Abstract
Background
An aerosol foam formulation of fixed combination calcipotriol 50 μg/g (Cal) and betamethasone 0.5 mg/g (as dipropionate; BD) has been developed for psoriasis vulgaris treatment.
Objective
To compare Cal/BD aerosol foam pharmacodynamic activity with Cal/BD ointment and with other topical corticosteroids of different potencies by assessing vasoconstrictor potential.
Methods
A Phase I, single‐centre, investigator‐blinded, vehicle‐controlled, intra‐individual comparison vasoconstriction study. Healthy volunteers received a single application on selected sites of: Cal/BD aerosol foam, clobetasol propionate 0.5 mg/g cream (CP; very potent), Cal/BD ointment (potent), fluocinolone acetonide 0.25 mg/g ointment (FA; moderately potent), BD aerosol foam and aerosol foam vehicle. A seventh untreated site acted as a negative control. Skin blanching was assessed by visual (primary response criterion) and colorimetric a* and L* measurements (secondary criteria), and was analysed over time (6–32 h post‐application).
Results
Thirty‐five healthy volunteers were included. All active treatments led to significantly greater skin blanching than control. By visual assessment, skin blanching with Cal/BD aerosol foam was significantly less compared with CP cream [mean AUC0–32 2560 vs. 3831; mean difference = −1272; 95% confidence interval (CI): −1598, −945; P < 0.001], similar to BD aerosol foam (mean AUC0–32 2560 vs. 2595; mean difference = −35; 95% CI: −362, 292; P = 0.83) and significantly greater than Cal/BD ointment (mean AUC0–32 2560 vs. 2008; mean difference = 552; 95% CI: 225, 878; P = 0.001) and FA ointment (mean AUC0–32 2560 vs. 1981; mean difference = 578; 95% CI: 251, 905; P < 0.001). Colorimetric assessments a* and L* also indicated significantly reduced skin blanching with Cal/BD aerosol foam compared with CP cream. No adverse events (AEs) were reported.
Conclusion
Cal/BD aerosol foam can be considered a more potent formulation than Cal/BD ointment and the moderately potent FA ointment, but less potent than the very potent corticosteroid, CP cream.
Introduction
Most patients with psoriasis vulgaris1 can effectively manage their disease with topical use of vitamin D3 analogues and corticosteroids, either as separate products used in combination or as fixed combination formulations.2, 3 The fixed combination of calcipotriol 50 μg/g (Cal) and betamethasone 0.5 mg/g (as dipropionate; BD) has demonstrated increased efficacy and fewer adverse events (AEs) compared with the individual active components.4, 5 An innovative alcohol‐free aerosol foam formulation of the fixed combination (Cal/BD aerosol foam) has been developed to enhance drug delivery and improve treatment acceptability and adherence for patients with psoriasis vulgaris. Enhanced drug delivery has been demonstrated in an in vitro skin penetration study, whereby steady‐state Cal/BD levels were significantly higher following Cal/BD aerosol foam application compared with the fixed combination ointment.6 Moreover, Phase II trials have demonstrated improved treatment efficacy.7
Many topical corticosteroids for psoriasis treatment are available in differing potencies and formulations. In Europe, corticosteroids are classified into four types according to increasing potency, from I (mild) to IV (very potent), whereas in the US, corticosteroids are divided into seven classes based on decreasing potency, from 1 (super‐potent) to 7 (lowest potency). Knowledge of topical corticosteroid potency is vital to ensure appropriate use. Although corticosteroids have favourable tolerability profiles when used within routine therapeutic ranges, excessive exposure to very potent corticosteroids is associated with an increased risk of cutaneous and systemic side effects.8, 9 Corticosteroid potency is often assessed by vasoconstriction assays.10 The McKenzie and Stoughton vasoconstriction assay (human skin blanching test)11 evaluates the blanching (whitening) response induced in healthy skin by topical corticosteroid application. Since it is a measure of corticosteroid diffusion through the outermost layer of the skin (stratum corneum), the intensity of blanching has been shown to be directly related to the inherent potency of topical corticosteroids. Furthermore, corticosteroid potency, as determined by the vasoconstriction assay, correlates with clinical activity in psoriasis,12 and as such is considered a measure of corticosteroid efficacy.
