKD and 37 healthy cats. The other 8 cats were not cooperative enough, to reliably determine SBP. Four CKD cats were hypertensive, of which only 1 was proteinuric. Also 4 of the healthy cats had SBP >160 mmHg. Although fundic exam was not undertaken to distinguish white‐coat hypertension from true hypertension, white‐coat hypertension was a likely explanation, since those cats were very stressed during the measurement.28 Systolic blood pressure, sCr, serum urea, sCysC, USG, UPC, of both CKD and healthy cats are presented in Table 1.
Table 1.
Descriptive statistics of both CKD and healthy cats. The variables are presented as mean ± SD
| Variable (unit) | CKD (n = 49) | Healthy (n = 41) | P‐value |
|---|---|---|---|
| SBP (mmHg) | 135 ± 27 | 142 ± 20 | P = .06 |
| sCr (mg/dL) | 4.06 ± 2.53 | 1.23 ± 0.26 | P < .001 |
| Serum Urea (mg/dL) | 74.5 ± 54.3 | 26.3 ± 5.9 | P < .001 |
| sCysC (mg/L) | 1.4 ± 0.5 | 1.0 ± 0.3 | P < .001 |
| USG | 1.019 ± 0.009 | 1.045 ± 0.007 | P < .001 |
| UPC | 0.67 ± 0.92 | 0.21 ± 0.14 | P = .003 |
| uCysC/uCr (mg/mol) | 291 ± 411 | 0.32 ± 0.97 | P < .001 |
CKD, chronic kidney disease; SBP, systolic blood pressure; sCr, serum creatinine; sCysC, serum cystatin C; USG, urine specific gravity; UPC, urinary protein:creatinine ratio; uCysC/uCr, urinary cystatin C:creatinine ratio.
Comparison of sCysC and uCysC between cats with CKD and healthy cats
Serum CysC was measured in all cats and uCysC in 44 CKD cats and all healthy cats. Urinary CysC was <LOD (0.049 mg/L) in 15/44 CKD cats and in all but 5 healthy cats. We observed that there was a significant effect (P < .001) of the status (CKD or healthy) on sCysC and uCysC/uCr with significantly higher concentrations in CKD cats (Table 1). The IRIS stage also had a significant positive effect (P < .001) on both sCysC and uCysC/uCr, with increasing mean concentrations as IRIS stage increased (Table 2). However, R 2 between IRIS stage and CysC was weak (0.31 for sCysC and 0.29 for uCysC). Also UPC had a significant effect (P < .001) on uCysC/uCr in the whole population and in cats with CKD. R 2 was 0.54 for the whole population and 0.50 for the cats with CKD.
Table 2.
Serum CysC (sCysC) and urinary cystatin C to creatinine ratio (uCysC/uCr) measured met the nephelometric assay in the healthy cats and cats with CKD for each IRIS stage. Data are presented as mean ± SD. n represents number of cats
| Status | sCysC (mg/L) | uCysC/uCr (mg/mol) |
|---|---|---|
| Healthy (n = 41) | 1.0 ± 0.28 | 0.32 ± 0.95 |
| CKD IRIS stage 1 (n = 1) | 1.065 | 7.40 |
| CKD IRIS stage 2 (n = 17) | 1.26 ± 0.40 | 123.62 ± 374.4 |
| CKD IRIS stage 3 (n = 11) | 1.50 ± 0.50 | 254.0 ± 206.0 |
| CKD IRIS stage 4 (n = 15) | 1.67 ± 0.57 | 526.80 ± 489.87 |
CKD, chronic kidney disease; IRIS, international renal interest society; SD, standard deviation LOD, limit of detection.
Comparison of correlation between GFR and sCysC versus sCr
The PEC‐ICT was performed in 17 CKD and 15 healthy cats. The mean ± SD Cr, endo‐ and exo‐iohexol clearances of the CKD and healthy cats are presented in Table 3. In 1 healthy cat, the serum sample 60 minutes after injection was not available, and therefore the GFR of that cat was calculated based on 9 samples instead of 10 samples. Both for the PENIA and the PETIA, there was a significant correlation between GFR and sCysC. The scatter plots of sCr and sCysC PENIA, sCysC PETIA versus PexICT are presented in Fig 1. The other GFR‐markers showed comparable results. The regression coefficients with P‐values are presented in Table 4.
Table 3.
