Abstract
Psychiatric hospitals are increasingly adopting smoke-free policies. Tobacco use is common among persons with mental illness, and nicotine withdrawal (NW), which includes symptoms of depression, anxiety, anger/irritability, and sleep disturbance, may confound psychiatric assessment and treatment in the inpatient setting. This study aimed to characterize NW and correlates of NW severity in a sample of smokers hospitalized for treatment of mental illness in California. Participants (N = 754) were enrolled between 2009 and 2013, and averaged 17 (SD = 10) cigarettes/day prior to hospitalization. Though most (70%) received nicotine replacement therapy (NRT) during hospitalization, a majority (65%) reported experiencing moderate to severe NW. In a general linear regression model, NW symptoms were more severe for women, African American patients, and polysubstance abusers. Though invariant by psychiatric diagnostic category, greater NW was associated with more severe overall psychopathology and greater cigarette dependence. The full model explained 46% of the total variation in NW symptom severity (F [19, 470] = 23.03 p < 0.001). A minority of participants (13%) refused NRT during hospitalization. Those who refused NRT reported milder cigarette dependence and stated no prior use of NRT. Among smokers hospitalized for mental illness, NW severity appears multidetermined, related to cigarette dependence, demographic variables, psychiatric symptom severity, and other substance use. Assessment and treatment of NW in the psychiatric hospital is clinically warranted and with extra attention to groups that may be more vulnerable or naïve to cessation pharmacotherapy.
1. Introduction
Psychiatric hospitals are increasingly adopting smoking bans (Lawn and Campion, 2013). Tobacco use is two to three times more common among people with a mental illness, when compared to the general population, and almost five times greater for those with schizophrenia (de Leon and Diaz, 2005; Lawrence et al., 2009). Hence, many patients admitted for psychiatric care are likely to experience nicotine withdrawal (NW) during their hospitalization.
The symptoms of NW can range in severity from minor to severely debilitating, and include increased feelings of anger, anxiety, restlessness, depressed mood, sleep disturbances, increased appetite, difficulty concentrating, and craving (American Psychiatric Association, APA, 2013; Hughes and Hatsukami, 1986, 1992). These symptoms generally emerge within the first 24 h of nicotine deprivation and are typically most severe during the first week of abstinence (Hughes, 2007).
To properly manage the care of hospitalized smokers, the US Department of Health and Human Services (2008) suggests that every patient's tobacco use status be assessed on admission, and that hospital staff provide all tobacco users (without medical contraindications) with nicotine replacement therapy (NRT), such as nicotine gum or patches, as well as cessation counseling. Similarly, both the APA (2006) and the Royal College of Psychiatrists (2013) have identified the hospital setting as opportune for managing NW and for initiating treatment.
Despite these recommendations, research suggests that NW is not routinely assessed in psychiatric facilities or incorporated into treatment planning. Our earlier review of 250 medical records of smokers and nonsmokers hospitalized on a smoke-free psychiatric unit found: (1) NW was never diagnosed and smoking was never included on the treatment plan; (2) only about half of smokers were offered NRT during their hospitalization; and (3) though many of the symptoms of NW can manifest with other mental disorders, hospitalized smokers were significantly more likely than nonsmokers to have symptoms of irritability and agitation documented in their medical record and were more likely to have four or more NW symptoms documented in their nursing progress notes, hence meeting the criteria of tobacco withdrawal (APA, 2013; Prochaska et al., 2004). In our more recent studies, we continue to find that NRT prescription at psychiatric hospital discharge is rare (Schuck et al., 2016); and, even when NRT is prescribed, it is often administered at an insufficient dose relative to patients' heaviness of smoking (Leyro et al., 2013). The same has been observed in other developed Western nations with smoke-free hospital policies and cessation recommendations similar to those in the U.S. (Ashton et al., 2010; Wye et al., 2010).
Failure to appraise and attend to NW could negatively impact several components of patients' care (Prochaska, 2009). NW is defined by a cluster of symptoms, many of which overlap with the criteria for common psychological disorders, such as depression and anxiety. Increased symptoms of depression, anxiety, or difficulty concentrating, caused by NW, could alter clinical interpretation of the presenting condition. This imprecision could contribute to incorrect or incomplete diagnosis, and as NW symptoms slowly abate, to an incorrect perception of the efficacy of treatment. Further, anger, agitation, and restlessness, which can be heightened during NW, are associated with behaviors that may disrupt psychiatric treatment. In locked psychiatric units, agitation has been found to increase the likelihood of assaultive behavior (Hankin et al., 2011) and the need for physical restraint (Bowers et al., 2003). Untreated NW also has been associated with hospital discharges against medical advice (AMA), ostensibly due to patients wanting to leave to smoke; notably, the AMA rate among smokers offered NRT was no different from nonsmokers (Prochaska et al., 2004). Attention to changes in patients' tobacco use also is important because tobacco smoke induces the metabolism of several psychotropic medications, altering therapeutic blood levels (Zevin and Benowitz, 1999). In the case of the atypical antipsychotic clozapine, case reports have documented adverse clinical outcomes resulting from elevated blood levels post smoking cessation when dosages are not adjusted (Bondolfi et al., 2005; Derenne and Baldessarini, 2005).
