Summary
Aims
Concomitant renin–angiotensin–aldosterone system blockade and natriuretic peptide system enhancement may provide unique therapeutic benefits to patients with heart failure and reduced ejection fraction (HFrEF). This study assessed the pharmacodynamics and pharmacokinetics of LCZ696 in patients with HFrEF.
Methods
This was an open‐label, noncontrolled single‐sequence study. After a 24‐h run‐in period, patients (n = 30) with HFrEF (EF ≤ 40%; NYHA class II–IV) received LCZ696 100 mg twice daily (bid) for 7 days and 200 mg bid for 14 days, along with standard treatment for heart failure (HF) (except angiotensin‐converting enzyme inhibitors [ACEIs] or angiotensin receptor blockers [ARBs]).
Results
On Day 21, significant increases were observed in the plasma biomarkers indicative of neprilysin and RAAS inhibition (ratio‐to‐baseline: cyclic guanosine monophosphate [cGMP], 1.38; renin concentration and activity, 3.50 and 2.27, respectively; all, P < 0.05). Plasma NT‐proBNP levels significantly decreased at all the time points on Days 7 and 21; plasma aldosterone and endothelin‐1 levels significantly decreased on Day 21 (all, P < 0.05). Following administration of LCZ696, the Cmax of sacubitril (neprilysin inhibitor prodrug), LBQ657 (active neprilysin inhibitor), and valsartan were reached within 0.5, 2.5, and 2 h. Between 100‐ and 200‐mg doses, the Cmax and AUC 0–12 h for sacubitril and LBQ657 were approximately dose‐proportional while that of valsartan was less than dose‐proportional.
Conclusions
Treatment with LCZ696 for 21 days was well tolerated and resulted in plasma biomarker changes indicative of neprilysin and RAAS inhibition in patients with HF. The pharmacokinetic exposure of the LCZ696 analytes in patients with HF observed in this study is comparable to that observed in the pivotal Phase III study.
Keywords: Heart failure, LCZ696, Pharmacodynamics, Pharmacokinetics, Sacubitril/valsartan
Introduction
Sacubitril/valsartan (LCZ696) is an angiotensin receptor neprilysin inhibitor (ARNI) providing concomitant inhibition of neprilysin (via LBQ657, the active metabolite of the prodrug sacubitril) and blockade of the angiotensin II type‐1 (AT1) receptor (via valsartan) 1.
The rationale for neprilysin inhibition is based on the cardiorenal effects of natriuretic peptides (NPs) and other neprilysin substrates that oppose the effects of the activated renin–angiotensin–aldosterone system (RAAS) and the sympathetic nervous system (SNS). NPs exert their effects by activating membrane‐bound guanylyl cyclase‐coupled receptors (natriuretic peptide receptors [NPR]‐A and [NPR]‐B), thereby increasing the concentrations of the second messenger cyclic guanosine monophosphate (cGMP), which mediates several of the cardiovascular (CV) and renal effects of NPs, including blood pressure (BP) reduction, vasodilation, natriuresis, and diuresis, increase in glomerular filtration and renal blood flow, inhibition of renin and aldosterone release, reduction in sympathetic activity, antihypertrophic, and antifibrotic effects, and facilitation of lipolysis and mitochondrial biogenesis 2, 3, 4, 5. In addition, AT1‐receptor blockade by LCZ696 inhibits the adverse CV effects of angiotensin II and aldosterone 6. The complementary effect of neprilysin inhibition and AT1 receptor blockade has been confirmed in patients with hypertension 7. Moreover, in a recently completed Phase III outcomes trial (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure [PARADIGM‐HF]), LCZ696 was superior to the standard‐of‐care enalapril in reducing the CV and all‐cause mortality and heart failure (HF) hospitalizations in patients with HF and reduced ejection fraction (HFrEF).8 LCZ696 was approved recently for the treatment of patients with HFrEF based on the benefits of LCZ696 observed in the PARADIGM‐HF trial 9, 10.
The pharmacodynamic and pharmacokinetic properties following single‐ and multiple‐dose administration of LCZ696 in healthy subjects have been previously reported 1; however, similar data have not been reported for patients with HFrEF. This study aimed at investigating the pharmacokinetics of LCZ696 analytes in patient with heart failure along with the effects of LCZ696 on pharmacodynamic biomarkers related to its mechanism of action.
Methods
This was an open‐label, nonrandomized, noncontrolled, single‐sequence, dose‐titration Phase IIa study in patients with chronic HFrEF. The protocol was reviewed and approved by the Russian National Ethics Committee and the local ethics committees. Following written informed consent, eligible patients were admitted to the Center of Applied Clinical Pharmacology at Peoples Friendship University of Russia, Moscow, or the City Clinical Hospital #64, Moscow.