Mean ± SD of plasma clearance (mL/min/kg) of creatinine, exo‐iohexol and endo‐iohexol in CKD and healthy cats
| CKD (n = 17) | Healthy (n = 15) | |
|---|---|---|
| PECCT | 0.9 ± 0.3 | 2.3 ± 0.6 |
| PexICT | 0.9 ± 0.4 | 2.1 ± 0.5 |
| PenICT | 1.2 ± 0.5 | 2.9 ± 0.7 |
Cr, creatinine; CKD, chronic kidney disease; PECCT, plasma exogenous creatinine clearance test; PexICT, plasma exogenous iohexol clearance test; PenICT, plasma endogenous iohexol clearance test.
Figure 1.
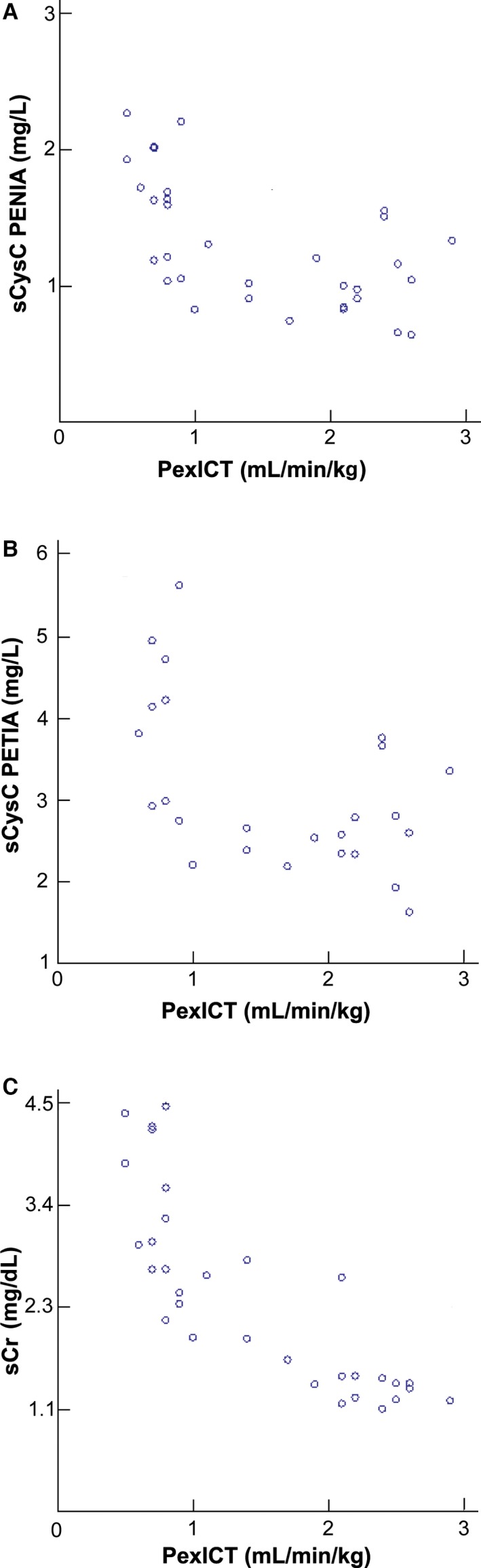
Scatter plots of the glomerular filtration rate (GFR) determined with a plasma exogenous iohexol clearance test (PexICT) and serum creatinine (sCr), cystatin C analyzed with the particle enhanced nephelometric immunoassay (sCysC PENIA) and cystatin C analyzed with the particle enhanced turbidimetric immunoassay (sCysC PETIA).
Table 4.
R 2 values and associated P‐values of sCr and sCysC measured with the PENIA and PETIA versus plasma clearance of creatinine, exo‐and endo‐iohexol
| PECCT | PexICT | PenICT | |
|---|---|---|---|
| sCysC PENIA | 0.46 (P < .001) | 0.34 (P < .001) | 0.37 (P < .001) |
| sCysC PETIA | 0.44 (P < .001) | 0.31 (P = .003) | 0.36 (P = .001) |
| sCr | 0.74 (P < .001) | 0.68 (P < .001) | 0.67 (P < .001) |
sCr, serum creatinine; sCysC, serum cystatin C; PENIA, particle enhanced nephelometric immunoassay; PETIA, particle enhanced nephelomtric immunoassay; PECCT, plasma exogenous creatinine clearance test; PexICT, plasma exo‐iohexol clearance test; PenICT, plasma endo‐iohexol clearance test.