If topical corticosteroids are used appropriately, the incidence of side effects is rare; however, used inappropriately, corticosteroids can cause serious local (e.g. skin atrophy) or systemic effects (e.g. suppression of the hypothalamic–pituitary–adrenal axis).13 The risk of side effects is higher the greater the potency of topical corticosteroid. Furthermore, a change in topical formulation can result in greater total absorption of corticosteroid, which could have important implications for the patient in terms of safety and/or efficacy.14, 15 Therefore, it is important to determine the steroid potency of topical therapies to ensure appropriate use and minimize the risks associated with treatment.
The aim of this vasoconstriction study in healthy volunteers was, therefore, to evaluate the steroid potency of BD in Cal/BD aerosol foam and compare it with other well‐known topical products containing corticosteroids of different potencies.
Materials and methods
Healthy volunteers
Enrolled healthy volunteers were 18–50 years old, with skin type I to IV (Fitzpatrick scale).16 All demonstrated adequate vasoconstriction prior to the study, which was defined as visual skin blanching score of at least one unit (scale 0–4) after non‐occlusive Cal/BD ointment application for 4–6 h on a 2.2‐cm diameter forearm test area (different to study test sites).
Volunteers were excluded if they received systemic treatments (including corticosteroids, other vasoactive drugs and acetylsalicylic acid) or any medications that could interfere with the blanching reaction within 2 weeks, or used topical corticosteroids on the test areas within 4 weeks prior to enrolment. Other exclusion criteria included: abnormal skin pigmentation; hypersensitivity to investigational product component(s); any systemic, cutaneous or calcium metabolism disorders; any skin infection or other inflammatory skin disease; participation in another investigational clinical trial. All volunteers provided written informed consent prior to study commencement.
Study design
This was a Phase I, single‐centre, investigator‐blinded, vehicle‐controlled, intra‐individual comparison study (NCT01946386). Each volunteer received a single application of each test product: Cal/BD aerosol foam, clobetasol propionate 0.5 mg/g cream [CP; very potent (EU); super‐potent (US)], Cal/BD ointment [BD; potent (EU and US)], fluocinolone acetonide 0.25 mg/g ointment [FA; moderately potent (EU); medium‐strength potency (US)], BD aerosol foam and aerosol foam vehicle. Treatment application was randomized per volunteer to six anterior forearm test sites (a seventh site remained untreated as a negative control; each circular test site was 2.2‐cm in diameter, delineated using a disposable device and outlined with an indelible marker). Each volunteer received a unique randomization number that determined application scheme; the randomization schedule was based on five Latin squares (7 × 7) generated by LEO Pharma. A calibrated Eppendorf® pipette (Eppendorf AG, Hamburg, Germany) was used to transfer 20 μL of CP cream, Cal/BD ointment and FA ointment. Cal/BD aerosol foam, BD aerosol foam and aerosol foam vehicle were sprayed onto pre‐weighed dishes and propellants allowed to evaporate for at least 30 s until 14.5–19.5 mg of active ingredients remained (corresponding to 20 μL ± 15%). Treatments were administered by trained study centre personnel, gently rubbed in with different gloved fingers, and treatment site protected with non−occlusive gauze. According to the McKenzie–Stoughton11 vasoconstriction trial design, the treatment period was 6 h, after which remaining treatments were removed.
The investigational site's independent ethics committee approved the protocol; the study conformed to the Declaration of Helsinki and Good Clinical Practice guidelines.
