Editor
Generally, redirecting hallmarks of cancer by communicative reprogramming leads to long‐term tumour control in histologically quite different neoplasias1, 2: A recently published randomized phase II trial on metastatic melanoma revealed significant impact of pioglitazone, a peroxisome proliferator‐activated receptorγ (PPARγ)3, 4 agonist, on inflammation control, progression‐free survival and overall survival when added to immune‐modulatory and angiostatic acting metronomic low‐dose chemotherapy.5, 6, 7
In the present phase I trial we studied tolerability and efficacy of the original combination pioglitazone daily 60 mg p.o, etoricoxib daily 60 mg p.o., plus low‐dose trofosfamide daily 50 mg three times p.o., and supplemented temsirolimus i.v. weekly at two dose levels, cohort 1, 15 mg, cohort 2, 25 mg (PETT schedule) (Fig. 1).8, 9 All medications were given continuously from day 1 until progression.
Figure 1.
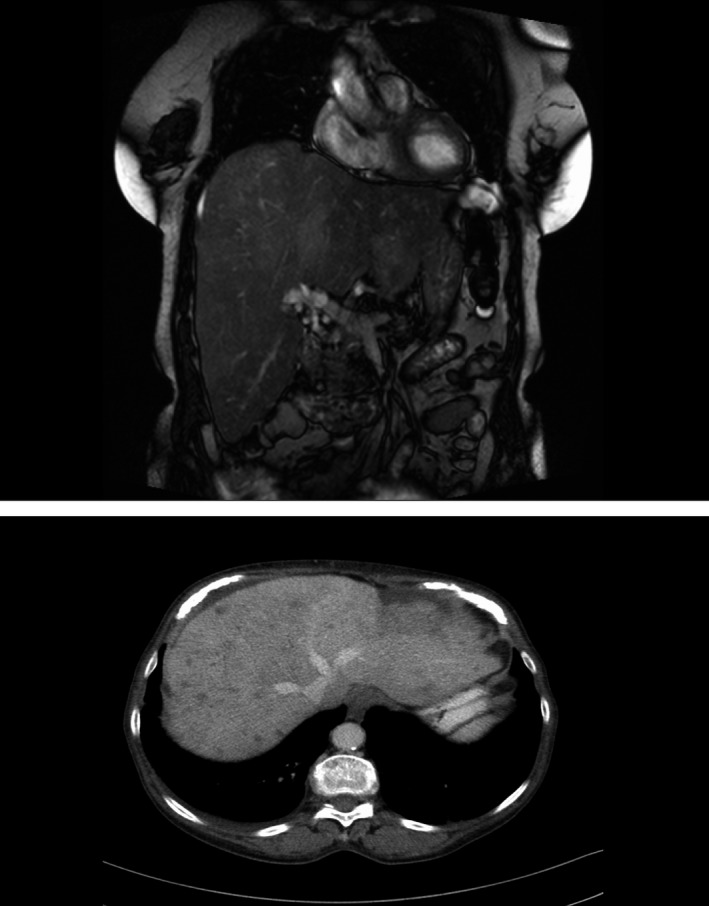
Hepatomegaly due to far advanced diffuse and multifocal metastases in patient No. 5 (Table 1) with uveal melanoma. Hepatomegaly did not decrease during PETT therapy, but disease could be stabilized for 1 year paralleled by a steep decrease of MIA in serum (Table 1).
Main inclusion criteria were age >18 years, histologically diagnosed metastatic melanoma with BRAF (the human gene that codes for the serine/threonine protein kinase B‐Raf) wild‐type, and LDH level >0.8 ULN, measurable lesions, and subjects had to receive study medication as first‐line therapy.
Dose‐limiting toxicity (DLT) was defined as any toxicity within the first 3 weeks of treatment with CTCAE (NCI‐CTCAE version 4.0) grade ≥3 and a causal relationship to the administration of one of the study drugs. At the end of phase I, patients were followed according RECIST criteria for progression‐free and overall survival. Computed tomography or magnetic resonance imaging (RECIST criteria) was performed every 8 weeks. The trial is approved by the local ethic committee and registered at ClinicalTrials.gov (NCT01614301).
Six elderly female patients (68–86 years old) with stage IV melanoma of uveal (n = 2) or cutaneous origin (n = 4), diffuse and multifocal liver metastases (n = 4) or tumour growth per continuitatem (n = 2) were enrolled during phase I. Local pretreatment and metastatic sites at study inclusion are listed in Table 1.
Table 1.