Eligibility Criteria
Male and female (nonchildbearing potential) patients (aged >18 years) with documented chronic HF, defined as New York Heart Association (NYHA) functional class II–IV, and a documented left ventricular ejection fraction (LVEF) of ≤40% were screened for enrollment. Patients had to receive standard‐of‐care HF medication at stable doses for ≥1 month before study entry. Other inclusion criteria were sitting systolic blood pressure (SBP) ≥110 mm Hg, estimated glomerular filtration rate ≥30 mL/min/1.73 m2, and serum potassium ≤5.2 mmol/L. The key exclusion criteria included isolated right HF, significant valvular disease, hypertrophic obstructive cardiomyopathy, secondary cardiomyopathy, previous or imminent cardiac transplantation, and unstable angina or recent myocardial infarction within 6 months before enrollment. Patients treated with combination therapy (i.e., angiotensin‐converting enzyme inhibitors [ACEIs] and angiotensin receptor blockers [ARBs]), or with a history of angioedema or significantly elevated liver enzymes (>3 times the upper limit of normal) were excluded.
Study Design
Eligible patients entered a 24‐h run‐in period during which ACEIs/ARBs were stopped while other concomitant HF medications were maintained throughout the study period. Following the run‐in period, patients could initiate treatment with LCZ696 at a dose of 50 or 100 mg twice daily (bid) based on their previously prescribed dose of ACEI/ARB for 7 days; the dose was subsequently up‐titrated to 100 mg for 7 days (in those initiating LCZ696 at 50 mg bid) or to the target dose of 200 mg bid for 14 days (in those completing the LCZ696 100‐mg bid treatment period, Figure 1).
Figure 1.
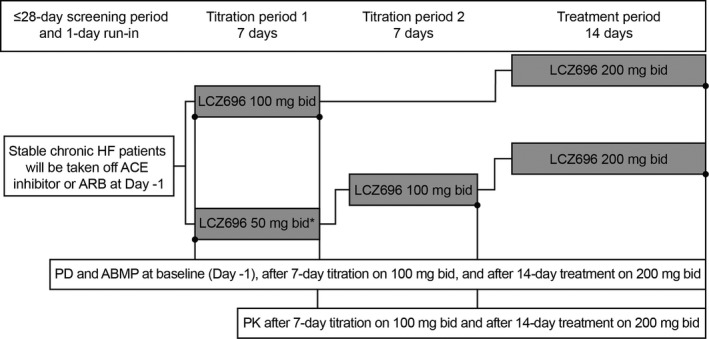
Study design. *No patients participated in the titration period 1. ABPM, ambulatory blood pressure monitoring; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; bid, twice daily; HF, heart failure; PD, pharmacodynamics; PK, pharmacokinetics.
To ensure patient safety and treatment compliance, patients were domiciled throughout the treatment period. A daily diet of 3 g sodium (1130 mEq) and 5 g potassium (128 mEq) was provided and standardized for all subjects throughout the study to eliminate the effect of dietary changes in measured pharmacodynamic parameters, including atrial natriuretic peptide [ANP]).
Key criteria requiring study discontinuation were as follows: serum potassium >5.5 mmol/L (confirmed by a repeat measurement), serum creatinine >3.0 mg/dL or doubled from baseline (confirmed by a repeat measurement), symptomatic or orthostatic hypotension, and uncontrolled hypertension (SBP >160 mm Hg).
Pharmacodynamic Assessments
Plasma pharmacodynamic assessments included ANP, cGMP, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), aldosterone, endothelin‐1 (ET‐1), and renin concentration (PRC) and activity (PRA). Plasma samples were collected at baseline, predose, and 4, 12, 16, and 24 h after the morning dose on Days 7 and 21 (i.e., after 7 and 14 days of LCZ696 100‐ and 200‐mg bid treatments, respectively). Urine samples were collected over 24 h in 12‐h time intervals (8:00–20:00 h and 20:00–8:00 h) to measure urine volume and biomarkers (sodium, potassium, creatinine, ANP, cGMP, and NT‐proBNP) at baseline, and Days 7 and 21.
Pharmacokinetic Assessments
Plasma concentration–time profiles and steady‐state pharmacokinetic parameters were determined for sacubitril, LBQ657, and valsartan following oral administration of LCZ696 on Days 7 and 21. Blood samples for pharmacokinetic analysis were collected at predose and 0.5, 1, 2, 3, 4, 6, 12, and 24 h after the morning dose on Days 7 and 21. Additional pharmacokinetic blood sampling was performed at 48 and 72 h after the morning dose on Day 21. Noncompartmental analysis was performed for the pharmacokinetic parameters: maximum plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), area under the concentration–time curve from time zero to 12 h at steady state (AUC0–12 h), and elimination half‐life (T1/2).