Study objectives and assessments
The objective was to compare the pharmacodynamic activity of Cal/BD aerosol foam with CP cream, Cal/BD ointment, FA ointment, BD aerosol foam and aerosol foam vehicle using the human skin blanching test (based on the McKenzie–Stoughton11 vasoconstriction assay). Colorimetric and visual vasoconstriction assessments occurred at 6 h and 10 min (allowing for remaining topical product to be removed after 6 h) and at 8, 10, 12, 24, 28 and 32 h post‐application. A colorimetric assessment occurred within 30 min prior to application to determine baseline skin colour. For visual assessments, two independent, trained observers scored the degree of skin blanching for each test site and the mean was calculated. Visual assessment of skin blanching was evaluated on a 5‐point scale [0, no change in skin colour; 1, slight (barely visible) blanching; 2, obvious blanching; 3, intense blanching; 4, maximal blanching]. Colorimetric measurements were performed with a chromameter (Konica Minolta CR 400) using the L* a* b* system, commonly used for evaluation of the human skin blanching test.17, 18, 19 This study evaluated the L* value (luminance), which represents relative brightness ranging from total black (L* = 0) to total white (L* = 100), and the a* value, representing the balance between red (positive values) and green (negative values); b* was not analysed as it is not modified by the blanching phenomenon. Before use, the chromameter was calibrated to a standard white calibration plate (CR‐A); calibration was performed each time the instrument was switched on. Two successive measurements were recorded for each test site and the mean was calculated.
Safety and tolerability were assessed at baseline and throughout the study by evaluating AEs, adverse drug reactions (ADRs) and vital signs.
Statistical analysis
A 30‐volunteer sample size was required to achieve an 80% power of rejecting the null hypothesis of no difference between treatments, with a 5% significance level, assuming a 20% standard deviation of the mean and a minimum 10% relative difference. The primary response criterion was area under the curve from time zero to 32 h (AUC0–32) of visual score assessing skin blanching. Secondary response criteria were AUC0–32 of baseline‐adjusted, untreated control site‐corrected values of skin colour change due to blanching for colorimetric parameters a* and L* (described as Δa* and ΔL* AUC0–32 throughout this manuscript). Due to a Food and Drug Administration regulatory requirement, post‐hoc analysis of visual skin blanching assessment was performed for measurements recorded 2 h after investigational product removal (using 8‐h assessment measurements). For each treatment site and time point, colorimetric parameters a* and L* were normalized to baseline and then to the untreated site to eliminate diurnal variation in skin colour. All AUC0–32 scores were compared between treatment groups using analysis of variance (ANOVA) with individuals, treatments and sites as factors. All significance tests were two‐sided, presented with 95% confidence intervals (CIs) without correction for multiplicity. Multiplicity was accounted for by primary comparison definition and corrected for using Tukey's honestly significant difference method.
Results
Volunteers
In total, 35 healthy volunteers with a median age of 36.0 years (range 21–49) were included in the study; 40% were male. Three volunteers (8.6%) had Fitzpatrick16 skin type II and 32 (91.4%) had skin type III; median baseline skin blanching score was 1.0 (range 1.0–2.0). All volunteers received each treatment; all completed the study.
Assessment of skin blanching
All active treatments led to significantly greater skin blanching compared with aerosol foam vehicle according to visual and colorimetric measurements, and compared with untreated negative controls according to visual measurements.
Visual assessment of skin blanching
Over the 32‐h study period, the degree of skin blanching with Cal/BD aerosol foam was significantly lower compared with CP cream (mean AUC0–32 2560 vs. 3831; mean difference = −1272; 95% CI: −1598, −945; P < 0.001), similar to BD aerosol foam (mean AUC0–32 2560 vs. 2595; mean difference = −35; 95% CI: −362, 292; P = 0.83) and significantly greater than Cal/BD ointment (mean AUC0–32 2560 vs. 2008; mean difference = 552; 95% CI: 225, 878; P = 0.001) and FA ointment (mean AUC0–32 2560 vs. 1981; mean difference = 578; 95% CI: 251, 905; P < 0.001) (Fig. 1).