Previous research has highlighted several factors, beyond mere patterns of tobacco consumption, which may influence the severity of NW. Preexisting psychiatric conditions (Pomerleau et al., 2000; Xian et al., 2005); exposure to trauma (Dedert et al., 2012); alcohol and other drug use (Weinberger et al., 2010); female gender (Xu et al., 2008); African American ethnicity (Bello et al., 2015); and genetic vulnerability (Xian et al., 2003; Lazary et al., 2014) have been linked with greater intensity of NW. That individuals experience and present with NW in different forms is of interest clinically and perhaps particularly so in an environment in which the cues to smoke have been removed, as is the case for hospitals with complete smoking bans. Identifying risk factors for NW in the inpatient psychiatric setting may improve the clinical care of hospitalized smokers with mental illness. The current study aimed to characterize NW and correlates of NW severity in a sample of smokers hospitalized for treatment of mental illness.
2. Method
2.1. Design
Participants were part of a larger clinical trial (N = 956) examining stage-tailored smoking cessation interventions initiated in an inpatient psychiatry setting. Participants were enrolled between 2009 and 2013, and desire to quit smoking was not required to participate. The study design has been described elsewhere (Schuck et al., 2016). The current study analyzed data from the baseline survey, which was conducted prior to treatment condition assignment and experimental intervention, and as such, provided a naturalistic representation of the general treatment patterns provided by hospital staff and the NW experiences of the participants.
2.2. Sample
Adult current smokers of at least 5 cigarettes per day were recruited during a psychiatric hospitalization on one of seven smoke-free units at three San Francisco Bay Area hospitals (67% of patients who were screened as eligible enrolled in the parent trial): one community-based hospital (four locked units) and two academic hospitals (two locked units, one unlocked). Patients had to have the capacity to consent in English. Exclusion criteria were quitting smoking more than a week prior to hospitalization; serious contraindications to NRT (e.g., recent heart attack, pregnancy/breastfeeding); overly aggressive, disorganized, or somnolent behavior that did not sufficiently resolve while hospitalized to allow for re-approach; or plans to relocate from the greater Bay Area during the 18-month study.
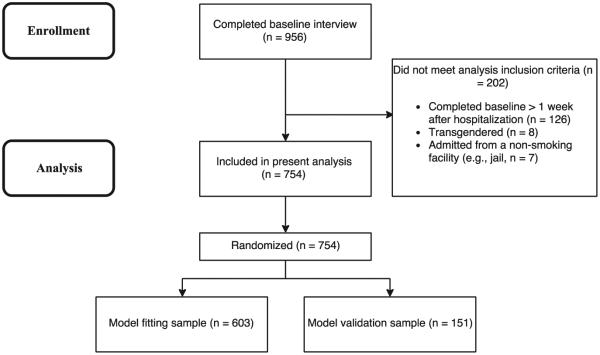
To reduce variability due to differences in the length of time from last cigarette to the baseline interview, participants who were abstinent from cigarettes for more than 24 h before their hospitalization (e.g., due to extended ER treatment, incarceration) or enrolled in the study more than 7 days after hospital admission were excluded from the present analysis. Eight participants who identified as transgender were also excluded due to inadequate numbers for gender-based comparisons, leaving a final n = 754 (Fig. 1).
Fig. 1.
Flow of participant enrollment and inclusion in analysis. California, 2009–2013. Flow of participant enrollment and inclusion in analysis. Adapted from “Smokers with serious mental illness and requests for nicotine replacement therapy post-hospitalization,” by R. K. Schuck et al., 2016. Tobacco Control, 25, 27–32.