Assay Methods
Pharmacodynamics
Plasma and urinary levels of cGMP were measured using Amersham cGMP Enzyme immunoassay (EIA) Biotrak System (GE Healthcare); plasma levels of ET‐1 were measured using Human Endothelin‐1 QuantiGlo® ELISA Kit (QET00B; R&D SYSTEMS); aldosterone plasma levels were measured using Aldosterone RIA kit (DSL‐8600 ACTIVE®; Diagnostic Systems Laboratories Inc. ); PRC was measure using Renin III Generation RIA kit (RENINE; Cis Biointernational); and PRA was measured using Angiotensin I RIA kit (REN‐CT2; Cis Biointernational). Plasma and urinary levels of ANP and NT‐proBNP were measured using Atrial Natriuretic Factor (1–28) EIA kit (S‐1131; Bachem) and Elecsys NT‐proBNP immunoassay (Roche Diagnostics), respectively. Sodium, potassium, and creatinine were analyzed using standard laboratory techniques.
Pharmacokinetics
Plasma levels of sacubitril, LBQ657, and valsartan were analyzed using a validated liquid chromatography with tandem mass spectrometry method with a lower limit of quantification of 1.0 ng/mL for all the analytes 1.
Safety Assessments
The safety and tolerability assessments included electrocardiogram monitoring, vital signs, and electrolyte and laboratory panels for renal and hepatic safety. Details regarding BP measurements are provided in the Appendix S1.
Statistical Analysis
All patients who received at least one dose of the study drug were included in the safety and tolerability assessments. For the pharmacokinetic and pharmacodynamic analyses, patients who received at least one dose of the study drug and had at least one postbaseline assessment were included. For the plasma and urine pharmacodynamic biomarkers, geometric means were calculated at baseline and on Days 7 and 21. The geometric mean ratio‐to‐baseline on Day 21 was calculated according to the baseline values for patients who completed the study. The geometric mean ratios and 95% confidence interval (CI) and the two‐sided P values for the ratio‐to‐baseline were calculated using the paired t‐test for log‐transformed data.
Results
Patients
In total, 36 patients with chronic HF were screened, of whom 30 (NYHA functional class II [n = 17, 57%] and III [n = 13, 43%]) were enrolled in the study between May and July 2009. The mean (±standard deviation [SD]) age was 62 (9.3) years, and 83.3% (n = 25) were men. The mean (±SD) LVEF was 33.6% (4.56), and most patients had a history of MI (n = 27, 90%) and arterial hypertension (n = 26, 87%). The most common background medications were ACE inhibitors or ARBs (all patients), beta‐blockers (n = 27, 90%), and acetylsalicylic acid (n = 27, 90%). Baseline demographics, medical history, and background or concomitant medications are summarized in Table 1. All the patients initiated LCZ696 treatment at 100 mg bid for 7 days, and the dose was subsequently up‐titrated to the target dose of 200 mg bid for 14 days.
Table 1.
Summary of baseline demographics, medical history, and background/concomitant medications
| Variable | LCZ696 100 or 200 mg bid N = 30 |
|---|---|
| Age, years (mean ± SD) | 62.0 ± 9.3 |
| Male/female | 25 (83.3)/5 (16.7) |
| Body mass index, kg/m2 (mean [min–max]) | 30.66 (22.8–38.9) |
| NYHA | |
| Class II | 17 (56.7) |
| Class III | 13 (43.3) |
| LVEF %, mean (±SD) | 33.6 (4.56) |
| Systolic BP, mm Hg (mean ± SD) | 133.0 ± 14.59 |
| Diastolic BP, mm Hg (mean ± SD) | 79.6 ± 8.36 |
| Medical history (by preferred term) | |
| Heart rate, beats per min (mean ± SD) | 70.3 ± 11.18 |
| Myocardial infarction | 27 (90.0) |
| Arterial Hypertension | 26 (86.7) |
| Atrial fibrillation | 6 (20.0) |
| Diabetes mellitus | 5 (16.7) |
| Renal failure chronic | 3 (10.0) |
| Background/concomitant medications | |
| ACE inhibitorsa | 30 (100.0) |
| Beta‐blocker | 27 (90.0) |
| Spironolactone | 10 (33.3) |
| Furosemide | 19 (63.3) |
| Hydrochlorothiazide | 8 (26.7) |
| Indapamide | 1 (3.3) |
| Digoxin | 4 (13.3) |
| Nifedipine | 5 (16.7) |
| Aspirin | 27 (90.0) |
| Nitrates | 10 (33.3) |
| Statin | 15 (50.0) |
ACE, angiotensin‐converting enzyme; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
Data are presented as n (%), unless otherwise specified.
Treatment with ACE inhibitors was discontinued before the study initiation.