Characteristics of patients enrolled in phase I part of the study
| Patient no/age (year) | Histology, initial stage | Prior treatment | Clinical status before | Metastatic sites at study inclusion | Treatment duration (months) | Best response/tumor marker initially | Grade 3/4 toxicities following phase I (NCI‐CTCAE version 4.0) | Overall survival (months)/outcome |
|---|---|---|---|---|---|---|---|---|
| 1/68 y | Primary metastatic melanoma Stage IV |
|
ECOG 0 |
|
8 |
SD MIA 16/21 ng/mL |
|
13 Progression in the radiation field |
| 2/83 y | Mixed desmo‐plastic melanoma Glandula parotis pT4 pN1a |
|
ECOG 1 |
|
9 MIA 8.1–8.5 |
SD S‐100 0.45/0.1 μg/L |
No |
14 Progression at metastatic sites |
| 3/71 y | Uveal melanoma right eye, (metastatic) T3, N0, M1c Stage IV |
|
ECOG 0 |
|
13 |
PR MIA 8.6/8.5 ng/mL |
|
20 Progression liver |
| 4/71 y | Superficial spreading melanoma dorsal, pT1a, R0, N0, M0, Stage Ia | For metastatic disease:
|
ECOG 0 |
|
4 Inhibition of further spreading |
SD MIA 22/23 ng/mL |
Perianalnumbness, Anal and urinary incontinence:
|
8 Progression in the radiation field and vertebra 11 |
| 5/79 y | Uveal melanoma, (right eye, metastatic) Multiple liver metastases Stage IV |
|
ECOG 2 |
|
12 |
SD MIA 465/82 ng/mL |
|
13 Progression at original metastatic sites |
| 6/86 y | Melanoma DIG II left pT2a N3 M0 R0, Stage IIIb |
|
ECOG 0 |
|
5 |
Mixed response S‐100 0.45/0.1 μ/L |
No |
14 Progression in the radiation field |
ECOG, Eastern Cooperative Oncology Group performance status; SIRT, selective internal radiation therapy; MIA, melanoma inhibitory activity; SD, stable disease; PR, partial response; SAE, severe adverse event.
The 25 mg dose of temsirolimus was chosen for the randomized phase II part of the study as no DLTs occurred in cohort 1 or 2. Two severe adverse events (SAEs) were observed during phase I part of the study, one due to pneumonia and another to pleural effusion. Both SAEs resolved during continuation of study medication. Grade 3 (no grade 4) toxicities during the follow‐up phase are listed in Table 1. According to scheduled dose reductions, trofosfamide was reduced to 50 mg twice daily in four patients. Due to oedema, the dose of pioglitazone was reduced to 30 mg daily in two patients.
Progression‐free survival was 4–13 months, with four disease stabilizations, one mixed response (no objective response in the radiation field) and one partial response (PR) (Table 1). One stable disease in a patient with diffuse as well as measurable liver metastases of uveal melanoma was associated with a steep decline of melanoma inhibitory activity (MIA) and an improvement of Eastern Cooperative Oncology Group (ECOG )status from ECOG 2 to 1 (Fig. 1, Table 1). PR was not associated with a significant decline of MIA (Table 1). Interestingly, two patients with extensive metastatic liver involvement from uveal melanoma had a comparatively long progression‐free survival.
In all patients, progression took place at the original metastatic sites. Patients, who were prior irradiated, presented with progression in the radiation field.
To our knowledge, this is the first report describing the use of PETT schedule, to successfully control both, metastatic growth in cutaneous and uveal melanoma by simultaneously targeting multiple hallmarks of melanoma: Communicative reprogramming may overcome (molecular‐)genetic heterogeneity among melanomas of quite different origin as well as at different metastatic sites.2, 5 PETT schedule provides a modest toxicity profile and omits maximal tolerable dosages of single drugs by concertedly modulating melanoma plus adjacent stroma cell functions.3, 5, 8, 10 These promising data with PETT combination therapy are currently studied in a randomized phase II trial in second‐line.
A.R., C.H. designed the research, analysed the data and wrote the paper; C.H., M.V., Ch.H., M.L., M.B., S.H. and A.R. treated the patients; W.H. critically reviewed the manuscript.
References
- 1. Larkin J, Chiarion‐Sileni V, Gonzalez R et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart C, Vogelhuber M, Wolff D et al Anakoinosis: communicative reprogramming of tumor systems – for rescuing from chemorefractory neoplasia. Cancer Microenviron 2015; 8: 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer S, Vogt T, Landthaler M et al Cyclooxygenase 2 (COX2) and Peroxisome Proliferator‐Activated Receptor Gamma (PPARG) are stage‐dependent prognostic markers of malignant melanoma. PPAR Res 2009; 2009: 848645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herwig MC, Bergstrom C, Wells JR, Höller T, Grossniklaus HE. M2/M1 ratio of tumor associated macrophages and PPAR‐gamma expression in uveal melanomas with class 1 and class 2 molecular profiles. Exp Eye Res 2013; 107: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reichle A, Vogt T, Coras B et al Targeted combined anti‐inflammatory and angiostatic therapy in advanced melanoma: a randomized phase II trial. Melanoma Res 2007; 17: 360–364. [DOI] [PubMed] [Google Scholar]
- 6. Schadendorf D. Peroxisome proliferator‐activating receptors: a new way to treat melanoma? J Invest Dermatol 2009; 129: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 7. Emmenegger U, Chow A, Bocci G. The biomodulatory capacities of low‐dose metronomic chemotherapy: complex modulation of the tumor microenvironment In: Reichle A, ed. Reichle's From Molecular to Modular Tumor Therapy, 1st edn Springer, Dordrecht, 2010: 234–262. [Google Scholar]
- 8. Stahl JM, Sharma A, Cheung M et al Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res 2004; 64: 7002–7010. [DOI] [PubMed] [Google Scholar]
- 9. Luke JJ, Triozzi PL, McKenna KC et al Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res 2015; 28: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paulitschke V, Gruber S, Hofstätter E et al Proteome analysis identified the PPARγ ligand 15d‐PGJ2 as a novel drug inhibiting melanoma progression and interfering with tumor‐stroma interaction. PLoS One 2012; 7: e46103. [DOI] [PMC free article] [PubMed] [Google Scholar]


