Abstract
Immunological memory has traditionally been attributed as a unique trait of the adaptive immune system. Nevertheless, there is evidence of immunological memory in lower organisms and invertebrates, which lack an adaptive immune system. Despite their innate ability to rapidly produce effector cytokines and kill virally infected or transformed cells, NK cells also exhibit adaptive characteristics such as clonal expansion, longevity, self-renewal, and robust recall responses to antigenic or non-antigenic stimuli. This review highlights the intracellular and extracellular requirements for memory NK cell generation, and describes the emerging evidence for “memory precursor” NK cells, and their derivation.
Introduction
The immune system has classically been divided into two arms, innate and adaptive immunity. Innate immunity is poised for swift, short-lived effector responses mediated through recognition of evolutionarily conserved signals via germline-encoded receptors (1). Although initially slow in onset, adaptive immunity is considered highly “specialized” based on the ability to somatically rearrange antigen receptor genes to generate a diverse repertoire of T and B cells that can amplify antigen-specific responses through prolific clonal expansion (2-4). Because adaptive immune cells can persist long-term following recognition of cognate antigen and execute a quantitatively and qualitatively more robust response following re-challenge with the same antigen, T and B cells were thought to be the only immune population capable of generating “memory”.
The emergence of immunological memory in the adaptive immune system can be traced to lower vertebrates, including the jawless fish such as lamprey and hagfish. Early studies demonstrated that lampreys immunized with the killed bacterium Brucella abortus produce long-lived “antibody” capable of agglutinating Brucella cells upon re-challenge but not capable of agglutinating typhoid-paratyphoid cells, underscoring the antigen-specific nature of these antibody titers (5). The lamprey can also mediate delayed-type hypersensitivity (DTH) reactions following stimulation with Mycobacterium tuberculosis-fortified complete Freund's adjuvant (5). The basis for these phenomena may be attributable to the recently discovered lymphocyte-like populations and variable lymphocyte receptors, akin to primordial T and B lymphocytes and their antigen receptors, respectively (6, 7).
However, evidence also exists for immunological memory in invertebrates devoid of an adaptive immune system. Protection of the American cockroach Periplaneta americana against the bacterium Pseudomonas aeruginosa was enhanced by prior immunization with killed P. aeruginosa but not with saline or immunization with an array of other gram-negative organisms (8). Interestingly, this protection against P. aeruginosa re-challenge persisted for 14 days post-immunization (8), demonstrating both specificity and memory. Similar findings were demonstrated in the copepod Macrocyclops albidus, in which exposure to larvae of their natural parasite, the tapeworm Schistocephalus solidus, resulted in fewer infections from sibling but not unrelated parasites, the first evidence of innate immune memory in crustaceans (9). Similar protective memory responses against bacteria, parasites and fungi in other invertebrates add to the mounting evidence for innate immunity's capacity for memory responses in lower organisms (10-16), suggesting that the ability to “remember” may be evolutionarily conserved across both innate and adaptive immunity.
Natural killer cells: innate lymphocytes with adaptive features
NK cells were first described in 1975, when several groups identified a lymphocyte population in athymic (nude) mice capable of mediating “natural” (i.e. without the requirement of prior target exposure) cytotoxicity against both syngeneic and allogeneic tumor cell lines (17-20). Cytotoxic activity was maintained despite filtration of splenocytes through an anti-immunoglobulin column or treatment with carbonyl iron/magnet, excluding a contribution from T and B lymphocytes or macrophages (18, 20). Because of their capacity to rapidly secrete lytic molecules and the proinflammatory cytokine IFN-γ upon sensing pathogen-derived or host stress ligands through a repertoire of germline-encoded receptors, and because they lack RAG-mediated rearrangement of receptor genes, NK cells were characterized as a component of innate immunity (21-25).
