Abstract
In the adjuvant setting for malignant melanoma, interferon (IFN)‐α‐2b and pegylated (PEG) IFN‐α‐2b were approved in several countries including the USA before these were approved in Japan. To resolve the “drug‐lag” issue, this phase I study was designed to evaluate the safety and tolerability in Japanese patients with stage II or III malignant melanoma who had undergone surgery, by treating with PEG IFN‐α‐2b. As with a previously reported phase III study, patients were to receive PEG IFN‐α‐2b 6 μg/kg per week s.c. during an 8‐week induction phase, followed by a maintenance phase at a dose of 3 μg/kg per week up to 5 years. Dose‐limiting toxicity and pharmacokinetics were assessed during the initial 8 weeks. Of the nine patients enrolled, two patients had dose‐limiting toxicities that resolved after discontinuation of treatment. The most frequently reported drug‐related adverse events (DRAE) included pyrexia, decreased neutrophil and white blood cell counts, and arthralgia. Grade 3 DRAE included decreased neutrophil count. No deaths, serious adverse events and grade 4 adverse events were reported. Distant metastasis occurred in one patient. No apparent differences in area under the concentration–time curve and maximum observed serum concentration were observed between Japanese and historical non‐Japanese pharmacokinetic data, suggesting no marked racial differences. No neutralizing antibody was detected in these patient samples. PEG IFN‐α‐2b was tolerated in Japanese patients, and eventually approved in Japan in May 2015 for adjuvant therapy in patients with stage III malignant melanoma. Because the number of patients was limited, further investigation would be crucial.
Keywords: adjuvant therapy, Japanese, melanoma, peg‐interferon α‐2b, phase I
Introduction
Although malignant melanoma can be curatively resected if detected early, it is a highly malignant disease that becomes fatal due to tendency to recur and metastasize with disease progression. Complications may also cause problems in daily life. Hence, adding postoperative adjuvant therapy is considered important in preventing tumor recurrence and metastasis after excision.1
In a comparative study of adjuvant therapy using interferon (IFN)‐α‐2b, extension of overall survival (OS) was confirmed when compared with an observation arm (Eastern Cooperative Oncology Group [ECOG] 1684 study).2 Based on this study result, IFN‐α‐2b has been utilized as a global standard for adjuvant therapy in patients with high‐risk malignant melanoma.3, 4 Recently, pegylated (PEG) IFN‐α‐2b became available in several countries including the USA and made treatments easier.5
Pegylated IFN‐α‐2b in patients with stage III malignant melanoma who have undergone surgery demonstrated statistically meaningful extension of recurrence‐free survival (RFS) in a large phase III (European Organization for Research and Treatment of Cancer [EORTC] 18991) study when administrated at 6 μg/kg per week (8 weeks) in the induction phase and 3 μg/kg per week (up to 5 years in total) in the maintenance phase.6 ECOG 1684 and EORTC 18991 were randomized, multinational, comparative studies with confirmed efficacies; however, in Japan, both IFN‐α‐2b and PEG IFN‐α‐2b remained unapproved for adjuvant treatment of malignant melanoma after surgery.
Meanwhile, Japan has independently adopted therapies with IFN‐β and DAV Feron (dacarbazine, nimustine, vincristine and IFN‐β) for malignant melanoma after surgery in stage IIA or later patients based on results from a phase II single arm study. Nevertheless, no randomized comparative study has been performed to prove the efficacy of these therapies.7 Therefore, it was necessary to quickly introduce globally standardized treatment to Japan so that differences in medical environment could also be resolved.
Accordingly, the Japanese Skin Cancer Society and others submitted an application to the Ministry of Health, Labour and Welfare for development of IFN‐α‐2b which was already an approved drug outside Japan at that time. A review was conducted by a unique domestic organization called the Review Committee on Unapproved Drugs and Indications with High Medical Needs, and the committee requested evaluation of the safety of IFN‐α‐2b in Japanese patients with malignant melanoma after surgery.
Subsequently, PEG IFN‐α‐2b was also approved in the USA as adjuvant therapy in patients with stage III malignant melanoma who have undergone surgery. We then proposed to the Pharmaceuticals and Medical Devices Agency that development be switched from IFN‐α‐2b to PEG IFN‐α‐2b (MK‐4031, SCH 54031). After receiving permission, a phase I (P370, P08556) study was conducted (ClinicalTrials.gov: NCT01636960).
Methods
The P370 study was a multicenter, open‐label, uncontrolled phase I study in Japanese patients who underwent surgery for malignant melanoma. The primary objective was to evaluate the safety and tolerability of PEG IFN‐α‐2b in the induction phase (8 weeks). The safety and tolerability were evaluated from the appearance rate of patients who exhibited dose‐limiting toxicity (DLT). The secondary objectives were to evaluate the safety in the maintenance phase (up to 5 years) and to obtain pharmacokinetic parameters in the induction phase. As exploratory objectives, efficacy and developments of antidrug antibody (ADA) and neutralizing antibodies were examined. The 8‐week induction phase was regarded as an evaluation period for DLT and pharmacokinetics.