2.3. Materials
Measures from the baseline interview relevant to the present analyses included participant demographics; measures of tobacco use, dependence and withdrawal; psychiatric diagnosis and symptom severity; and patient reported receipt of NRT upon admission. Demographics included gender, age, and ethnicity. We assessed usual number of cigarettes smoked per day prior to hospitalization and number of years smoking. The Fagerström Test for Cigarette Dependence (FTCD; Heatherton et al., 1991; Fagerstrom, 2012) assessed severity of cigarette dependence. The Minnesota Nicotine Withdrawal Scale (MNWS) measured the severity of 8 NW symptoms during the 24 h prior to the baseline interview, rated on an ordinal scale ranging from 0 (none) to 4 (severe). The symptoms were depression, insomnia, anger, anxiety, difficulty concentrating, restlessness, increased appetite, and craving to smoke. The MNWS was presented as a generic “behavioral rating scale”, not a withdrawal scale, to reduce the possibility that participants might not report a symptom that they did not attribute to NW. In previous studies using the MNWS, a total score, inclusive of all eight symptoms, has been regarded as the optimal representation of NW (Toll et al., 2007). An electronic version of the Mini International Neuropsychiatric Interview (eMINI; Sheehan et al., 1998) assessed current psychiatric disorders and screened for past exposure to trauma. The Behavior and Symptom Identification Scale (BASIS-24; Eisen et al., 2004), with six subscales, assessed past week: depression/functioning, interpersonal relationships, self-harm, emotional lability, psychosis, and substance abuse and yielded an overall psychiatric symptom severity score. NRT use during hospitalization was patient reported. The baseline survey asked whether the participant had been offered NRT since being admitted and in what form (patch, gum, lozenge) and dose. The survey did not specify the frequency of NRT use during the hospitalization. Patients who refused offered NRT were asked to report why.
2.4. Procedure
Research staff identified newly admitted study-eligible smokers by reviewing hospital admission records, and clinical staff asked these patients if they would like to hear about the study. Those who were interested and provided informed consent were enrolled. Individuals who had been assigned a legal guardian (conservator) due to having diminished mental capacity during recruitment were allowed to participate provided their conservator consented. Research staff conducted the baseline interviews in the hospital. The Institutional Review Boards of the participating hospitals and universities approved all study procedures, and study procedures were standardized across sites.
2.5. Data analysis
We graphed distributions of the items assessing the individual NW symptoms and then analyzed the total MNWS score comprised of all eight symptoms. To avoid excluding participants without complete responses to all eight measures, we averaged the total MNWS score (range: 0–4) for participants who responded to a minimum of six items. General linear modeling was used to assess the relationship between the averaged total withdrawal score and participant variables.
The participant variables used as correlates in the model were: gender (dichotomized as male/female), age, ethnicity (African American/Black, non-Hispanic Caucasian, mixed/other), primary psychiatric diagnosis (psychotic disorder, bipolar depression, unipolar depression, other [e.g. eating or anxiety disorder]), current diagnosis of a substance use disorder (none, alcohol, other drugs, both), reported history of trauma exposure (no/yes), BASIS-24 score of psychiatric symptom severity (ranging from 0 to 4), FTCD score (ranging from 0 to 10), and type of NRT received upon hospital admission (none, oral, patch, both). Hospital unit and length of time hospitalized before completing the baseline assessment were controlled for in the model.
Cross-validation analysis was performed to gauge how well the model predicted NW severity with a validation subset of the data not used in model fitting (20% of sample data). To obtain the most accurate estimate, the cross-validation was repeated 1000 times using bootstrap sampling, a technique used to estimate population statistics by randomly resampling with replacement from the sample population, simulating new samples (Efron, 1979). This analysis indicated no significant difference in model error during the fitting and validation periods (p > 0.10), indicating that the model was likely not over-fit, and should generalize well to new data. All data analyses were performed using R (V.3.1.2; R Core Team, 2014).
3. Results
3.1. Sample characteristics
Of the full study sample, n = 754 participants (79%) were included in the current analyses (see Fig. 1). The full sample and analysis sample groups did not differ meaningfully on any of the variables of interest (Table 1). The analysis sample (M age = 38.7, SD = 13.4, 48.5% female) was 25% African American/Black, 51% non-Hispanic Caucasian, and 24% mixed/other ethnicity. The average length of time participants were hospitalized before completing the baseline assessment was 52.7 h (SD = 36.6). Prior to hospitalization, participants smoked an average of 16.6 (SD = 9.8) cigarettes per day for a mean of 18.7 (SD = 13.4) years; 26% reported using tobacco products other than cigarettes, most frequently cigars, cigarillos, and chewing tobacco. Over a quarter (26%) of the sample had a primary diagnosis of psychotic disorder, 31% bipolar depression, 27% unipolar depression, and 16% other diagnoses. Over half of participants had a diagnosed substance use disorder (15% alcohol, 26% other drug, 27% alcohol and other drug), and 41% reported a history of trauma exposure. BASIS-24 scores ranged from 0.02 to 3.87, with an average of 2.07 (SD = 0.77), higher than the norms reported for inpatient psychiatric samples (Dawood et al., 2008). FTCD scores ranged from 0 to 10, with an average of 4.78 (SD = 2.23), indicating moderate dependence (Table 1). Notably, 78% of participants reported smoking within 30 min of waking.
Table 1.