Determination of Sensitivity and Specificity of sCysC
Results from the clearance test demonstrated that one cat classified as “healthy” actually had borderline GFR and another cat classified as “healthy” had low GFR. In addition, one “CKD” cat actually had borderline GFR and another had normal GFR. The boxplots of sCysC and sCr from cats classified with normal, borderline, and low GFR are presented in Fig 2. For sCysC, the overlap was much larger compared to sCr between cats with normal GFR, cats with borderline and low GFR. Serum CysC exceeded the RI previously established by our group, in only 4 of 16 cats with low GFR. In contrast, sCr exceeded the RI in all of them. Indeed, the sensitivity of detecting decreased GFR (<1.7 mL/min/kg) was 22% for sCysC compared with 83% for sCr. In contrast, the specificity for sCysC was 100% compared with 93% for sCr. The ROC is presented in Fig 3.
Figure 2.
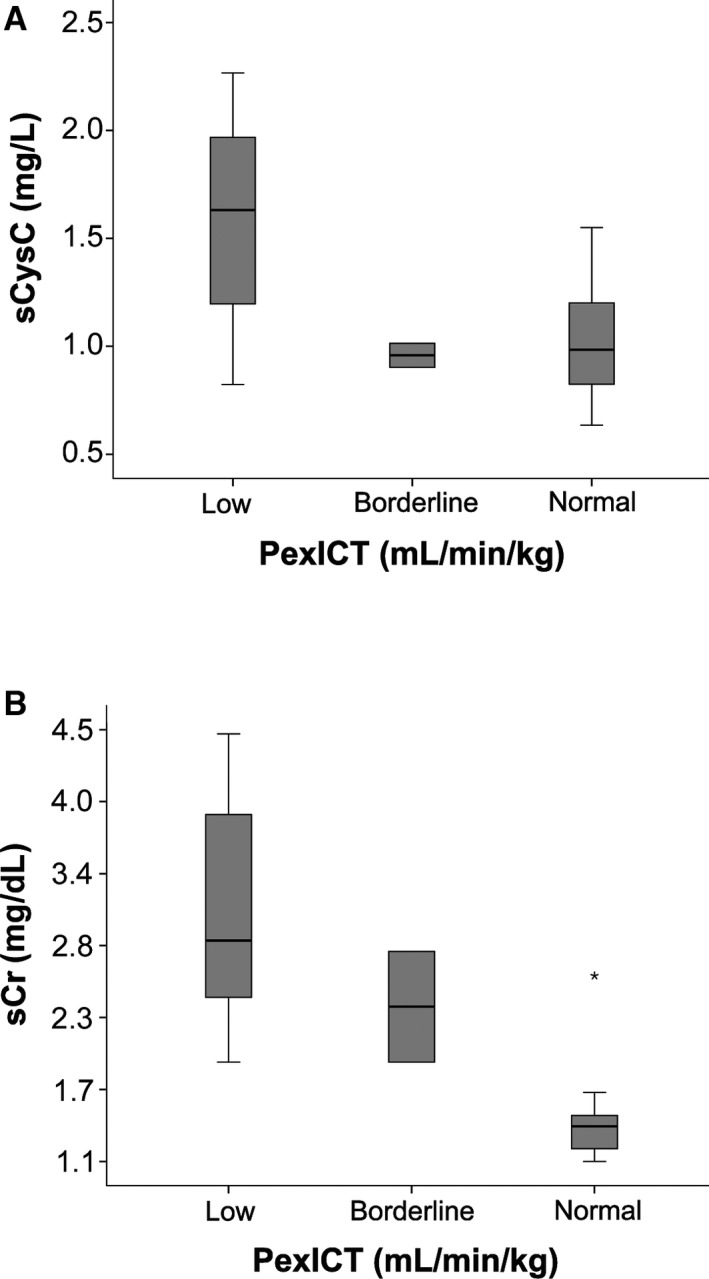
Boxplot of serum cystatin C (sCysC) for cats with normal GFR (GFR ≥ 1.7 mL/min/kg) determined with a plasma exo‐iohexol clearance test (PexICT); borderline GFR (GFR (1.2–1.7 mL/min/kg)) and low GFR (GFR < 1.2 mL/min/kg).
Figure 3.