Patients
This study was performed for Japanese patients with malignant melanoma who underwent surgery, had histologically confirmed American Joint Committee on Cancer (AJCC) 2009 Stage II or III malignant melanoma, were 20–74 years of age, had a lesion completely excised with adequate surgical margins, in whom a full lymphadenectomy had been performed within 84 days prior to initiation of study treatment and had an ECOG Performance Status (PS) of 0 or 1.
A patient meeting any of the criteria listed below were excluded from participating in the trial: Patients with ocular or mucous membrane melanoma, in‐transit melanoma (i.e. TanyN2c,3M0), a history of prior malignancy within the past 5 years (other than surgically cured squamous, basal cell carcinoma of the skin, early stage cutaneous melanoma [T0, T1, T2a], cervical carcinoma in situ), thyroid dysfunction not responsive to therapy, a pre‐existing psychiatric condition, a concurrent or past history of interstitial lung disease, hepatic decompensation (Child–Pugh score >6 [class B and C]), uncontrolled diabetes mellitus with hemoglobin A1c level of 8.0% or more (Japan Diabetes Society value), in addition to patients previously treated with IFN‐α/β, chemotherapeutic drug, hormone, radiation, immunotherapeutic drug or vaccine for melanoma, or who exhibited retinopathy such as retinal hemorrhage.
The trial (Protocol P370) was approved by the institutional review board at each center and complied with the Good Clinical Practice guidelines, the Declaration of Helsinki and local laws. All patients provided written informed consent.
Treatment plan
Dose and administration in this study consisted of PEG IFN‐α‐2b in the induction phase at 6 μg/kg once weekly s.c. for 8 weeks and in maintenance phase at 3 μg/kg once weekly s.c. from the ninth week up to 5 years. The treatment was planned to continue until completion of the 5‐year therapy or confirmation of distant metastases. In the EORTC 18991 study, keeping ECOG PS within 0–1 level by adjusting the dose of PEG IFN‐α‐2b was considered important for the longest treatment possible. Likewise, the same conception was adopted in this study by establishing criteria for dose reduction and discontinuation, defining the action plans and reduced dose level (Table 1).
Table 1.
Criteria for dose reduction and discontinuation, with action plans and reduced dose level
| Criteria | Action | Dose reduction | |
|---|---|---|---|
|
ANC <500/μL PLT <50 000/μL ECOG PS ≥2§ Grade 3 non‐hematological toxicity§ |
Withhold PEG IFN‐α‐2b. When all criteria below are met, resume treatment reducing the dose: ANC ≥500/μL PLT ≥50 000/μL ECOG PS 0–1 Non‐hematological toxicity resolves to grade 1 or baseline. |
Induction phase (weeks 1–8) 6 μg/kg per week |
First reduction: 3 μg/kg per week Second reduction: 2 μg/kg per week Third reduction: 1 μg/kg per week Discontinue if did not tolerate at 1 μg/kg per week |
|
Maintenance phase (weeks 9–260) 3 μg/kg per week† |
First reduction: 2 μg/kg per week Second reduction: 1 μg/kg per week Discontinue if did not tolerate at 1 μg/kg per week |
||
|
Grade 4 non‐hematological toxicity Not tolerated at 1 μg/kg per week. New retinopathy |
Discontinue PEG IFN‐α‐2b. | ||
§Except transient ECOG PS ≥2 and the following grade 3 events: (i) influenza‐like symptoms such as fever, chill, myalgia, arthralgia, fatigue or headache; (ii) manageable toxicity by adequate supportive care or non‐prohibited therapies (e.g. nausea, vomiting); and (iii) laboratory events that may have no clinical significance (e.g. transient decreased lymphocyte, transient electrolytes abnormality, increased liver γ‐glutamyltransferase or alkaline phosphatase). †If the dose was reduced to ≤3 μg/kg per week in the induction phase, the following maintenance phase started with the last dose in the induction. ANC, absolute neutrophil count; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PEG IFN, pegylated interferon; PLT, platelet count.
Therapy was discontinued if toxicity persisted or recurred in spite of dose reductions until 1 μg/kg per week; otherwise, treatment was continued until unacceptable adverse events (AE), development of distant metastases, persistent or worsening grade 3 neuropsychiatric disorders, new retinopathy, protocol violation, pregnancy, patient's request or investigator's judgment. The trial was to be terminated if regulatory approval was obtained in Japan during the maintenance phase.