Figure 1.
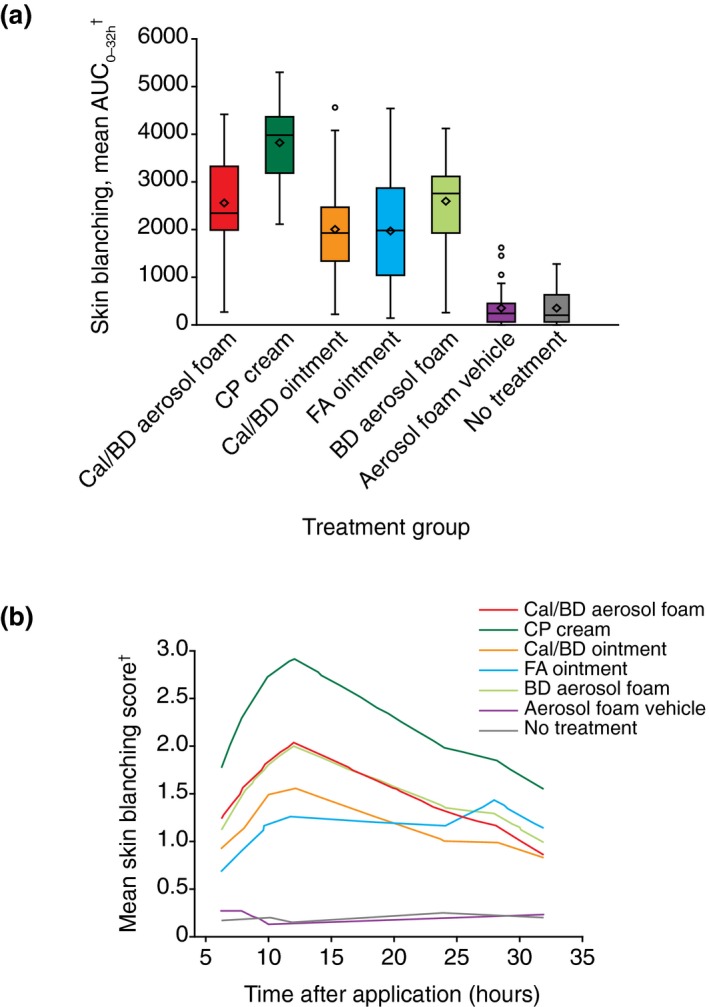
Visual assessment of skin blanching. (a) Box plots of the visual assessment of skin blanching area under the curve (AUC0–32). The horizontal line represents the median and the diamond represents the mean, with the box representing the IQR, the whiskers representing the range within 1.5 × IQR and the circle showing the outliers (observations falling outside of 1.5 × IQR). (b) Mean visual assessment of skin blanching score obtained at each time point from two independent, trained observers. †Higher score indicates greater degree of skin blanching. BD, betamethasone 0.5 mg/g (as dipropionate); Cal, calcipotriol 50 μg/g; CP, clobetasol propionate 0.5 mg/g cream; FA, fluocinolone acetonide 0.25 mg/g ointment; IQR, interquartile range.
In addition to the multipoint AUC0–32 vasoconstriction assessment analysis, a single‐point post‐hoc analysis of visual assessment was performed for measurements recorded 2 h after removal of investigational products (8‐h time point). The results were consistent with the earlier measurements of AUC0–32. As assessed at a single time point, skin blanching with Cal/BD aerosol foam was significantly less compared with CP cream (mean difference = −0.78; 95% CI: −1.04, −0.52; P < 0.001), similar to BD aerosol foam (mean difference = 0.04; 95% CI: −0.22, 0.29; P = 0.78) and significantly greater than Cal/BD ointment (mean difference = 0.43; 95% CI: 0.17, 0.69; P = 0.001) and FA ointment (mean difference = 0.64; 95% CI: 0.39, 0.90; P < 0.001).