Characteristics of (a) all study participants and (b) analysis sample. California, 2009– 2013.
| All participants |
Analysis sample |
|||
|---|---|---|---|---|
| Total N | 956 | 754 | ||
| Age: M (SD) | 38.7 | (13.5) | 38.7 | (13.4) |
| Gender: n (%) | ||||
| Women | 464 | (48.7) | 366 | (48.5) |
| Men | 481 | (50.1) | 388 | (51.5) |
| Transgender | 8 | (< 1) | NA | |
| Ethnicity: n (%) | ||||
| African-American | 226 | (24.2) | 185 | (25.1) |
| Non-Hispanic Caucasian | 477 | (51.2) | 374 | (50.7) |
| Other | 229 | (24.6) | 178 | (24.2) |
| Psychiatric diagnosis: n (%) | ||||
| Psychotic disorder | 253 | (26.5) | 198 | (26.3) |
| Bipolar depression | 304 | (31.8) | 235 | (31.2) |
| Unipolar depression | 256 | (26.8) | 202 | (26.8) |
| Other | 143 | (14.9) | 119 | (15.8) |
| Trauma history: n (%) | ||||
| No | 537 | (59.1) | 422 | (58.7) |
| Yes | 372 | (40.9) | 297 | (41.3) |
| Substance use disorder: n (%) | ||||
| None | 303 | (32.1) | 239 | (32) |
| Alcohol only | 137 | (14.5) | 113 | (15.1) |
| Other drugs only | 254 | (26.9) | 196 | (26.2) |
| Alcohol and other drugs | 250 | (26.5) | 199 | (26.6) |
| BASIS-24 total score (0– 4): M (SD) | 2.06 | (0.77) | 2.07 | (0.77) |
| Cigarettes smoked per day: M (SD) | 16.6 | (10.4) | 16.6 | (9.8) |
| Fagerström test of cigarette dependence (0– 10): M (SD) | 4.66 | (2.22) | 4.66 | (2.23) |
Eight participants who identified as transgender were excluded from the analysis sample duetoinadequate numbers for gender-based comparisons. BASIS-24 = The Behavior and Symptom Identification Scale.
Most participants (73%) reported being offered NRT upon admission: 17% offered gum or lozenge only, 23% offered the patch only, and 32% offered both forms. Most (69%) participants offered the patch were offered 21 mg, and 97% of participants offered gum or lozenge was the 2 mg dose. Exploratory post-hoc analyses indicated participants with previous NRT use and more severe cigarette dependence were more likely to be offered NRT; older participants and those identifying as African American were significantly less likely to be offered NRT (p-values < .05). A minority (13%) refused NRT, and “not needed” was the most common reason. Those refusing NRT reported milder cigarette craving and less cigarette dependence and were less likely to have ever used NRT (p-values < .05). There was no difference by hospital unit in the provision or patient acceptance of NRT.
A review of the medical records for n = 607 participants confirmed self-reported use of NRT during hospitalization for 91% of those who reported NRT receipt.
3.2. Characteristics of NW symptoms
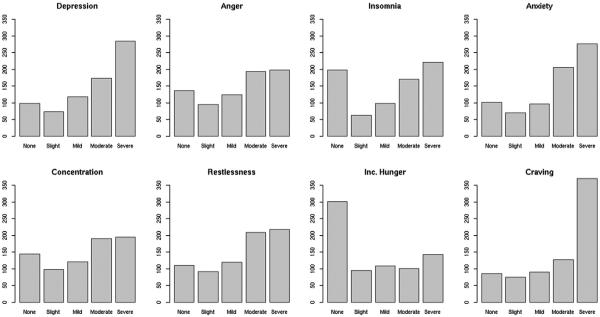
The response distributions for participants' self-reported severity of individual NW symptoms are summarized in Fig. 2. For depression, anger, insomnia, anxiety, difficulty concentrating, restlessness, and craving to smoke, there was a reliable pattern of negative skew; severe was the most common response. For increased hunger, the inverse trend was observed.
Fig. 2.
Distribution of participant-reported nicotine withdrawal symptom severity. California, 2009–2013.
3.3. Averaged NW symptom severity
Participants' (n = 745, 9 excluded due to incomplete MNWS) averaged withdrawal scores spanned the full range from 0 to 4 (IQR = 1.63–3). The distribution of responses was approximately normal, with a mean of 2.4 (SD = 0.93). Less than 1% of participants indicated complete absence of withdrawal symptoms, 10% rated their NW symptoms as slight, 24% as mild, 40% as moderate, and 25% as severe.