Patient evaluation and study design
The number of patients planned was nine to 18. During induction, which is an 8‐week DLT evaluation phase, it was planned to enroll three patients initially at 6 μg/kg per week dose level. If one or less of the three patients exhibited DLT, six additional patients were to be enrolled at the same dose. If four or less of the nine patients in total manifested DLT, the dose level of induction phase was considered to be tolerated. On the other hand, if two or more of the initial three patients or five or more of the nine patients exhibited DLT during the induction phase, it was planned to reduce the dose to 3 μg/kg per week to continue the study (Fig. 1).
Figure 1.
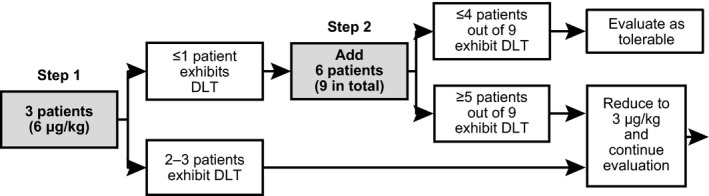
Dose‐limiting toxicity (DLT) was evaluated stepwise: step 1 in three patients, and step 2 in nine patients, adding 6. If four or less of the nine patients exhibited DLT, the dose was considered tolerable.
Based on toxicity development rate in the EORTC 18991 study, the target DLT rate was designated to be 35.38% or less. When DLT rate was 35.38%, the probability of four or less patients developing DLT among nine patients was 82.1%. A method by Ji et al.8 was used for DLT evaluation, and toxicity was graded as per Common Terminology Criteria for Adverse Events by National Cancer Institute version 4.0.
Among the following toxicities which occurred in the DLT evaluation period, the events evaluated as drug‐related were defined as DLT: absolute neutrophil count of less than 500/μL, platelet count of less than 50 000/μL, ECOG PS of 2 or more, non‐hematological toxicity of grade 3 or more, new retinopathy and toxicity causing therapy discontinuation. Transient ECOG PS of 2 or more and the following grade 3 events were excluded from the DLT: influenza‐like symptoms such as fever, chill, myalgia, arthralgia, fatigue or headache, manageable toxicity by adequate supportive care or non‐prohibited therapies such as nausea and vomiting, and laboratory events that might have had no clinical significance.
Patients who had a DLT or who received 75% or more of planned doses of study medications in the induction phase without a DLT were evaluable for determining the DLT. Appropriateness of the tolerability and safety during the DLT evaluation period was finally evaluated by the Efficacy and Safety Evaluation Committee. Treatment after week 9 (maintenance phase) was continued until there was evidence of distant metastases, unacceptable toxicity or patient decision to discontinue. Adverse events were listed in preferred term provided by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Medical Dictionary for Regulatory Activities (MedDRA) Terminology version 17.1.
Serum concentration and pharmacokinetic parameters were also evaluated for an 8‐week administration performed as the secondary objective. Serum PEG IFN‐α‐2b concentrations were analyzed using a validated electrochemiluminescence (ECL) immunoassay.9 The range of quantitation was 30.0–8000 pg/mL.
Non‐compartment pharmacokinetic analysis was performed for the calculation of area under the concentration–time curve (AUC), maximum observed serum concentration (Cmax) and time to maximum observed serum concentration (Tmax), using Phoenix 64 WinNonlin version 6.3 (Certara, Princeton, NJ, USA), a pharmacokinetic analysis program. Terminal phase (elimination) half‐life (t 1/2), apparent total body clearance (CL/F), apparent volume of distribution (V/F) and accumulation factor (R) based on AUC were also estimated, if applicable.
Exploratory evaluation was performed for RFS and distant metastasis‐free survival (DMFS), as well as occurrence of ADA and neutralizing antibodies during the study treatment. The ADA and neutralizing antibodies were also measured using a validated ECL immunoassay.9
Results
Study treatment was started in February 2013, and nine patients from four medical institutions were enrolled by January 2014. PEG IFN‐α‐2b was administrated in all nine patients, and seven of them moved into the maintenance phase. During the maintenance phase, regulatory approval in Japan was obtained (26 May 2015) for adjuvant treatment in patients with stage III malignant melanoma who underwent surgery, and the study was terminated (Fig. 2).
Figure 2.
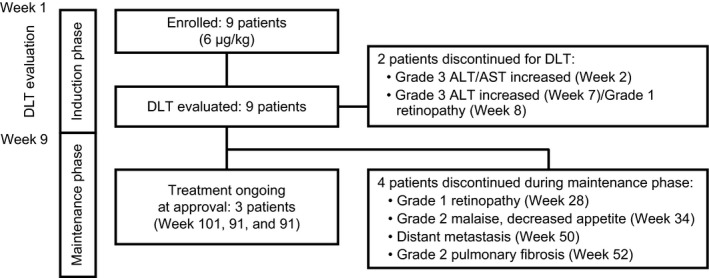
Overall study flow. Two patients discontinued in the induction phase because of dose‐limiting toxicity (DLT). Four patients discontinued in the maintenance phase by adverse events or distant metastasis. Treatment was ongoing at approval in three patients. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Patient characteristics
Patient characteristics are provided in Table 2. These nine patients were 24–61 years of age, including five males and three stage II malignant melanoma patients who had undergone surgery. All patients were eligible for analyses.