Colorimetric assessment of skin blanching
The skin blanching effect leads to a decrease in the a* value (redness) and an increase in the L* value (luminance) compared with baseline. Colorimetric a* skin blanching with Cal/BD aerosol foam was significantly less compared with CP cream (mean Δa* AUC0–32 −2192 vs. −3154; mean difference = 962; 95% CI: 567, 1357; P ≤ 0.001) and BD aerosol foam (mean Δa* AUC0–32 −2192 vs. −2661; mean difference = 469; 95% CI: 74, 863; P = 0.020), and similar to Cal/BD ointment (mean Δa* AUC0–32 −2192 vs. −2068; mean difference = −124; 95% CI: −519, 270; P = 0.53) and FA ointment (mean Δa* AUC0–32 −2192 vs. −1862; mean difference = −330; 95% CI: −725, 65; P = 0.10) (Fig. 2).
Figure 2.
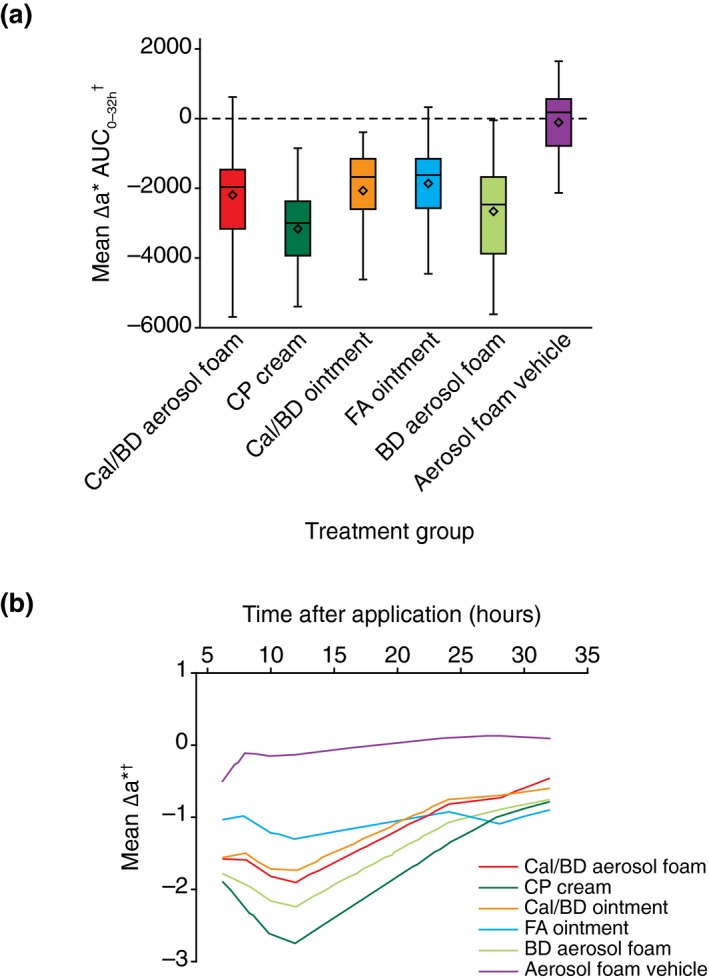
Colorimetric assessment of skin blanching. (a) Box plots of baseline‐adjusted, untreated control site‐corrected values of skin colour change measured by the colorimetric parameter a* (Δa*) area under the curve (AUC0–32). The horizontal line represents the median and the diamond represents the mean, with the box representing the IQR and the whiskers showing the range within 1.5 × IQR. (b) Mean Δa* skin blanching score obtained from two successive measurements at each time point. †Lower score indicates greater degree of skin blanching. BD, betamethasone 0.5 mg/g (as dipropionate); Cal, calcipotriol 50 μg/g; CP, clobetasol propionate 0.5 mg/g cream; FA, fluocinolone acetonide 0.25 mg/g ointment; IQR, interquartile range.