Pharmacodynamic Effects of LCZ696
Biomarkers Related to Neprilysin Inhibition
Plasma
Significant increases from baseline, sustained throughout the dosing interval, were observed in plasma cGMP levels on Days 7 and 21 (Table 2). Plasma ANP was not significantly different from baseline during either of the treatment periods.
Table 2.
Plasma and urine levels of NP and RAAS biomarkers at baseline and Days 7 and 21 during LCZ696 treatment
| Biomarker | Baseline Na = 30 | Day 7 (LCZ696 100 mg bid) Na = 30 | Day 21 (LCZ696 200 mg bid) Na = 27 | ||
|---|---|---|---|---|---|
| Predose | Ratio‐to‐baseline (95% CI), P value | Predose | Ratio‐to‐baseline (95% CI), P value | ||
| Plasma NP biomarkers | |||||
| cGMP, nmol/L | 11.13 | 13.83 | 1.24 (1.06–1.45) P = 0.008 | 15.07 | 1.38 (1.16–1.65) P < 0.001 |
| ANP, pg/mL | 114.31 | 105.20 | 0.92 (0.80–1.05) P = 0.223 | 110.83 | 1.00 (0.80–1.26) P = 0.986 |
| Urine NP biomarkers | |||||
| cGMP, nmol | 937.96 | 1096.09 | 1.17 (0.97–1.40) P = 0.090 | 1180.57 | 1.22 (1.01–1.47) P = 0.040 |
| ANP, ng | 209.60 | 353.42 | 1.69 (1.40–2.03) P < 0.001 | 378.48 | 1.82 (1.54–2.17) P < 0.001 |
| Plasma RAAS biomarkers | |||||
| PRC, pg/mL | 9.92 | 42.64 | 4.30 (2.78–6.64) P < 0.001 | 34.11 | 3.50 (2.13–5.76) P < 0.001 |
| PRA, ng/mL/h | 0.69 | 2.70 | 3.94 (2.27–6.87) P < 0.001 | 1.64 | 2.27 (1.20–4.32) P = 0.014 |
ANP, atrial natriuretic peptide; bid, twice daily; cGMP, cyclic guanosine monophosphate; CI, confidence interval; NP, natriuretic peptide; PRA, plasma renin activity; PRC, plasma renin concentration; RAAS, renin–angiotensin–aldosterone system.
Data are presented as geometric means. The ratio‐to‐baseline after LCZ696 200 mg bid was calculated according to the baseline values for patients who completed the study.
Data for PRA are presented for 29 patients at baseline and on Day 7 and for 26 patients on Day 21.
Urine
Urinary cGMP levels showed a trend toward an increase by Day 7 and were significantly increased by Day 21; urinary ANP levels significantly increased by the end of each treatment period (Table 2).
Biomarkers Related to AT1 Receptor Blockade
The plasma renin markers (PRC and PRA) significantly increased from baseline after the 7‐day treatment with LCZ696 100‐mg bid and the 14‐day treatment with LCZ696 200 mg bid (Table 2).
Biomarkers Indicative of Beneficial Pharmacodynamic Effects in HF
Plasma
There was a trend toward a reduction in predose plasma aldosterone and ET‐1 levels on Day 7 as compared with baseline, which reached a statistical significance on Day 21 following LCZ696 200‐mg bid treatment for 14 days (ratio‐to‐baseline [95% CI]: aldosterone, 0.79 [0.65–0.95]; P = 0.017 and ET‐1, 0.80 [0.71–0.91]; P = 0.001; Figure 2). Plasma NT‐proBNP levels significantly decreased at all the time points on Days 7 and 21 (ratio‐to‐baseline [95% CI]: Day 7, 0.53 [0.45–0.62]; P < 0.001; Day 21, 0.56 [0.45–0.70]; P < 0.001; Figure 2).
Figure 2.
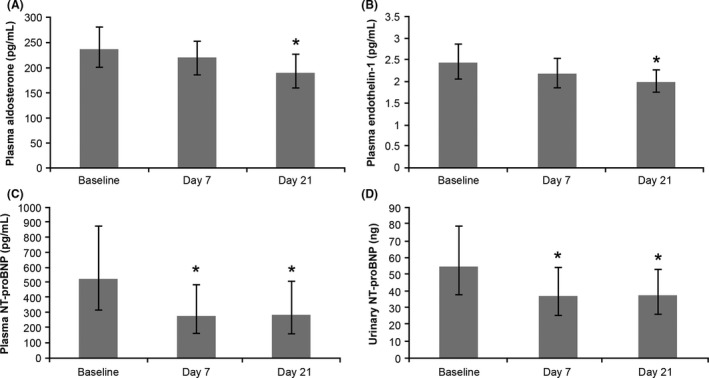
Mean (±SD) levels of (A) plasma aldosterone, (B) plasma endothelin‐1 and (C and D) plasma and urine NT‐proBNP for patients receiving LCZ696 treatment. Data are presented as geometric mean and 95% confidence intervals; *P < 0.05. NT‐proBNP, N‐terminal pro‐hormone B‐type natriuretic peptide; SD, standard deviation.