Table 2.
Patient characteristics
| Subjects in population (n, %) | 9 (100) |
|---|---|
| Sex (n, %) | |
| Male | 5 (56) |
| Female | 4 (44) |
| Age (years) | |
| Median | 46.0 |
| Range | 24–61 |
| Weight (kg) | |
| Median | 72.5 |
| Range | 46.6–87.9 |
| ECOG PS (n, %) | |
| 0 | 8 (89) |
| 1 | 1 (11) |
| Stage of melanoma at study entry (n, %) | |
| IIB | 2 (22) |
| IIC | 1 (11) |
| IIIA | 2 (22) |
| IIIB | 4 (44) |
All patients were Japanese who had the malignant melanoma resected with appropriate margins. ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Treatment
The mean treatment duration of the study drug for the induction phase was 47.4 days (range, 14–56), and the mean treatment duration for the entire treatment period was 338.3 days (range, 14–658). The compliance rate with the drug administration for the induction phase was 75% or more in all patients. The compliance rate for the maintenance phase was less than 75% in one patient due to AE‐related dosing interruption; however, no dosing problems occurred in the other patients.
As of the time of approval on 26 May 2015, treatment with PEG IFN‐α‐2b was ongoing for three patients who subsequently continued the same treatment with marketed PEG IFN‐α‐2b. On the other hand, five patients discontinued the study treatment due to AE, and one patient discontinued the study treatment because of distant metastasis.
Safety evaluation
The primary objective was to evaluate the safety and tolerability of PEG IFN‐α‐2b in the induction phase from the appearance rate of patients who exhibited DLT. The secondary objective was to evaluate safety in the maintenance phase. Among the initial three patients who enrolled at 6 μg/kg per week of PEG IFN‐α‐2b in the induction phase, one patient exhibited DLT.
Because the number of patients who exhibited DLT was one or fewer out of three, an additional six patients were enrolled at the same dose level. Of the total nine patients, two patients exhibited DLT in induction phase. As the patients who exhibited DLT were four or fewer out of nine, the dose level was considered tolerable in Japanese patients with malignant melanoma who underwent surgery (Fig. 2).
The DLT observed in one patient were grade 3 increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels at week 2, and in the other patient were grade 3 increased ALT level at week 7 and grade 1 retinopathy at week 8. These patients discontinued the therapy in the induction phase and all toxicities resolved after discontinuation.
As retinopathy was observed, the protocol was amended for patient safety to add complete eye (visual acuity and fundus) examination to be performed prior to start of induction and maintenance phase, and every 3 months subsequently. The other seven patients who did not exhibit DLT moved into the maintenance phase (Fig. 2).
Throughout the treatment period, drug‐related AE (DRAE) in any grade occurred in all nine patients. Pyrexia, decreased neutrophil and white blood cell counts, and arthralgia occurred in nine patients, increased ALT and AST levels occurred in eight patients, and injection site reaction occurred in seven patients. Grade 3 or more DRAE occurred in eight patients, and grade 3 decreased neutrophil count developed in seven patients. Details on DRAE are described in Table 3. Grade 4 AE, death or serious AE were not observed.
Table 3.
Drug‐related adverse events in entire treatment period
| Any grade, n (%) | Grade 3, n (%) | |
|---|---|---|
| Hematological | ||
| Decreased neutrophil count | 9 (100) | 7 (78) |
| Decreased white blood cell count | 9 (100) | 2 (22) |
| Decreased platelet count | 5 (56) | 0 |
| Anemia | 2 (22) | 1 (11) |
| Decreased lymphocyte count | 2 (22) | 0 |
| Non‐hematological | ||
| Pyrexia | 9 (100) | 0 |
| Arthralgia | 9 (100) | 0 |
| Increased ALT level | 8 (89) | 2 (22) |
| Increased AST level | 8 (89) | 2 (22) |
| Injection site reaction (erythema, pruritus, joint pain) | 7 (78) | 0 |
| Chills | 6 (67) | 0 |
| Malaise | 6 (67) | 0 |
| Myalgia | 6 (67) | 0 |
| Fatigue | 5 (56) | 1 (11) |
| Hypertriglyceridemia | 4 (44) | 2 (22) |
| Weight decreased | 4 (44) | 0 |
| Decreased appetite | 4 (44) | 0 |
| Headache | 4 (44) | 0 |
| Alopecia | 4 (44) | 0 |
| Nausea | 3 (33) | 0 |
| Rash | 3 (33) | 0 |
| Retinopathy | 2 (22) | 0 |
| Constipation | 2 (22) | 0 |
| Stomatitis | 2 (22) | 0 |
| γ‐GT increased | 2 (22) | 0 |
| Hypocalcemia | 2 (22) | 0 |
| Oropharyngeal pain | 2 (22) | 0 |
Grades were evaluated based on Common Terminology Criteria for Adverse Events version 4.0. DRAE (any grade) with a frequency of ≥20% are presented. Patients were counted only once and categorized by the highest DRAE grade reported. No grade 4 or 5 AE were reported. DRAE (any grade) exhibited in a patient (n = 1): eye pain, photophobia, diarrhea, dyspepsia, gastritis, vomiting, adenoiditis, gingivitis, pericoronitis, hyperglycemia, hypoalbuminemia, limb discomfort, dizziness, dysgeusia, insomnia, cough, epistaxis, pharyngeal inflammation, pleuritic pain, pulmonary fibrosis, dry skin, pruritus, seborrheic dermatitis, skin disorder, skin hypopigmentation and urticaria. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DRAE, drug‐related adverse event; γ‐GT, γ‐glutamyltransferase.