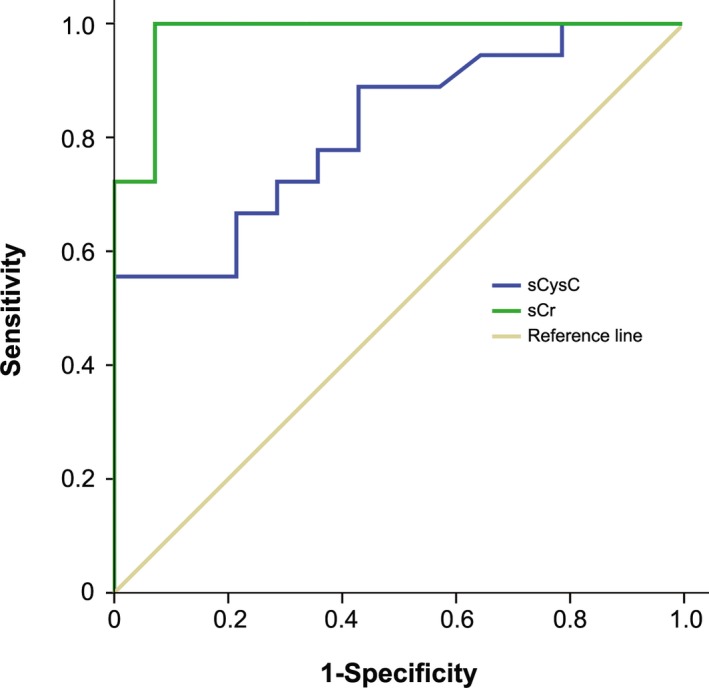
Nonparametric receiver operating characteristics (ROC) plots of the sensitivity and specificity of sCysC and sCr for distinguishing cats with normal and reduced GFR (<1.7 mL/min/kg) determined with plasma exo‐iohexol clearance test (PexICT).
Comparison between PETIA and PENIA for sCysC Analysis
The two methods were highly correlated (R 2 = 0.94 P < .001), but sCysC concentrations measured with PETIA were significantly higher (P < .001) than those measured with PENIA. No significantly better correlation between sCysC PETIA and GFR (whatever the marker) than between sCysC PENIA and GFR could be observed (Fig 2, Table 4). Fig 4 presents the Bland‐Altman Plot in which PENIA and PETIA are compared. The mean ± SD difference was calculated as 1.84 ± 0.95 mg/L. The limits of agreement were wide and the difference increases as the mean increases.
Figure 4.
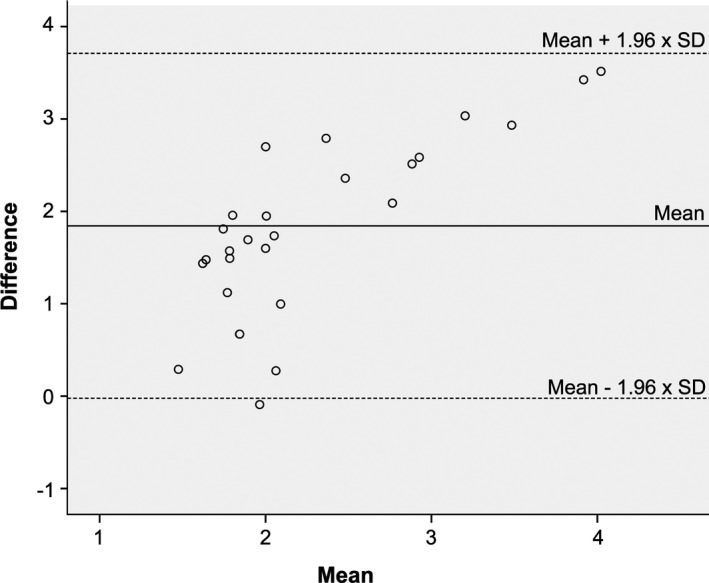
Bland‐Altman Plot of serum cystatin C analyzed with the particle enhanced tubidimetric immunoassay (PETIA) and particle enhanced nephelometric immunoassay (PENIA).
Discussion
The main results of this study are: (1) sCysC was significantly higher in cats with CKD, compared with healthy cats, but an important overlap was present; (2) urinary CysC was not present in all cats with CKD; (3) the correlation between GFR measured with PEC‐ICT and sCysC was weaker than that between GFR and sCr, regardless of the sCysC assay.
Although sCysC was significantly higher in CKD versus healthy cats, several other findings argue against the use of sCysC in cats: an obvious overlap in sCysC between both groups was present, as previously described in cats22, 36 and dogs.37 In addition, only 6 of the 49 CKD cats had sCysC above the upper limit (1.95 mg/L) of the RI, determined in a previous study.7 Furthermore, although significant, the correlations between sCysC and IRIS stage were weak and probably not clinically relevant, since not all cats with CKD IRIS stage 3 or 4 had a higher sCysC concentration compared with the healthy cats or cats with CKD IRIS stage 2.