Colorimetric L* skin blanching with Cal/BD aerosol foam was significantly less compared with CP cream (mean ΔL* AUC0–32 1849 vs. 2878; mean difference = −1029; 95% CI: −1457, −601; P < 0.001), similar to BD aerosol foam (mean ΔL* AUC0–32 1849 vs. 2185; mean difference = −336; 95% CI: −764, 92; P = 0.12) and significantly greater than Cal/BD ointment (mean ΔL* AUC0–32 1849 vs. 1418; mean difference = 431; 95% CI: 3, 859; P = 0.048) and FA ointment (mean ΔL* AUC0–32 1849 vs. 1353; mean difference = 496; 95% CI: 68, 924; P = 0.023).
Correction for multiplicity
For the visual assessments, the outcomes were unchanged after correction of P‐values for multiplicity. For the colorimetric parameter a*, Cal/BD aerosol foam no longer induced significantly less skin blanching but was similar to BD aerosol foam (Tukey's corrected 95% CI: −108, 1045; P = 0.18). For the less sensitive luminance parameter L*, Cal/BD aerosol foam was similar to Cal/BD ointment (Tukey's corrected 95% CI: −194, 1057; P = 0.35) and FA ointment (Tukey's corrected 95% CI: −129, 1122; P = 0.21).
Safety and tolerability
Throughout the study, there were no reports of AEs, serious AEs or ADRs, no clinically relevant changes in patient vital signs and no withdrawals due to AEs.
Discussion
The degree of skin blanching is a measure of the inherent potency of a corticosteroid and its ability to diffuse into the skin. This study demonstrates that the skin blanching response elicited by the innovative aerosol foam formulation of Cal/BD is significantly greater than Cal/BD ointment, but less than that elicited by CP cream, when evaluated by visual assessment. The greater skin blanching effect observed with Cal/BD aerosol foam compared with Cal/BD ointment, according to the colorimetric parameters, did not reach statistical significance. This could be due to the fact that the vasoconstriction test is usually performed to evaluate monocomponent steroid products, whereas in our study we have evaluated a combination formulation of steroid (BD) and vitamin D3 analogue (Cal). Therefore, an additional consideration was that Cal‐induced redness20 could have affected colorimetric measurement of the blanching effect of BD, such that the steroid potency may have been underestimated. Indeed, chromametric measurement non‐discriminately assesses skin colour over the entire test area, whereas by visual assessment, a trained reader may differentiate between blanching effect and red spots due to Cal irritation. Thus, the more pronounced blanching detected visually with Cal/BD may appear reduced when using the chromameter that evaluates concomitant skin blanching and redness.
Although the aerosol foam and the ointment both contain fixed combination Cal/BD, this study demonstrated enhanced potency of the aerosol foam formulation compared with the ointment. This could be attributed, in part, to the differing properties of each vehicle. It has been suggested that vehicle properties may impact upon drug delivery and local bioavailability.15 The explanation for this is not fully understood, although it has been suggested that formulations containing volatile components may improve drug delivery.15 Rapid evaporation of the vehicle may lead to ‘super‐saturation’ of active components on the skin and greater transfer into the skin compared with traditional formulations. Accordingly, an in vitro skin penetration model demonstrated increased penetration of Cal and BD into skin with the aerosol foam formulation compared with the ointment, resulting in significantly higher steady‐state levels of both individual components.6 This enhanced penetration of active ingredients following a change in vehicle type has been observed previously; clinical trials demonstrated that betamethasone valerate foam and clobetasol propionate foam were absorbed more rapidly with greater total absorption than their comparison formulations, betamethasone valerate lotion and clobetasol propionate solution, respectively.14
In addition to the enhanced potency demonstrated by this study, a Phase III study has demonstrated that Cal/BD aerosol foam is highly efficacious and well‐tolerated.21 Phase II studies have demonstrated significantly greater efficacy of Cal/BD aerosol foam compared with each individual active component used alone (Cal aerosol foam and BD aerosol foam),22 and compared with Cal/BD ointment.23 A further Phase III trial has been performed (NCT02132936). Enhanced delivery and increased bioavailability of the active components in the skin,6 as well as increased treatment efficacy,7, 22 may translate into less medication and exposure to corticosteroid being required for effective disease management; however, this requires further investigation.