In maintenance phase, three patients discontinued treatment due to DRAE. The DRAE leading to discontinuation were grade 1 retinopathy (week 28), grade 2 malaise and decreased appetite (week 34), and pulmonary fibrosis (week 52). Except pulmonary fibrosis, all of the toxicities resolved after discontinuation of study treatment.
Throughout the treatment period, DRAE requiring dose reduction or interruption of study treatment were observed in five patients: ALT increased in two patients, while increased AST level, decreased appetite, malaise, nausea, decreased weight and hypertriglyceridemia occurred in one patient each. All of the toxicities resolved after dose reduction or treatment interruption.
There was one occurrence of overdose. It was because the patient was erroneously prescribed a dose of induction phase for initial dosing of maintenance phase, but no AE was caused by the overdose.
Regulatory approval was obtained in May 2015 and this study was terminated. At that time, three patients had been receiving the study drug as treatment for 101, 91 and 91 weeks from the study initiation, respectively (Fig. 2).
Pharmacokinetics
As the secondary objective, pharmacokinetic parameters of PEG IFN‐α‐2b were obtained from seven to nine subjects for 8 weeks in the induction phase (Table 4, Fig. 3). The serum concentrations of PEG IFN‐α‐2b reached 23.6 and 23.7 h of median Tmax on weeks 1 and 8, respectively, and then decreased mono‐exponentially with the geometric mean t 1/2 of 46.1 and 61.8 h on weeks 1 and 8, respectively. The geometric mean on weeks 1 and 8 were 2252 and 3338 pg/mL for Cmax, and 330 and 702 pg/mL for trough values (C168 h), respectively. No obvious increase in C168 h was observed after week 7, indicating that pharmacokinetics of PEG IFN‐α‐2b had reached steady state by week 7.
Table 4.
Summary of pharmacokinetic parameters of serum concentration by 6 μg/kg per week dosing of PEG IFN‐α‐2b at weeks 1 and 8
| Week | Dose (μg/kg) | n | Tmax † (h) | Cmax (pg/mL) | C168 h (pg/mL) | AUC0–168 h (pg h/mL) | t 1/2 (h) | CL/F (mL/h per kg) | V/F (mL/kg) | R‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 9 | 23.6 (22.5–71.9) | 2252 (35) | 330 (104) | 195 173¶ (44) | 46.1¶ (35) | 26.9¶ (49) | 1787¶ (43) | NA |
| 8 | 6 | 7§ | 23.9 (22.4–47.8) | 3078 (53) | 719 (36) | 295 233 (25) | 65.3 (36) | 20.3 (25) | 1915 (60) | 1.63‡‡ (53) |
| 6§§ | 23.7 (22.4–47.8) | 3338 (52) | 702 (39) | 306 909 (25) | 61.8 (35) | 19.5 (25) | 1743 (58) | 1.78†† (49) |
†Median (range). ‡Arithmetical mean (%CV). §Excluded two patients who were not administrated PEG IFN‐α‐2b at week 8. ¶ n = 8, †† n = 5, ‡‡ n = 6. §§Excluded three patients who were not administrated PEG IFN‐α‐2b at weeks 7 or 8. Geometric mean (%CV based on geometric mean). AUC, area under the concentration–time curve; CL/F, apparent total body clearance; Cmax, maximum observed serum concentration; CV, coefficient of variation; PEG IFN, pegylated interferon; R, accumulation factor; t 1/2, terminal phase (elimination) half‐life; Tmax, time to maximum observed serum concentration; V/F, apparent volume of distribution.
Figure 3.