Urinary CysC was also significantly different between CKD and healthy cats. In contrast with sCysC, an overlap for uCysC was absent. As for sCysC, a significant but weak correlation between uCysC/uCr and the IRIS stage was present, and not all CKD cats with IRIS stage 3 or 4 had higher uCysC/uCr compared to healthy cats and CKD cats with IRIS stage 2. Indeed, 5 of the 41 healthy cats had a detectable uCysC concentration. With normal kidney function CysC is completely reabsorbed and catabolized in the tubules.38, 39 Small quantities can still be found in the urine, but one would expect this concentration to be <LOD.40 Two of the 5 healthy cats with detectable uCysC were borderline proteinuric. None of those 5 cats had overt proteinuria, azotemia, or isostenuric urine, so CKD is unlikely. Nevertheless, no follow‐up or GFR results are available for those cats, so early CKD cannot be excluded. Unexpectedly, 15 of the 49 CKD cats had uCysC <LOD, of which 2 cats were proteinuric and 2 borderline proteinuric. In a previous study41 of our group, uCysC could also not be detected in 5 of the 10 included cats with CKD. Those observations are surprising. Most of the cats with CKD have tubulo‐interstitial lesions. Therefore, we expected detectable uCysC in most of the CKD cats. However, without histopathology, a less typical form of CKD cannot be excluded. In contrast, uCysC seemed to be valuable as marker for local proximal damage in hyperthyroid cats.42 Therefore, the prognostic value and ability to detect early tubular damage of uCysC should be evaluated further with investigation of renal biopsies.
The major objective of the present study was to compare the correlation of GFR with sCysC and sCr. In contrast with human studies14, 15, 43 and studies in dogs17, 18 a weaker correlation was found between PECCT, PenICT, PexICT, and sCysC measured with PENIA or PETIA compared with sCr. In addition, the use of the inverse values increased R 2 value for sCr, but not for sCysC PETIA or sCysC PENIA. From these findings we can conclude that sCysC does not appear to be advantageous over sCr for detection of CKD in cats.
Our results are different from a similar study in cats, demonstrating a significantly better correlation of sCysC with PICT compared to that with sCr.36 In the study of Poświotowska‐Kaszczyszyn a one‐compartmental model and the slope‐intercept method corrected with the Brochner‐Mortensen formula was used to calculate GFR.44 It has been shown that one‐compartmental models overestimate true GFR, because of underestimation of AUC and the slope‐intercept method with the Brochner‐Mortensen formula can cause increasing errors with increasing clearances.45 Since we used a different method for GFR calculation, it is difficult to compare the results from the present study with the study from Poświotowska‐Kaszczyszyn. In addition, in this study we determined GFR with 3 different markers (Cr, endo‐and exo‐iohexol) to evaluate sCysC. However, no better correlation of each of the 3 methods with sCysC could be observed.
Early kidney impairment in some of the healthy cats could not be excluded. Therefore, in the subgroups in which GFR was measured, we correlated sCysC and sCr with renal function. Only 2 of the 15 cats previously classified as “healthy” had low and borderline GFR respectively. These 2 cats did not show high sCysC value exceeding the RI. Serum CysC overlapped between cats with low, borderline, and normal GFR, indicating that sCysC cannot distinguish between those three groups. For sCr, the overlap was less severe and could mainly be observed between cats with low and borderline GFR, as would have been expected. In dogs on the other hand, a significantly better correlation between sCysC and PECCT18 or PICT17 has been shown. However, an overlap in sCysC between healthy dogs and dogs with CKD was also present,17, 46, 47 and canine sCysC also seemed to overlap between dogs with normal and borderline GFR in the study from Wehner and co‐workers.17 Nevertheless, in contrast with studies in dogs,17, 18 no higher sensitivity of sCysC than of sCr to detect decreased GFR in cats could be observed in the present study. In contrast, a higher specificity was present, which means that if sCysC is increased in cats, CKD is definitely present.
The findings of the present study do not encourage the clinical use of CysC measurement in cats. However, suboptimal CysC determination in cats cannot be completely ruled out. We obtained a signal with the PETIA and PENIA, but we cannot exclude that measurement of CysC in cats by human assays is suboptimal. Western blot analysis with antibodies from the PENIA22 could not demonstrate good cross‐reactivity at 13 kDa, in contrast with the antibodies from the PETIA (Data S1). For both assays, bands at 26 and 52 kDa were visible. In humans, it has been shown that denaturing agents or high temperature can cause di‐and polymerization of CysC.48 It is unknown whether this occurs in dogs and cats. Alternatively, the human polyclonal anti‐human CysC antibodies might detect polymers and not 13 kDa CysC in cats. Another explanation for suboptimal testing could be the relatively limited homology between human and CysC in cats. A homology of 70% between cat and human CysC has been described,49, 50 but the epitope sequence to which the antibody binds is not provided by the manufacturers, which makes evaluation of cross‐reactivity between the anti‐human CysC antibodies and feline CysC difficult. Therefore, a cat assay should be developed, followed by a re‐evaluation of this marker. Until then, we do not recommend the use of CysC as renal marker in cats.