Urine
Urinary NT‐proBNP decreased significantly with a ratio‐to‐baseline (95% CI) of 0.68 (0.55–0.83; P < 0.001) and 0.74 (0.59–0.94; P < 0.017) after LCZ696 100‐mg bid treatment for 7 days and LCZ696 200‐mg bid treatment for 14 days, respectively (Figure 2).
No statistically significant changes were observed in the mean urinary sodium, potassium, and creatinine excretion during either of the treatment periods (Table S1 in Appendix S1).
Pharmacokinetics of LCZ696
Following oral administration of multiple doses of LCZ696 100 and 200 mg bid in patients with stable HF, plasma concentrations of sacubitril, LBQ657, and valsartan increased rapidly and reached peak plasma concentrations within 0.5, 2.5, and 2 h after the dose (median), respectively, in both the treatment periods (Figure 3 and Table 3). The Cmax and AUC0–12 h values for both sacubitril and LBQ657 were approximately dose‐proportional between the 100‐ and 200‐mg doses. However, the Cmax and exposure of valsartan appeared less than dose‐proportional between the 100‐ and 200‐mg doses. Plasma concentrations of sacubitril, LBQ657, and valsartan decreased with a mean T1/2 of approximately 4, 18, and 14 h, respectively (Table 3).
Figure 3.
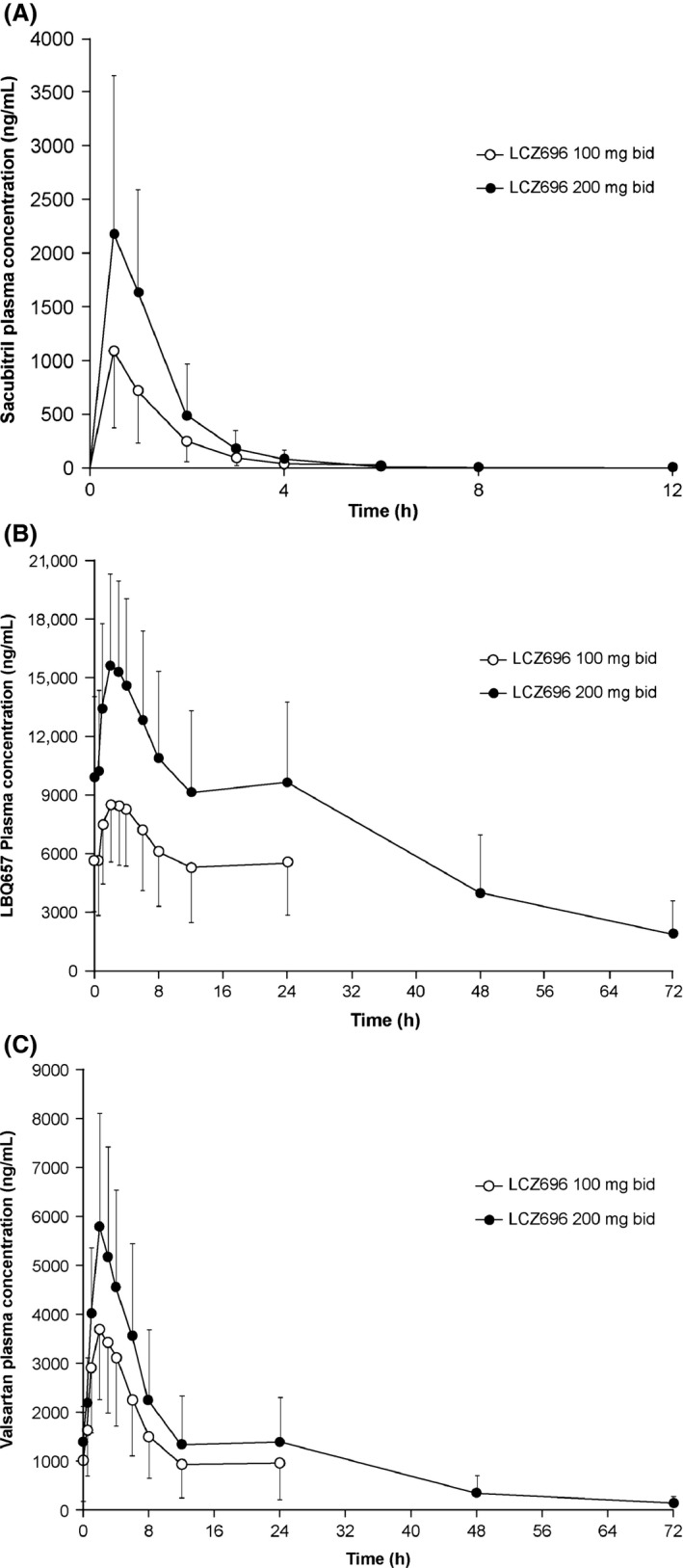
Mean (SD) plasma concentration–time profiles of (A) sacubitril, (B) LBQ657, and (C) valsartan at steady state following administration of LCZ696 100 and 200 mg bid. bid, twice daily; SD, standard deviation.