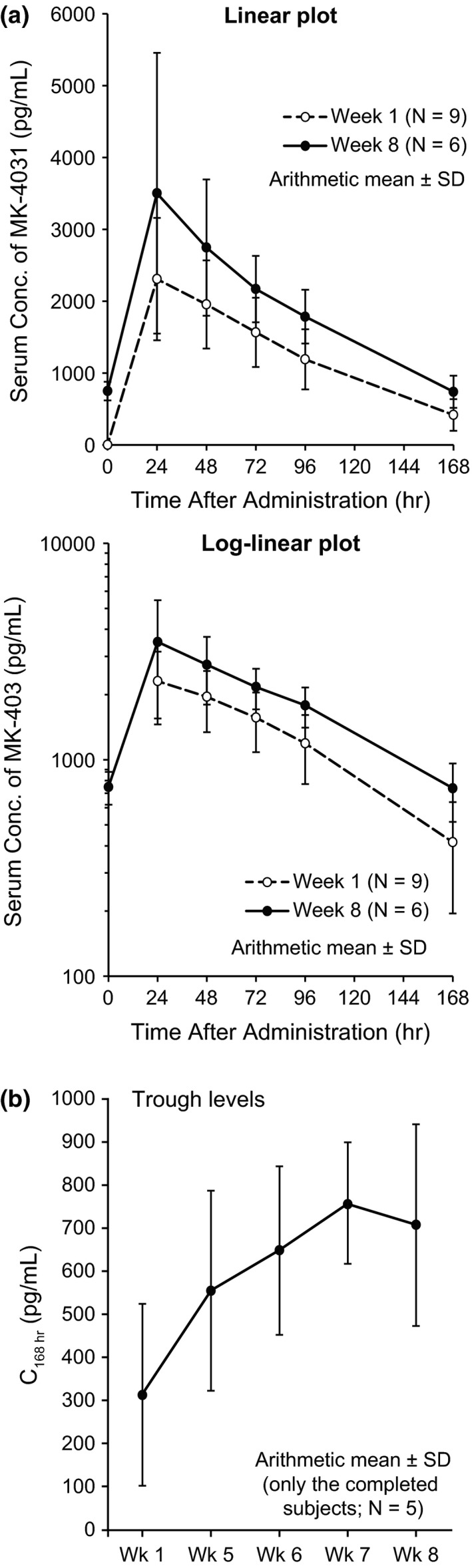
(a) Serum concentration–time profile by 6 μg/kg per week dosing of pegylated interferon‐α‐2b (MK‐4031) (arithmetical mean ± standard deviation [SD]). One patient data who was not administrated treatment at week 7 was excluded from calculation at week 8. (b) Trough level profile of serum concentration by 6 μg/kg per week dosing of pegylated interferon‐α‐2b (MK‐4031) (arithmetical mean). Data for five patients who completed administration from weeks 1 to 8 are shown.
The geometric mean AUC0–168 h on weeks 1 and 8 were 195 173 and 306 909 pg h/mL, respectively, and arithmetic mean of accumulation factor (R) based on the AUC ratio (AUC0–168 h week 8/AUC0–168 h week 1) was 1.78. The coefficients of variation for Cmax and AUC0–168 h were 35–52% and 25–44%, respectively, suggesting that inter‐individual variability in the systemic exposure was moderate to high.
No apparent change in either CL/F and V/F was observed after repeated dosing. Geometric means on weeks 1 and 8 were 26.9 and 19.5 mL/h per kg for CL/F, respectively, and 1787 and 1743 mL/kg for V/F, respectively.
Efficacy
The RFS and DMFS during the treatment period were evaluated exploratorily. Regional and distant metastases were confirmed in one patient who was enrolled after resection of primary stage IIC (T4bN0M0) malignant melanoma in leg. The drug administration was interrupted as metastasis confined to the inguinal lymph node was found on day 205 of the study treatment, which was then resumed after performing complete lymphadenectomy. Subsequently, distant metastases to lung and bone were observed on day 350, and the treatment was discontinued.
No recurrence or distant metastasis was observed in the other patients. Kaplan–Meier analysis was not performed as only one patient exhibited RFS and DMFS event.
Expressions of ADA and neutralizing antibodies were also evaluated exploratorily. For two samples, antibodies for PEG IFN‐α‐2b and IFN‐α‐2b were both positive and anti‐PEG antibody was negative; however, neutralizing antibody responses in both samples were negative.
Discussion
This P370 is the first study of administrating PEG IFN‐α‐2b after surgery to Japanese stage II or III malignant melanoma patients. The primary objective was to evaluate safety and tolerability, and nine patients received the study treatment. DLT occurred in two out of nine patients who received 6 μg/kg per week of PEG IFN‐α‐2b s.c. during the DLT evaluation period (8‐week induction phase). One patient exhibited grade 3 increased ALT/AST levels and the other patient exhibited grade 3 increased ALT level and grade 1 retinopathy. All toxicities resolved after the treatment discontinuation.
As the patients who manifested DLT were four or less out of nine, the dose level and administration in the induction phase was considered tolerable (Fig. 2). Because the reported DLT were also observed in phase III (EORTC 18991) study,10 they were adequately considered as known and predictable events. After week 9, the treatment was continued at 3 μg/kg per week of PEG IFN‐α‐2b in seven patients as maintenance phase.