Conclusion
Serum CysC was not able to distinguish healthy cats from cats with CKD. Furthermore, uCysC was not present in all CKD cats. A limitation of this study was the requirement to fix the value of CysC concentration when it was below LOD. There is no consensus regarding how to manage this situation but whichever value is chosen, it will have the effect of decreasing variability and inducing a statistical bias. An alternative option would have been to use a value other than 0, i.e. half of LOD, however, overall this would not have altered the significant difference identified between the healthy and CKD cats. Whatever the marker for GFR determination and assay for CysC measurement, a markedly weaker correlation between GFR and sCysC compared with sCr was demonstrated. Therefore, we do not advise to use sCysC in cats as an indirect marker for GFR.
Supporting information
Data S1. Materials and methods.
Fig S1. Western Blot analysis with chemiluminiscent detection of the polyclonal rabbit anti‐human cystatin C antibody from the particle enhanced turbidimetric immunoassay (PETIA) in feline serum (1A) and urine (1B).
Fig S2. Sequential dilution of serum (2A) and urine (2B) of a cat with chronic kidney disease (CKD) analyzed with PETIA illustrating linearity.
Acknowledgments
For this work support was received from the institute for the promotion of innovation by science and technology in Flanders (IWT) through a bursary to L.F.E. Ghys. The authors thank MedVet, AML Veterinary Medicine, for the analyses of the samples. The authors also thank Ms. Bieke Weyn, Mrs. Saar Muylaert, Mrs. Elke Lecocq, Mrs. Kristel Demeyere, Mrs. Siegrid De Baere, and Ms. Jelle Lambrecht for their technical assistance.
This article received support from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT) (grant number: 11070).
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This paper was presented at the 25th ECVIM Congress, September 10–12, 2015, Lisbon, Portugal.
Footnotes
Advia 2120, Siemens, Brussels, Belgium
Architect C16000, Abbott Max‐Planck‐Ring, Wiesbaden, Germany
Atago, Tokyo, Japan
Iricell velocity, chemical system, Instrumentation Laboratory, Zaventem, Belgium
IQ 200 SPRINT, Instrumentation Laboratory, Zaventem, Belgium
Immulite 2000 system, Siemens Healthcare Diagnostics, Marburg, Germany
Wask Copan, MLS, Vitek 2 system, Biomerieux, Brussels, Belgium
Anhydrous creatinine, Sigma Chemical Co, St Louis, MO
Omnipaque 300, GE Healthcare, Amersham Health, Wemmel, Belgium
Particle enhanced nephelometric assay, Siemens Healthcare Diagnostics, Marburg, Germany
BN Prospec Nephelometer, Siemens Healthcare Diagnostics, Marburg, Germany
Particle enhanced turbidimetric assay, Dako, Glostrup, Denmark
Cobas C system, Roche Diagnostics Gmbh, Mannheim, Germany
Vettest, Idexx laboratories Europe B.V., Amsterdam, the Netherlands
Med Vet Lab, Antwerp, Belgium
WinNonlin version 4.0.1., Scientific Consulting Inc. Apex, NC)
Systat 12, Systat Software Inc
References
- 1. Marino CL, Lascelles BDX, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014;16:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lulich JP, Osborne CA, Obrien TD, et al. Feline renal failure: Questions, answers, questions. Comp Cont Educ Pract 1992;14:127–154. [Google Scholar]
- 3. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999;214:1336–1341. [PubMed] [Google Scholar]
- 4. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973‐1984). J Am Vet Med Assoc 1987;190:1196–1202. [PubMed] [Google Scholar]
- 5. Paepe D, Daminet S. Feline CKD: Diagnosis, staging and screening‐ what is recommeded? J Feline Med Surg 2013;15:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun JP, Lefebvre HP. Kidney Function and Damage In: Kaneko JJ, Harvey JW, Bruss ML, ed. Clinical Biochemistry of Domestic Animals, sixth ed London: Elsevier; 2008:485–528. [Google Scholar]
- 7. Ghys L, Paepe D, Duchateau L, et al. Biological validation of feline cystatin C: Effect of breed, age and gender. Vet J 2015;204:168–173. [DOI] [PubMed] [Google Scholar]
- 8. James GD, Sealey JE, Alderman M, et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens 1988;1:124–131. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Berg RL, Gassman JJ, et al. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl 1989;27:S73–S80. [PubMed] [Google Scholar]