Table 3.
Summary of mean (SD) pharmacokinetic parameters at steady state for sacubitril, LBQ657, and valsartan after LCZ696 100‐ and 200‐mg bid administration in patients with stable HF
| Sacubitril | LBQ657 | Valsartan | ||||
|---|---|---|---|---|---|---|
| LCZ696 100 mg bid | LCZ696 200 mg bid | LCZ696 100 mg bid | LCZ696 200 mg bid | LCZ696 100 mg bid | LCZ696 200 mg bid | |
| Tmax, h | 0.5 (0.5–2) | 0.5 (0.5–2) | 2.5 (1–8) | 2 (1–6) | 2 (1–4) | 2 (1–3) |
| Cmax, ng/mL | 1229 (621) | 2408 (1357) | 9103 (3174) | 16,345 (4703) | 3814 (1504) | 6044 (2502) |
| T1/2, h | ND | 3.9 (3.6) | ND | 18.4 (6.8) | ND | 13.7 (5.0) |
| AUC0–12, ng × h/mL | 1537 (731) | 3153 (1377) | 82,633 (33,740) | 147,111 (51,762) | 25,888 (12,096) | 38,807 (18,129) |
For Tmax, data are presented as median (range).
bid, twice daily; HF, heart failure; ND, not determined; SD, standard deviation.
Safety and Tolerability
All 30 patients completed the 7‐day treatment with LCZ696 100 mg bid and started the 14‐day treatment with LCZ696 200 mg bid. In total, three patients (one patient with hyperkalemia at baseline) discontinued the study because serum potassium reached the prespecified upper threshold of 5.5 mmol/L. The remaining 27 (90%) patients completed the study treatment as per protocol.
Overall, adverse events (AEs) were reported in 21 (70.0%) of the 30 patients; in 16 (53.3%) and 15 (50.0%) patients during the 7‐day titration period and the 14‐day treatment period, respectively. No deaths or serious AEs occurred during the study. The most frequently reported AEs that occurred in four patients each were increased serum potassium and serum creatinine, PR prolongation, and dizziness. The frequency of AEs was not dose related, and most of the AEs resolved by the end of the study.
Reductions in the supine, mean 24‐h ambulatory, and daytime and night‐time SBP and DBP were noted on Day 7, and these changes were sustained up to Day 21 (Table S3 in Appendix S1). BP reduction was well tolerated; none of the patients experienced BP‐related symptoms that required treatment discontinuation, and hypotension was not reported as an AE.
Further details on AEs and the effects of LCZ696 on BP are provided in the Appendix S1.
Discussion
This study investigated the pharmacodynamics and pharmacokinetics of LCZ696 in patients with HFrEF. The study included patients who were receiving stable doses of standard‐of‐care HF medication including an ACE inhibitor and a beta‐blocker at screening. A bid treatment schedule, similar to that used in the PARADIGM‐HF trial, was used to ensure sustained 24‐h neprilysin inhibition, and to reduce the risk of hypotension in patients with HF.
The changes observed in the plasma and urinary biomarkers are consistent with the anticipated mechanism of action of LCZ696. The increases in urinary cGMP and ANP levels were consistent with neprilysin inhibition as reported in the previous studies 1, 11. Furthermore, the significant increase in plasma cGMP levels was sustained throughout the dosing interval, indicating effective neprilysin inhibition and further supporting a bid dosing regimen in patients with HF. Notably, no significant changes in plasma ANP levels were observed; however, ANP is a labile analyte, requires careful sample handling including immediate freezing, and plasma samples were analyzed after expiry of the sample stability dates due to a technical error; therefore, changes in plasma ANP levels remain to be defined. RAAS inhibition by LCZ696 was evidenced by increases in PRA and PRC, which were similar to those observed in earlier studies with an ARB 12. No significant changes were noted in the natriuresis; however, it remains challenging to determine the true effect of LCZ696 on natriuresis in the presence of concomitant use of diuretics.
Neprilysin has a high affinity for biologically active NPs 13 but does not degrade NT‐proBNP 14, which therefore remains a cardiac biomarker reflecting the hemodynamic status and prognosis in patients with HFrEF receiving a neprilysin inhibitor. The potential hemodynamic improvement with LCZ696 treatment was corroborated by an early and sustained reduction in NT‐proBNP levels, similar to that observed following hemodynamic unloading of the left ventricle 15.