Drug‐related adverse events that developed during the entire treatment period (Table 3), and AE leading to study discontinuation, dose reduction or treatment interruption, were almost the same as those observed in the EORTC 18991 study10 and the events reported when PEG IFN‐α‐2b was administrated to Japanese patients with chronic hepatitis C. No unexpected toxicity was observed. After discontinuation, dose reduction or interruption of the study treatment, all the toxicities (retinopathy, malaise, decreased appetite, increased ALT/AST levels, nausea, decreased weight and hypertriglyceridemia) except pulmonary fibrosis have resolved. Grade 4 AE, death or serious AE were not observed. Depression, known to be a related AE of PEG IFN‐α‐2b,6, 10 was not observed in this study.
From the above results, no significant difference was observed in the safety profiles of adjuvant PEG IFN‐α‐2b treatment between Japanese and non‐Japanese patients with malignant melanoma who underwent surgery. No major concern was hence raised. Nevertheless, as only nine patients were evaluated in this study, it is considered that safety information needs to be further collected from the post‐marketing surveillance.
In this study, retinopathy and pulmonary fibrosis occurred in two and one patient, respectively. We have to exercise caution against occurrence of these AE, and it would be essential to collaborate with other departments to manage the AE. Influenza‐like symptoms that occurred in all the patients may be alleviated or resolved quickly by prophylactic treatment with agents including non‐steroidal anti‐inflammatory drugs and appropriate supportive therapies.
For hepatic dysfunction, aggravation may be prevented by regular examination with therapy interruption or dose reduction depending on abnormality. Although depression was not observed in this study, it was reported that some patients exhibited the symptom found to be persistent in EORTC 18991 study.6, 10 Hence, depression must also be monitored carefully.
For pharmacokinetic parameters, geometric means of CL/F on weeks 1 and 8 were 26.9 and 19.5 mL/h per kg, respectively, which were similar to historical data in healthy Japanese adults (21.9–32.1 mL/h per kg) and hepatitis C patients (21.1–21.4 mL/h per kg).9 Also, almost all individual AUC and Cmax in this study fell within the distribution range of those shown in pharmacokinetic studies in non‐Japanese patients with malignant melanoma, suggesting no marked ethnic differences.9 However, AUC0–168 h and Cmax in Japanese patients were 30–40% lower than those in non‐Japanese patients, numerically.9, 11
As a result of exploratory efficacy evaluation in this study, regional lymph node and distant metastases were observed in one patient. ADA were positive in two patient samples, but neutralizing antibody was negative in both samples. Nevertheless, the number of patients in this study was limited to conclude the safety and efficacy of PEG IFN‐α‐2b in Japanese patients with malignant melanoma.
In the EORTC 18991 study, OS was not improved in all patients treated with PEG IFN‐α‐2b compared with observation. However, an analysis performed with 7.6‐year follow‐up data showed statistically significant OS extension in patients who ulcerated with less tumor burden.12
The EORTC 18952 study13 did not improve the outcome for patients with stage IIB and III malignant melanoma treated with IFN‐α‐2b. However, the therapeutic effect for stage IIB and stage III‐N1 (patients with microscopic nodal disease) subgroups tended to be higher than stage III and stage III‐N2 (patients with palpable nodal disease) subgroups, respectively.
A meta‐analysis of EORTC 18952 and EORTC 18991 studies also indicated that both tumor stage and ulceration were predictive factors for the efficacy of adjuvant IFN‐α‐2b and PEG IFN‐α‐2b.14 Therefore, it was suggested that IFN‐α‐2b and PEG IFN‐α‐2b could be particularly effective, including OS improvement, in patients ulcerated and with relatively low tumor burden such as stage IIB or III‐N1.15
Based on the background, the EORTC 18081 study is in progress in patients with stage II ulcerated malignant melanoma for the purpose of confirming efficacy of PEG IFN‐α‐2b (ClinicalTrials.gov: NCT01502696). It would be important to evaluate continuously the efficacy of PEG IFN‐α‐2b in Japanese malignant melanoma patients who underwent surgery while analyzing the EORTC 18081 study data, as well as obtaining information from the post‐marketing surveillance and further research.
The approval of PEG IFN‐α‐2b in Japan made it possible to introduce global standard treatment and thereby resolve difference in medical environment.
Recently, immune checkpoint inhibitors including anti‐cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) and anti‐programmed cell death 1 (PD‐1) antibodies have been approved for unresectable metastatic malignant melanoma.16 These drugs are also developed for adjuvant therapy, and ipilimumab (anti‐CTLA‐4 antibody)17 was approved by the US Food and Drug Administration for adjuvant treatment of patients with stage III malignant melanoma who underwent surgery. In the future, it would be possible to promote further international collaborative clinical researches using such drugs with PEG IFN‐α‐2b for the adjuvant treatment in malignant melanoma.