- 10. Abrahamson M. Human cysteine proteinase inhibitors. Isolation, physiological importance, inhibitory mechanism, gene structure and relation to hereditary cerebral hemorrhage. Scand J Clin Lab Invest Suppl 1988;191:21–31. [PubMed] [Google Scholar]
- 11. Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J 1990;268:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seronie‐Vivien S, Delanaye P, Pieroni L, et al. Cystatin C: Current position and future prospects. Clin Chem Lab Med 2008;46:1664–1686. [DOI] [PubMed] [Google Scholar]
- 13. Grubb A, Simonsen O, Sturfelt G, et al. Serum concentration of cystatin C, factor D and beta 2‐microglobulin as a measure of glomerular filtration rate. Acta Med Scand 1985;218:499–503. [DOI] [PubMed] [Google Scholar]
- 14. Kyhse‐Andersen J, Schmidt C, Nordin G, et al. Serum cystatin C, determined by a rapid, automated particle‐enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 1994;40:1921–1926. [PubMed] [Google Scholar]
- 15. Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 1995;47:312–318. [DOI] [PubMed] [Google Scholar]
- 16. Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma‐trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 1985;45:97–101. [DOI] [PubMed] [Google Scholar]
- 17. Miyagawa Y, Takemura N, Hirose H. Evaluation of the measurement of serum cystatin C by an enzyme‐linked immunosorbent assay for humans as a marker of the glomerular filtration rate in dogs. J Vet Med Sci 2009;71:1169–1176. [DOI] [PubMed] [Google Scholar]
- 18. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc 2008;44:131–138. [DOI] [PubMed] [Google Scholar]
- 19. Uchida K, Gotoh A. Measurement of cystatin‐C and creatinine in urine. Clin Chim Acta 2002;323:121–128. [DOI] [PubMed] [Google Scholar]
- 20. Conti M, Moutereau S, Zater M, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med 2006;44:288–291. [DOI] [PubMed] [Google Scholar]
- 21. Monti P, Benchekroun G, Berlato D, et al. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J Small Anim Pract 2012;53:254–259. [DOI] [PubMed] [Google Scholar]
- 22. Ghys LFE, Meyer E, Paepe D, et al. Analytical validation of a human particle‐enhanced nephelometric assay for cystatin C measurement in feline serum and urine. Vet Clin Pathol 2014;43:226–234. [DOI] [PubMed] [Google Scholar]
- 23. Ghys LFE, Paepe D, Lefebvre H, et al. The effect of feeding, storage and anticoagulant on feline serum cystatin C. Vet J 2015;206:91–96. In press. doi: 10.1016/j.tvjl.2015.07.00. [DOI] [PubMed] [Google Scholar]
- 24. Ishiguro H, Ohkubo I, Mizokami M, et al. The use of monoclonal antibodies to define levels of cystatin C in normal human serum. Hybridoma 1989;8:303–313. [DOI] [PubMed] [Google Scholar]
- 25. Finney H, Newman DJ, Gruber W, et al. Initial evaluation of cystatin C measurement by particle‐enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 1997;43:1016–1022. [PubMed] [Google Scholar]
- 26. Randers E, Erlandsen E. Serum cystatin C as an endogenous marker of the renal function‐a review. Clin Chem Lab 1999;37:389–395. [DOI] [PubMed] [Google Scholar]
- 27. Polzin DJ. Chronic kidney disease In: Ettinger SJFEC, ed. Textbook of Veterinary Internal Medicine, 7th ed Philadelphia: WB Saunders; 2010:1990–2021. [Google Scholar]
- 28. DiBartola SP. Clinical approach and laboratory evaluation of renal disease In: Ettinger SJFEC, ed. Textbook of Veterinary Internal Medicine. Philadelphia: WB Saunders; 2010:1955–1969. [Google Scholar]
- 29. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 30. Paepe D, Smets P, van Hoek I, et al. Within‐ and between‐examiner agreement for two thyroid palpation techniques in healthy and hyperthyroid cats. J Feline Med Surg 2008;10:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paepe D, Verjans G, Duchateau L, et al. Routine healthy screening findings in apparently healthy middle‐aged and old cats. J Feline Med Surg 2013;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Hoek I, Vandermeulen E, Duchateau L, et al. Comparison and reproducibility of plasma clearance of exogenous creatinine, exo‐iohexol, endo‐iohexol, and 51Cr‐EDTA in young adult and aged healthy cats. J Vet Intern Med 2007;21:950–958. [DOI] [PubMed] [Google Scholar]