General linear regression was used to model variable associations. Analysis of correlates of the averaged NW score was conducted with the 490 participants from the model-fitting period with observations for all model variables. The full model explained 46% of the total variation in NW symptom severity (F [19, 470] = 23.03, p < 0.001). The regression model indicated significantly greater total NW scores among women (β = 0.20, t (470) = 3, p = 0.003), African American participants (β = 0.17, t (470) = 2.15, p = 0.03), those with a higher BASIS-24 total score of psychiatric symptom severity (β = 0.76, t (470) = 17.13, p < 0.001), greater FTCD score of cigarette dependence (β = 0.04, t (470) = 2.69, p = 0.007), and those with a diagnosis of both alcohol and other drug use disorders, relative to alcohol only, other drug only, or no substance use diagnosis (β = 0.22, t (470) = 2.58, p = 0.01) (Table 2).
Table 2.
A general linear regression model of nicotine withdrawal scores among smokers hospitalized for mental illness on units with complete smoking bans. California, 2009–2013.
| Variable | β | SE | p |
|---|---|---|---|
| NRT upon admission | |||
| None | Ref | ||
| Oral | −0.02 | 0.10 | |
| Patch | −0.14 | 0.09 | |
| Oral and patch | 0.02 | 0.08 | |
| Gender | |||
| Men | Ref | ||
| Women | 0.20 | 0.07 | ** |
| Race/ethnicity | |||
| Non-Hispanic Caucasian | Ref | ||
| African American | 0.17 | 0.08 | * |
| Other race/ethnicity | 0.003 | 0.08 | |
| Psychiatric diagnosis | |||
| Psychotic disorder | −0.18 | 0.13 | |
| Bipolar depression | −0.22 | 0.13 | |
| Unipolar depression | −0.08 | 0.13 | |
| Other | Ref | ||
| Trauma history | |||
| No | Ref | ||
| Yes | 0.09 | 0.07 | |
| Age | 0.02 | 0.03 | |
| Fagerström test of cigarette dependence | 0.04 | 0.01 | ** |
| BASIS-24 total score psychiatric disturbance | 0.76 | 0.04 | *** |
| Substance use disorder | |||
| None | Ref | ||
| Alcohol only | −0.07 | 0.10 | |
| Drugs only | −0.04 | 0.08 | |
| Alcohol and drugs | 0.22 | 0.09 | * |
Ref=reference groupinregression model. BASIS-24=The Behavior and Symptom Identification Scale. NRT=provision of oral or transdermal nicotine replacement upon hospital admission.
= p < 0.05.
= p < 0.01.
= p < 0.001.
4. Discussion
Accurate assessment is critical for treatment planning and medical management. Due to the high prevalence of smoking among people with mental illness, psychiatric assessment is likely to be confounded by NW in a smoke-free inpatient setting. The current findings indicate that NW is commonly experienced among hospitalized smokers with mental illness, with 65% of participants reporting moderate to severe NW, even while a majority were receiving nicotine replacement.
Among individual NW symptoms, the largest proportions of severe ratings were given for cigarette craving, depression, and anxiety. While psychiatric inpatients experience many of the symptoms associated with NW, our results indicate they may be most likely to experience internalizing symptoms during early nicotine deprivation. In light of evidence that internalizing symptoms are associated with an increased risk of relapse during smoking cessation (Piper et al., 2010; Morris et al., 2014), proactively managing NW would likely improve health outcomes. Reports of increased hunger were not observed in this sample; potentially explained by provision of regular meals and snacks during hospitalization.
Most studies of NW have been conducted with participants whose abstinence from smoking was a voluntary condition of study participation. Participants in the current study were hospitalized on a smoke-free unit, most involuntarily. This lack of agency could affect the expression of NW. In situations of involuntary abstinence, NW severity may be determined by biological responses to the absence of nicotine (Perkins et al., 2002) as well as psychological responses to external controls. Providing NRT to hospitalized smokers is advised, and larger reductions in NW may be achieved by adding behavioral counseling and strategies for managing withdrawal (e.g., deep breathing, distraction, exercise).
Most (73%) participants reported being offered NRT at hospital admission. While encouraging, it remains that over one quarter of hospitalized smokers in this study were not offered any NRT. Participants of older age and African American ethnicity were significantly less likely to be offered NRT. Given that these participants did not refuse NRT at higher rates, parity in NRT provision would likely improve the management of NW in these groups. Further, less than a third of participants were offered both oral and patch NRT, despite strong support that combination NRT treatment is more beneficial than single form therapy and is comparable to the efficacy of varenicline (Cahill et al., 2013). Increasing the adequate provision of NRT during hospitalization remains an important clinical goal.
We found that some hospitalized smokers experienced more severe NW than others. Specifically, NW was most severe among women, African American participants, polysubstance abusers, and those with more severe overall psychopathology and cigarette dependence. The observed gender difference may represent actual differences in the experience of NW and/or a difference in patterns of reporting (e.g., perhaps an underreporting bias among men; Pomerleau et al., 1994). The gender difference observed here is consistent with prior studies of NW (Leventhal et al., 2007; Xu et al., 2008; Cosgrove et al., 2012; Hogle and Curtin, 2006). The literature on racial and ethnic variation in NW is equivocal, with some studies of African American smokers reporting more severe NW (Bello et al., 2015) and others reporting less severity (Riedel et al., 2003; Robinson et al., 2014). That African American participants in the current study were less likely to be offered NRT upon hospital admission may account for the observed effects.