No AEs were reported during this study, although it must be considered that the study was in healthy volunteers and the treatment period was very short (32 h), meaning that very few AEs would be expected. However, Phase II7, 22 and Phase III21 trials have demonstrated that Cal/BD aerosol foam maintains the favourable safety and tolerability profile established with Cal/BD ointment and gel.5, 24, 25, 26
In conclusion, in this vasoconstriction study in healthy volunteers, the fixed combination Cal/BD aerosol foam showed a significantly greater skin blanching response than Cal/BD ointment but less than CP cream, indicating that the corticosteroid potency of fixed combination Cal/BD aerosol foam is greater than that of Cal/BD ointment (a potent corticosteroid) but less than that of a very potent corticosteroid, CP 0.5 mg/g cream. The enhanced potency of Cal/BD aerosol foam is not associated with compromised safety, as demonstrated by Phase II and Phase III clinical trials. As such, Cal/BD aerosol foam may provide greater efficacy than Cal/BD ointment, highlighting the importance of vehicle formulation in topical psoriasis treatments.
Acknowledgements
This study was sponsored by LEO Pharma. We thank Magali Procacci‐Babled for coordinating the study. Additionally, we thank Catherine Risebro, PhD, from Mudskipper Business Ltd, who provided medical writing support, funded by LEO Pharma.
Conflicts of interest
CQ‐R reports subject fees paid by LEO Pharma; J‐PL reports reimbursement for conference attendance and honoraria for consultancy activity; BB and FC are employees of LEO Pharma.
Funding source
This study was sponsored by LEO Pharma. Medical writing support was provided by Catherine Risebro, PhD, from Mudskipper Business Ltd, funded by LEO Pharma.
Previous presentation
Poster presented at the 73rd Annual Meeting of the American Academy of Dermatology, San Francisco, California, USA (20–24 March 2015), corresponding abstract published in J Am Acad Dermatol 2015;72 (Suppl 1):AB243.
Trial registration
References
- 1. Schön MP, Boehncke W‐H. Psoriasis. N Engl J Med 2005; 352: 1899–1912. [DOI] [PubMed] [Google Scholar]
- 2. Menter A, Korman NJ, Elmets CA et al Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60: 643–659. [DOI] [PubMed] [Google Scholar]
- 3. Samarasekera E, Sawyer L, Parnham J, Smith CH. Assessment and management of psoriasis: summary of NICE guidance. BMJ 2012; 345: e6712. [DOI] [PubMed] [Google Scholar]
- 4. Fleming C, Ganslandt C, Guenther L et al Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. Eur J Dermatol 2010; 20: 465–471. [DOI] [PubMed] [Google Scholar]
- 5. McCormack PL. Calcipotriol/betamethasone dipropionate: a review of its use in the treatment of psoriasis vulgaris of the trunk, limbs and scalp. Drugs 2011; 71: 709–730. [DOI] [PubMed] [Google Scholar]
- 6. Hollesen Basse L, Olesen M, Lacour JP, Queille‐Roussel C. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol 2014; 134: S33: abst 192. [Google Scholar]
- 7. Koo J, Tyring S, Werschler WP et al Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris ‐ A randomized phase II study. J Dermatolog Treat 2016; 27: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levin E, Gupta R, Butler D, Chiang C, Koo JY. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat 2014; 25: 501–506. [DOI] [PubMed] [Google Scholar]