- 33. Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin excretion corrected by creatinine and specific gravity. Clin Chim Acta 2000;294:139–155. [DOI] [PubMed] [Google Scholar]
- 34. Watson AD, Lefebvre HP, Concordet D, et al. Plasma exogenous creatinine clearance test in dogs: Comparison with other methods and proposed limited sampling strategy. J Vet Intern Med 2002;16:22–33. [DOI] [PubMed] [Google Scholar]
- 35. Paepe D, Lefebvre HP, Concordet D, et al. Simplified methods for estimating glomerular filtration rate in cats and for detection of cats with low or borderline glomerular filtration rate. J Feline Med Surg 2015;17:889–900. Epub ahead of print December 17, 2014: doi: 10.1177/1099612X14561106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Poswiatowska‐Kaszczyszyn I. Usefulness of serum cystatin C measuremen for assessing renal function in cats. Bull Vet Inst Pulawy 2012;56:235–239. [Google Scholar]
- 37. Almy FS, Christopher MM, King DP, et al. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Intern Med 2002;16:45–51. [DOI] [PubMed] [Google Scholar]
- 38. Roald AB, Aukland K, Tenstad O. Tubular absorption of filtered cystatin‐C in the rat kidney. Exp Physiol 2004;89:701–707. [DOI] [PubMed] [Google Scholar]
- 39. Tenstad O, Roald AB, Grubb A, et al. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 1996;56:409–414. [DOI] [PubMed] [Google Scholar]
- 40. Lofberg H, Grubb AO. Quantitation of gamma‐trace in human biological fluids: Indications for production in the central nervous system. Scand J Clin Lab Invest 1979;39:619–626. [DOI] [PubMed] [Google Scholar]
- 41. Paepe D, Ghys LF, Smets P, et al. Routine kidney variables, glomerular filtration rate and urinary cystatin C in cats with diabetes mellitus, cats with chronic kidney disease and healthy cats. J Feline Med Surg 2015;17:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghys LFE, Paepe D, Taffin ERL, et al. Serum and urinary cystatin C in cats with feline immunodeficiency virus infection and cats with hyperthyroidism. J Feline Med Surg 2015; Epub ahead of Print June 22, 2015, doi: 10.1177/1098612X15592343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stickle D, Cole B, Hock K, et al. Correlation of plasma concentrations of cystatin C and creatinine to inulin clearance in a pediatric population. Clin Chem 1998;44:1334–1338. [PubMed] [Google Scholar]
- 44. Bröchner‐Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J of Clin Lab Invest 1972;30:271–274. [DOI] [PubMed] [Google Scholar]
- 45. Finch NC, Syme HM, Elliott J, et al. Glomerular filtration rate estimation by use of a correction formula for slope‐intercept plasma iohexol clearance in cats. Am J Vet Res 2011;72:1652–1659. [DOI] [PubMed] [Google Scholar]
- 46. Antognoni MT, Siepi D, Porciello F, et al. Use of serum cystatin C determination as a marker of renal function in the dog. Vet Res Commun 2005;29(Suppl 2):265–267. [DOI] [PubMed] [Google Scholar]
- 47. Braun JP, Perxachs A, Péchereau D, et al. Plasma cystatin C in the dog: Reference values and variations with renal failure. Comp Clin Pathol 2002;11:44–49. [Google Scholar]
- 48. Ekiel I, Abrahamson M. Folding‐related dimerization of human cystatin C. J Biol Chem 1996;271:1314–1321. [DOI] [PubMed] [Google Scholar]
- 49. Nakata J, Nakahari A, Takahashi C, et al. Molecular cloning, expression in Escherichia coli, and development of monoclonal antibodies to feline cystatin C. Vet Immunol Immunopathol 2010;138:231–234. [DOI] [PubMed] [Google Scholar]
- 50. Pearson WR, Wood T, Zhang Z, et al. Comparison of DNA sequences with protein sequences. Genomics 1997;46:24–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and methods.
Fig S1. Western Blot analysis with chemiluminiscent detection of the polyclonal rabbit anti‐human cystatin C antibody from the particle enhanced turbidimetric immunoassay (PETIA) in feline serum (1A) and urine (1B).
Fig S2. Sequential dilution of serum (2A) and urine (2B) of a cat with chronic kidney disease (CKD) analyzed with PETIA illustrating linearity.