That the quality of an individual's psychopathology may affect their experience of NW is an intuitive expectation, attributed to symptom amplification as well as shared symptom profiles. In the event of symptom overlap (e.g., severity of depression), it would be a mistake to make a default attribution of the symptom to other mental illness. A substantial literature has well-established the symptom profile of NW in persons both with and without comorbid mental illness (Hughes et al., 1994; Hughes, 2007; McLaughlin et al., 2015). Further, to seek a conception of NW that is completely separate from other psychopathology, while appealing from a diagnostic standpoint, would perpetuate a false dualism that ignores the reality of NW presentation. Importantly, after adjusting for psychiatric symptom severity, we still found predictable variation in NW symptoms by level of cigarette dependence, gender, and ethnicity, while primary psychiatric diagnosis was not associated with variability in NW. The latter findings are consistent with those from a recent analysis of the National Epidemiologic Survey on Alcohol and Related Conditions data (Smith et al., 2013), which indicated that NW symptoms were similar across categories of mental illness.
Some caution is warranted in interpreting the current findings. The rate of comorbid psychiatric diagnoses was high in this sample, and the regression model was insufficiently powered to investigate the effects of co-occurring disorders, so only primary diagnosis was used. Moreover, the presentation of many psychiatric disorders can vary widely. Individuals with bipolar depression, for example, may be hospitalized for severe depression or severe mania. Hence, the parsimonious view of psychiatric diagnosis used in this study may be masking a more nuanced relationship between mental illness and NW.
The association between polysubstance abuse and severe NW is consistent with what has been reported elsewhere (Grant et al., 2004; Dani and Harris, 2005). The observed effects have important clinical implications for the management of hospitalized smokers. Substance use disorders are common as both primary and comorbid psychiatric diagnoses. Due to the often-extreme presentation of these disorders, clinicians on a psychiatric unit may be inclined to delay (or ignore entirely) NW management in these patients. Under-treatment of NW may decrease the likelihood of future smoking cessation attempts and serve as a maintaining risk factor for future psychopathology. This interpretation is supported by Allen et al. (2011) who reported better cooperation and less agitation in hospitalized smokers with schizophrenia when treated for their NW.
The current study was limited by the nature of the data collected. The measured covariates were pre-existing, not randomly assigned, and while this increases the ecological validity of the findings, it prevents causal mechanisms from being identified. Needed is a prospective study of NW among smokers with mental illness. Longitudinal studies are needed to identify risk (and protective) factors associated with NW and to model dynamic relationships that may change over time. Investigation also is needed within other settings where smokers with co-occurring disorders are involuntarily prevented from smoking (e.g., prisons, drug and alcohol rehabilitation centers).
Patient-reported provision of NRT was used as the exposure variable of interest and was found to concur with chart review. The baseline interview did not quantify the dose or frequency of NRT use, though did assess single versus combination forms. With interest in gender-based comparisons, due to small numbers, we had to exclude participants identifying as transgender. Tobacco use tends to be greater among adults who identify as transgender, and barriers to preventive medical care have been identified (Grant et al., 2010), highlighting the need for future research inclusive of this vulnerable group.
5. Conclusion
Due to the high prevalence of smoking among people with mental illness, an acute psychiatric hospitalization presents a unique opportunity to address tobacco use. Providing the highest standards of psychiatric diagnosis and treatment includes accurate assessment of NW symptoms. This study identified key demographic, behavioral, and psychiatric factors that can be used to improve the assessment of NW. Tobacco use is the leading cause of preventable death among smokers with mental health concerns, and attention to NW is needed to support treatment and future cessation attempts.
Footnotes
This research was supported by grants from the National Institute of Health, Bethesda, MD, USA; NIMH R01 MH083684 and NIDA P50 DA009253.