- 9. Bruner CR, Feldman SR, Ventrapragada M, Fleischer AB Jr. A systematic review of adverse effects associated with topical treatments for psoriasis. Dermatol Online J 2003; 9: 2. [PubMed] [Google Scholar]
- 10. Kirkland R, Pearce DJ, Balkrishnan R, Feldman SR. Critical factors determining the potency of topical corticosteroids. J Dermatolog Treat 2006; 17: 133–135. [DOI] [PubMed] [Google Scholar]
- 11. McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol 1962; 86: 608–610. [Google Scholar]
- 12. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol 1985; 121: 63–67. [PubMed] [Google Scholar]
- 13. Castela E, Archier E, Devaux S et al Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol 2012; 26(Suppl 3): 47–51. [DOI] [PubMed] [Google Scholar]
- 14. Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol 2005; 53: S39–S49. [DOI] [PubMed] [Google Scholar]
- 15. Surber C, Smith EW. The mystical effects of dermatological vehicles. Dermatology 2005; 210: 157–168. [DOI] [PubMed] [Google Scholar]
- 16. Fitzpatrick TB. Soleil et peau. J Méd Esthét 1975; 2: 33–34. [Google Scholar]
- 17. Queille‐Roussel C, Poncet M, Schaefer H. Quantification of skin‐colour changes induced by topical corticosteroid preparations using the Minolta Chroma Meter. Br J Dermatol 1991; 124: 264–270. [DOI] [PubMed] [Google Scholar]
- 18. Pierard GE. EEMCO guidance for the assessment of skin colour. J Eur Acad Dermatol Venereol 1998; 10: 1–11. [DOI] [PubMed] [Google Scholar]
- 19. Taylor S, Westerhof W, Im S, Lim J. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol 2006; 54: S282–S290. [DOI] [PubMed] [Google Scholar]
- 20. Fullerton A, Serup J. Topical D‐vitamins: multiparametric comparison of the irritant potential of calcipotriol, tacalcitol and calcitriol in a hairless guinea pig model. Contact Dermatitis 1997; 36: 184–190. [DOI] [PubMed] [Google Scholar]
- 21. Leonardi C, Bagel J, Yamauchi P et al Efficacy and safety of an innovative aerosol foam formulation of the fixed combination calcipotriene plus betamethasone dipropionate in patients with psoriasis – the PSO‐FAST study. J Am Acad Dermatol 2015; 72(Suppl 1): AB232. [Google Scholar]
- 22. Lebwohl M, Tyring S, Bukhalo M et al A novel aerosol foam formulation of calcipotriene (Cal) 0.005% plus betamethasone dipropionate (BD) 0.064% is more efficacious than Cal and BD foam alone in treating psoriasis vulgaris: a randomized, double‐blind, multicenter, three‐arm, Phase II study. J Am Acad Dermatol 2015; 72(Suppl 1): AB222. [PMC free article] [PubMed] [Google Scholar]
- 23. Koo J, Tyring S, Werschler WP. Superior efficacy of the fixed combination calcipotriene plus betamethasone dipropionate in an innovative aerosol foam versus ointment, in patients with psoriasis vulgaris. Semin Cutan Med Surg 2015; 34(Suppl 1): PA–42. [Google Scholar]
- 24. Douglas WS, Poulin Y, Decroix J et al A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol 2002; 82: 131–135. [DOI] [PubMed] [Google Scholar]
- 25. Kragballe K, Austad J, Barnes L et al A 52‐week randomized safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris. Br J Dermatol 2006; 154: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 26. Langley RG, Gupta A, Papp K, Wexler D, Østerdal ML, Curcic D. Calcipotriol plus betamethasone dipropionate gel compared with tacalcitol ointment and the gel vehicle alone in patients with psoriasis vulgaris: a randomized, controlled clinical trial. Dermatology 2011; 222: 148–156. [DOI] [PubMed] [Google Scholar]