Neprilysin degrades numerous additional vasoactive peptides, including ET‐1 and angiotensin II 16, 17. Although elevation of NPs through neprilysin inhibition is expected to result in beneficial CV and renal effects in HF, potential increases in ET‐1 and angiotensin II may potentially counteract these benefits. In this study, we demonstrated that LCZ696 treatment for 21 days in patients with HFrEF was associated with a decrease in ET‐1 levels from baseline, suggesting that neprilysin‐mediated ET‐1 degradation may not be clinically relevant in this patient population or that ET‐1 production may have been attenuated due to improved vascular homeostasis and endothelial function following LCZ696 treatment. Angiotensin II was not measured in this study; however, a potential increase in angiotensin II following neprilysin inhibition provides the rationale for concomitant AT1‐receptor blockade with LCZ696.
Aldosterone antagonists effectively reduce adverse CV outcomes in patient with HFrEF 18. LCZ696 decreased plasma aldosterone levels by 21% at Day 21. As both NPs and RAAS blockers may decrease aldosterone levels, it is not clear to what extent these findings reflect AT1‐receptor blockade or NP facilitation, or both combined. Of note, a recent preclinical report showed that combined neprilysin and AT1 blockade enhanced the aldosterone suppression effects of ANP and BNP in Ang‐II‐sensitized human adrenocortical cells 19. Similarly, combined neprilysin and AT1 blockade more effectively attenuated Ang‐II mediated hypertrophic and fibrotic effects on cardiac and renal cells compared with stand‐alone neprilysin inhibition and AT1 blockade 2, 3. These data underscore the complementary effects of combined neprilysin and AT1 blockade as the potential mechanism for the observed benefits with LCZ696.
Based on prior pharmacokinetic characterization of LCZ696, it is well established that concentrations of the three analytes are at steady state after 3 days of dosing 20. Hence, in the current study, all analytes would have been at steady state by the seventh day of dosing. The observed exposure for sacubitril, LBQ657, and valsartan in patients with HF was approximately 55%, 110%, and 132% higher, respectively, compared with the exposure observed in healthy subjects after LCZ696 200‐mg bid administration 20. This exposure is consistent with the increase in the estimated T1/2 for sacubitril, LBQ657, and valsartan in patients with HF compared with healthy subjects, reflecting a reduction in the clearance of LCZ696 analytes in patients with HF potentially due to altered hepatic and/or renal function. The observed valsartan exposure following administration of LCZ696 200 mg bid in this study is similar to that observed when valsartan 160 mg bid was administered in patients with HF 21. Furthermore, the observed exposure is similar to the exposure of LCZ696 analytes observed in the recently concluded PARADIGM‐HF trial [Novartis data on file], which established the efficacy and safety of LCZ696 at doses up to 200 mg bid in patients with HF 8.
Overall, treatment with LCZ696 100 mg bid for 7 days and 200 mg bid for 14 days was well tolerated, including the transition from previous ACE inhibitors or ARB therapy. In total, three patients withdrew from the study because of increased serum potassium values; however, baseline renal impairment in two patients and the use of an aldosterone antagonist in the third patient reflect a predisposition to increased serum potassium levels in these patients. In the PARADIGM‐HF trial, LCZ696 treatment was associated with a lower incidence of hyperkalemia compared with enalapril 8. Moreover, in a recent meta‐analysis, combined neprilysin and RAAS inhibition (omapatrilat and LCZ696) was associated with better preservation of renal function compared with stand‐alone RAAS inhibition 22.
The main limitations of this study are the open‐label, noncontrolled, single‐sequence study design, and small sample size, which do not allow generalization of the safety and tolerability results or differentiation of the effects of neprilysin inhibition from those of ARBs on the pharmacodynamic endpoints.
In conclusion, the exposure to LCZ696 analytes in this study with intense PK sampling was comparable to the LCZ696 PK profile observed in the recently completed PARADIGM‐HF trial utilizing sparse PK sampling. This study further demonstrates that administration of LCZ696 resulted in increased levels of plasma and urinary cGMP and increased urinary ANP, thereby providing evidence of neprilysin inhibition in patient with HFrEF. Additionally, treatment with LCZ696 decreased aldosterone and endothelin‐1 plasma levels. Overall, these findings support the benefits of LCZ696 with regard to reduction in CV death, HF hospitalizations and all‐cause mortality compared with enalapril as demonstrated in the PARADIGM‐HF trial 8.
Funding
This study was funded by Novartis Pharma AG, Basel, Switzerland. Novartis contributed to the study design, data analysis, and interpretation of the study report.