In conclusion, no significant difference was observed in safety profiles between Japanese and non‐Japanese patients in this phase I study, which indicated that there were no major safety concerns about treatment with PEG IFN‐α‐2b in Japanese patients. Regarding pharmacokinetic parameters, it was suggested there were no marked ethnic differences. It was considered necessary to also continue evaluating the efficacy.
Pegylated IFN‐α‐2b was approved in Japan on 26 May 2015 on the basis of the evidence from this P370 and EORTC 18991 study, providing a new option for adjuvant treatment in patients with stage III malignant melanoma who underwent surgery. In the future, it is hoped that particular patients who exhibit more apparent efficacy to PEG IFN‐α‐2b will be identified, and new treatment options by combinations with new drugs will be developed.
Conflict of Interest
N. Y. and Y. K. received a fee for speaking from MSD K.K.. K. M., K. Y. and T. S. are employees of MSD K.K. All remaining authors have declared no conflicts of interest.
Acknowledgments
We thank the patients, their families and all of the investigators who participated in this study. The Efficacy and Safety Evaluation Committee comprised Drs Hideaki Tahara (Tokyo University), Koji Tsuboi (Toho University) and Koji Izutsu (Toranomon Hospital). This work was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA.
References
- 1. Coit DG, Andtbacka R, Anker CJ et al NCCN guidelines insights melanoma, version 2, 2013. J Natl Compr Canc Netw 2013; 11: 395–407. [DOI] [PubMed] [Google Scholar]
- 2. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa‐2b adjuvant therapy of high‐risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14: 7–17. [DOI] [PubMed] [Google Scholar]
- 3. Tarhini AA, Gogas H, Kirkwood JM. IFN‐α in the treatment of melanoma. J Immunol 2012; 189: 3789–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high‐risk melanoma: a systematic review and meta‐analysis. J Natl Cancer Inst 2010; 102: 493–501. [DOI] [PubMed] [Google Scholar]
- 5. Herndon TM, Demko SG, Jiang X et al U.S. Food and Drug Administration Approval: peginterferon‐alfa‐2b for the adjuvant treatment of patients with melanoma. Oncologist 2012; 17: 1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eggermont AM, Suciu S, Santinami M et al Adjuvant therapy with pegylated interferon alfa‐2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008; 372: 117–126. [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto T, Yokota K, Sawada M et al Postoperative DAV‐IFN‐β therapy does not improve survival rates of stage II and stage III melanoma patients significantly. J Eur Acad Dermatol Venereol 2013; 27: 1514–1520. [DOI] [PubMed] [Google Scholar]
- 8. Ji Y, Li Y, Bekele BN. Dose‐finding in phase I clinical trials based on toxicity probability intervals. Clin Trials 2007; 4: 235–244. [DOI] [PubMed] [Google Scholar]
- 9. Pharmaceuticals and Medical Devices Agency (PMDA) . Common Technical Document (CTD) of Peg‐interferon alpha‐2b for adjuvant treatment in melanoma, 2.7.1‐2. Available from URL: http://www.pmda.go.jp/drugs/2015/P201500047/.
- 10. Daud A, Soon C, Dummer R et al Management of pegylated interferon alpha toxicity in adjuvant therapy of melanoma. Expert Opin Biol Ther 2012; 12: 1087–1099. [DOI] [PubMed] [Google Scholar]
- 11. Daud AI, Xu C, Hwu WJ et al Pharmacokinetic/pharmacodynamic analysis of adjuvant pegylated interferon α‐2b in patients with resected high‐risk melanoma. Cancer Chemother Pharmacol 2011; 67: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eggermont AM, Suciu S, Testori A et al Long‐term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa‐2b versus observation in resected stage III melanoma. J Clin Oncol 2012; 30: 3810–3818. [DOI] [PubMed] [Google Scholar]
- 13. Eggermont AM, Suciu S, MacKie R et al Post‐surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 2005; 366: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 14. Eggermont AM, Suciu S, Testori A et al Ulceration and stage are predictive of interferon efficacy in melanoma: results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer 2012; 48: 218–225. [DOI] [PubMed] [Google Scholar]
- 15. Eggermont AM, Spatz A, Lazar V, Robert C. Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol 2012; 24: 137–140. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan RJ, Flaherty KT. Immunotherapy: anti‐PD‐1 therapies‐a new first‐line option in advanced melanoma. Nat Rev Clin Oncol 2015; 12: 625–626. [DOI] [PubMed] [Google Scholar]
- 17. Eggermont AM, Chiarion‐Sileni V, Grob JJ et al Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): a randomised, double‐blind, phase 3 trial. Lancet Oncol 2015; 16: 522–530. [DOI] [PubMed] [Google Scholar]