Conflict of interest statement
Dr. Prochaska has served as an expert witness against the tobacco companies in lawsuits for which she has received fees for the work and has provided consultation to Pfizer, which makes medications for quitting smoking. The other authors report no conflicts.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- Allen MH, Debanné M, Lazignac C, Adam E, Dickinson LM, Damsa C. Effect of nicotine replacement therapy on agitation in smokers with schizophrenia: a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry. 2011;168(4):395–399. doi: 10.1176/appi.ajp.2010.10040569. http://doi.org/10.1176/appi.ajp.2010.10040569. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Practice Guideline for the Treatment of Patients with Substance Use Disorders. second. Author; Washington, DC: 2006. Workgroup on substance use disorders. Accessed December 22, 2015: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. fifth Author; Washington, DC: 2013. [Google Scholar]
- Ashton M, Lawn S, Hosking JR. Mental health workers' views on addressing tobacco use. Aust. N. Z. J. Psychiatry. 2010;44(9):846–851. doi: 10.3109/00048674.2010.488637. http://doi.org/10.3109/00048674.2010.488637. [DOI] [PubMed] [Google Scholar]
- Bello MS, Pang RD, Cropsey KL, et al. Tobacco withdrawal amongst African American, Hispanic, and white smokers. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2015 doi: 10.1093/ntr/ntv231. http://dx.doi.org/10.1093/ntr/ntv231. [DOI] [PMC free article] [PubMed]
- Bondolfi G, Morel F, Crettol S, Rachid F, Baumann P, Eap CB. Increased clozapine plasma concentrations and side effects induced by smoking cessation in 2 CYP1A2 genotyped patients. Ther. Drug Monit. 2005;27(4):539–543. doi: 10.1097/01.ftd.0000164609.14808.93. [DOI] [PubMed] [Google Scholar]
- Bowers L, Simpson A, Alexander J. Patient-staff conflict: results of a survey on acute psychiatric wards. Soc. Psychiatry Psychiatr. Epidemiol. 2003;38(7):402–408. doi: 10.1007/s00127-003-0648-x. http://dx.doi.org/10.1007/s00127-003-0648-x. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. http://dx.doi.org/10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team, R. R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. URL http://www.R-project.org/ [Google Scholar]
- Cosgrove KP, Esterlis I, McKee SA, et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch. Gen. Psychiatry. 2012;69(4):418–427. doi: 10.1001/archgenpsychiatry.2011.1465. http://dx.doi.org/10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat. Neurosci. 2005;8(11):1465–1470. doi: 10.1038/nn1580. http://doi.org/10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Dawood N, Vaccarino V, Reid KJ, et al. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch. Intern. Med. 2008;168(18):1961–1967. doi: 10.1001/archinte.168.18.1961. http://dx.doi.org/10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. http://dx.doi.org/10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Harper LA, Dutton CE, McClernon FJ, Beckham JC. Smoking withdrawal in smokers with and without posttraumatic stress disorder. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2012;14(3):372–376. doi: 10.1093/ntr/ntr142. http://doi.org/10.1093/ntr/ntr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenne JL, Baldessarini RJ. Clozapine toxicity associated with smoking cessation: case report. Am. J. Ther. 2005;12(5):469–471. doi: 10.1097/01.mjt.0000146622.59764.dd. [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann. Stat. 1979;7(1):1–26. [Google Scholar]
- Eisen SV, Normand S-L, Belanger AJ, Spiro A, Esch D. The revised behavior and symptom identification scale (BASIS-R): reliability and validity. Med. Care. 2004;42(12):1230–1241. doi: 10.1097/00005650-200412000-00010. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nicotine Tob. Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. http://dx.doi.org/10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant JM, Mottet LA, Tanis J, Herman JL, Harrison J, Keisling M. National Center for Transgender Equality and National Gay and Lesbian Task Force. Washington, DC: 2010. National Transgender Discrimination Survey Report on Health and Health Care; pp. 1–23. [Google Scholar]
- Hankin CS, Bronstone A, Koran LM. Agitation in the inpatient psychiatric setting: a review of clinical presentation, burden, and treatment. J. Psychiatr. Pract. 2011;17(3):170–185. doi: 10.1097/01.pra.0000398410.21374.7d. http://dx.doi.org/10.1097/01.pra.0000398410.21374.7d. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43(4):344–356. doi: 10.1111/j.1469-8986.2006.00406.x. http://doi.org/10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2007;9(3):315–327. doi: 10.1080/14622200701188919. http://dx.doi.org/10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. The nicotine withdrawal syndrome: a brief review and update. Int. J. Smok. Cessat. 1992;1(2):21–26. [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction (Abingdon) 1994;89(11):1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Lawn S, Campion J. Achieving smoke-free mental health services: lessons from the past decade of implementation research. Int. J. Environ. Res. Public Health. 2013;10(9):4224–4244. doi: 10.3390/ijerph10094224. http://dx.doi.org/10.3390/ijerph10094224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. http://dx.doi.org/10.1186/1471-2458-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Dome P, Csala I, et al. Massive withdrawal symptoms and affective vulnerability are associated with variants of the CHRNA4 gene in a subgroup of smokers. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087141. http://dx.doi.org/10.1371/journal.pone.0087141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp. Clin. Psychopharmacol. 2007;15(1):21–36. doi: 10.1037/1064-1297.15.1.21. http://dx.doi.org/10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyro TM, Hall SM, Hickman N, Kim R, Hall SE, Prochaska JJ. Clinical management of tobacco dependence in inpatient psychiatry: provider practices and patient utilization. Psychiatr. Serv. (Wash.) 2013;64(11):1161–1165. doi: 10.1176/appi.ps.201200574. http://dx.doi.org/10.1176/appi.ps.201200574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA, De Biasi M. Nicotine withdrawal. Curr. Top. Behav. Neurosci. 2015;24:99–123. doi: 10.1007/978-3-319-13482-6_4. http://dx.doi.org/10.1007/978-3-319-13482-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CD, Burns EK, Waxmonsky JA, Levinson AH. Smoking cessation behaviors among persons with psychiatric diagnoses: results from a population-level state survey. Drug Alcohol Depend. 2014;136:63–68. doi: 10.1016/j.drugalcdep.2013.12.010. http://dx.doi.org/10.1016/j.drugalcdep.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, et al. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol. 2002;21(4):332–339. doi: 10.1037//0278-6133.21.4.332. http://dx.doi.org/10.1037/0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J. Consult. Clin. Psychol. 2010;78(1):13–23. doi: 10.1037/a0018065. http://doi.org/10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Tate JC, Lumley MA, Pomerleau OF. Gender differences in prospectively versus retrospectively assessed smoking withdrawal symptoms. J. Subst. Abus. 1994;6:433–440. doi: 10.1016/s0899-3289(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2000;2(3):275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ. Ten critical reasons for treating tobacco dependence in inpatient psychiatry. J. Am. Psychiatr. Nurses Assoc. 2009;15(6):404–409. doi: 10.1177/1078390309355318. http://dx.doi.org/10.1177/1078390309355318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Gill P, Hall SM. Treatment of tobacco use in an inpatient psychiatric setting. Psychiatr. Serv. (Wash.) 2004;55(11):1265–1270. doi: 10.1176/appi.ps.55.11.1265. http://dx.doi.org/10.1176/appi.ps.55.11.1265. [DOI] [PubMed] [Google Scholar]
- Riedel BW, Robinson LA, Klesges RC, McLain-Allen B. Ethnic differences in smoking withdrawal effects among adolescents. Addict. Behav. 2003;28(1):129–140. doi: 10.1016/s0306-4603(01)00220-9. http://dx.doi.org/10.1016/S0306-4603(01)00220-9. [DOI] [PubMed] [Google Scholar]
- Robinson CD, Pickworth WB, Heishman SJ, Waters AJ. The acute tobacco withdrawal syndrome among black smokers. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2014;28(1):173–181. doi: 10.1037/a0031950. http://doi.org/10.1037/a0031950. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians . Smoking and Mental Health. RCP. Royal College of Psychiatrists Council Report; London: 2013. Royal College of Psychiatrists; p. CR178. [Google Scholar]
- Schuck RK, Dahl A, Hall SM, et al. Smokers with serious mental illness and requests for nicotine replacement therapy post-hospitalisation. Tob. Control. 2016;25:27–32. doi: 10.1136/tobaccocontrol-2014-051712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM—IV and ICD—10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smith PH, Homish GG, Giovino GA, Kozlowski LT. Cigarette smoking and mental illness: a study of nicotine withdrawal. Am. J. Public Health. 2013;104(2):e127–e133. doi: 10.2105/AJPH.2013.301502. http://dx.doi.org/10.2105/AJPH.2013.301502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O'Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota nicotine withdrawal scale. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2007;21(2):216–225. doi: 10.1037/0893-164X.21.2.216. http://doi.org/10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services . Treating Tobacco Use and Dependence: 2008 Update. US Department of Health and Human Services; 2008. [Google Scholar]
- Weinberger AH, Desai RA, McKee SA. Nicotine withdrawal in U.S. smokers with current mood, anxiety, alcohol use, and substance use disorders. Drug Alcohol Depend. 2010;108(1–2):7–12. doi: 10.1016/j.drugalcdep.2009.11.004. http://dx.doi.org/10.1016/j.drugalcdep.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wye P, Bowman J, Wiggers J, et al. An audit of the prevalence of recorded nicotine dependence treatment in an Australian psychiatric hospital. Aust. N. Z. J. Public Health. 2010;34(3):298–303. doi: 10.1111/j.1753-6405.2010.00530.x. http://dx.doi.org/10.1111/j.1753-6405.2010.00530. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Madden PAF, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob. Res. 2003;5(2):245–254. http://dx.doi.org/10.1080/1462220031000073667. [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Madden PAF, et al. Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychol. Med. 2005;35(3):409–419. doi: 10.1017/s0033291704003289. [DOI] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2008;10(11):1653–1661. doi: 10.1080/14622200802412929. http://dx.doi.org/10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin. Pharmacokinet. 1999;36(6):425–438. doi: 10.2165/00003088-199936060-00004. http://dx.doi.org/10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]