Conflict of Interest
ZK, YK, OA, EP, and VM declare that they have no conflict of interests. DA, PC, MFP, PP, THL, PJ, SA, and IR were employees of Novartis at the time of the study conduct and may own company stocks.
Author Contributions
All the authors contributed to the protocol design or data analysis/interpretation, as well as had full access to the study data. All the authors contributed in critical review and revision of the manuscript and are in agreement with the publication, but thank Syed Abdul Haseeb and Rohini Verma (Novartis Healthcare Pvt. Ltd., Hyderabad, India) for their medical writing and editorial support and subsequent revisions based on authors' feedback.
Supporting information
Appendix S1. Urinary volume‐corrected levels of creatinine, potassium, and sodium excretion; adverse events and effects on blood pressure.
ClinicalTrials.gov Identifier: NCT00913653.
References
- 1. Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual‐acting angiotensin receptor‐neprilysin inhibitor (ARNi). J Clin Pharmacol 2010;50:401–414. [DOI] [PubMed] [Google Scholar]
- 2. Wang BH, Von Lueder TG, Kompa AR, et al. Combined angiotensin receptor blockade and neprilysin inhibition attenuates angiotensin‐II mediated renal cellular collagen synthesis. Int J Cardiol 2015;186:104–105. [DOI] [PubMed] [Google Scholar]
- 3. von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 2015;8:71–78. [DOI] [PubMed] [Google Scholar]
- 4. Mangiafico S, Costello‐Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: An evolving strategy in cardiovascular therapeutics. Eur Heart J 2013;34:886–893c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potter LR, Abbey‐Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate‐dependent signaling functions. Endocr Rev 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 6. Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007;292:C82–C97. [DOI] [PubMed] [Google Scholar]
- 7. Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double‐blind, placebo‐controlled, active comparator study. Lancet 2010;375:1255–1266. [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJ, Packer M, Desai AS, et al. Angiotensinneprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 9. Entresto Prescribing Information . US Food and Drug Administration Web Site [Internet]. 2015. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf.
- 10. Entresto Summary of Product Characteristics . EMA Web Site [Internet]. 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004062/WC500197536.pdf.
- 11. Liao WC, Vesterqvist O, Delaney C, et al. Pharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor, omapatrilat in healthy subjects. Br J Clin Pharmacol 2003;56:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azizi M, Menard J, Bissery A, Guyene TT, Bura‐Riviere A. Hormonal and hemodynamic effects of aliskiren and valsartan and their combination in sodium‐replete normotensive individuals. Clin J Am Soc Nephrol 2007;2:947–955. [DOI] [PubMed] [Google Scholar]
- 13. Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C‐type natriuretic peptide and some C‐receptor ligands by endopeptidase‐24.11. Biochem J 1993;291(Pt 1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez‐Rumayor A, Richards AM, Burnett JC, Januzzi JL Jr. Biology of the natriuretic peptides. Am J Cardiol 2008;101:3–8. [DOI] [PubMed] [Google Scholar]
- 15. Cioffi G, Tarantini L, Stefenelli C, et al. Changes in plasma N‐terminal proBNP levels and ventricular filling pressures during intensive unloading therapy in elderly with decompensated congestive heart failure and preserved left ventricular systolic function. J Card Fail 2006;12:608–615. [DOI] [PubMed] [Google Scholar]
- 16. Abassi Z, Golomb E, Keiser HR. Neutral endopeptidase inhibition increases the urinary excretion and plasma levels of endothelin. Metabolism 1992;41:683–685. [DOI] [PubMed] [Google Scholar]
- 17. Erdos EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 1989;3:145–151. [PubMed] [Google Scholar]
- 18. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 19. Miura S, Suematsu Y, Matsuo Y, et al. Angiotensin II Receptor‐Neprilysin Inhibitor, LCZ696 Blocked Aldosterone Synthesis in Human Adrenocortical Cell Line. Eur Heart J 2015;36(Suppl 1):662. [DOI] [PubMed] [Google Scholar]
- 20. Ayalasomayajula S, Jordaan P, Pal P, et al. Assessment of Drug Interaction Potential between LCZ696, an Angiotensin Receptor Neprilysin Inhibitor, and Digoxin or Warfarin. Clin Pharmacol Biopharm 2015;4:147. doi:10.4172/2167‐065X.1000147. [Google Scholar]
- 21. Prasad PP, Yeh CM, Gurrieri P, Glazer R, McLeod J. Pharmacokinetics of multiple doses of valsartan in patients with heart failure. J Cardiovasc Pharmacol 2002;40:801–807. [DOI] [PubMed] [Google Scholar]
- 22. Bodey F, Hopper I, Krum H. Neprilysin inhibitors preserve renal function in heart failure. Int J Cardiol 2015;179:329–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Urinary volume‐corrected levels of creatinine, potassium, and sodium excretion; adverse events and effects on blood pressure.