However, current evidence has revealed striking similarities between NK cells and adaptive immune cells. NK cells develop from the common lymphoid progenitor (CLP), from which T and B cells and the newly described lineage of innate lymphoid cells (ILCs) are also derived (26). Similar to T and B cells, NK cell development, homeostasis, survival, and function require common-γ-chain-dependent cytokine signaling, particularly IL-15 (27-29). Although NK cells do not require expression of the RAG recombinase for development or generation of their receptor repertoire, nearly a third of peripheral NK cells have a history of RAG expression during ontogeny (30, 31). Furthermore, analogous to thymic T cell education, NK cells acquire functional competence through a “licensing” or “arming” process; during development, NK cells become self-tolerant (and thus gain effector function) due to engagement of self-MHC by inhibitory receptors (32-34).
Lastly, NK cells can undergo clonal expansion and initiate antigen-specific recall responses. Perhaps the earliest evidence suggesting the possibility of NK cell memory was reported in 1964 in a model of F1 hybrid resistance. Adult F1 hybrid mice (B10 × B10.D2) reject parental (B10 or B10.D2) bone marrow grafts (35). When primed with a bone marrow graft from one parent (B10), F1 hybrid mice more rapidly rejected a second graft from that same parent (B10) compared with a graft derived from the other parent (B10.D2) (35). Conversely, priming the F1 hybrids with a B10.D2 or allogeneic graft did not accelerate B10 graft rejection (35). Together, these findings suggested that a non-T or -B cell responded in a qualitatively different manner upon re-exposure to previously encountered antigens. The later discovery of the NK cell and the “missing-self” hypothesis (that NK cells selectively kill cells lacking self-MHC class I) implicated NK cells as the cell type mediating F1 hybrid resistance (36). Since then, NK cells have been found to possess a number of adaptive features that have redefined their role in immunity.
Antigen-dependent NK cell memory
The first evidence of anamnestic NK cell responses was in the setting of contact hypersensitivity responses to chemical haptens. Rag2−/− mice lacking both T and B lymphocytes exhibited a severe inflammatory reaction when sensitized and re-challenged with the same hapten (either DNFB or oxazolone) (37). Depletion of NK cells abrogated the contact hypersensitivity, suggesting that NK cells either directly or indirectly were responsible for the observed recall response (37). Adoptive transfer of DNFB-sensitized NK cells into Rag2−/− Il2rg−/− mice was also sufficient to drive contact hypersensitivity upon recipient challenge with DNFB, although transferable hapten-specific recall was retained only in hepatic, but not splenic NK cells (37, 38). Specifically, contact hypersensitivity depended on a subset of hepatic NK cells expressing the chemokine receptor CXCR6, which was required for the homeostasis but not antigen-recognition of these cells (38). Thus, hepatic NK cells can generate antigen-specific recall responses to haptens, although whether these cells are truly mature NK cells or a distinct subpopulation of the type 1 ILC family is unresolved (39).
NK cells can also undergo recall responses against viral pathogens (40). During the “expansion” phase of the NK cell response to MCMV infection that peaks at day 7 post-infection, the antigen-specific NK cell compartment has been measured to undergo ~100-1000-fold growth in size (40). This proliferative burst is driven by antigen-specific interactions between the activating receptor Ly49H on NK cells and the MHC-I-like viral glycoprotein m157 on the surface of infected cells (41-43). Following robust expansion of Ly49H+ NK cells following MCMV infection, these effector cells contract and form a long-lived pool of memory NK cells in both lymphoid and non-lymphoid tissues that is detectable at least 70 days after MCMV infection (40). These memory NK cells exhibit enhanced IFN-γ production and degranulation compared to naïve NK cells (40). The response of memory NK cells re-challenged with MCMV was found to be comparable in both kinetics and magnitude to that of naïve NK cells, yet memory NK cells conferred greater protection to susceptible neonate mice against MCMV challenge (40). Thus, MCMV-experienced NK cells are capable of recall responses, enhanced functionality, and protection against repeated MCMV exposure.
Evidence for secondary NK cell responses against different viral pathogens continues to build. Analogous to hapten-specific memory NK cell-mediated contact hypersensitivity responses, adoptively transferred hepatic, but not splenic NK cells, from Rag1−/− mice immunized with virus-like particles expressing influenza A virus, vesicular stomatitis virus (VSV) or HIV-1 antigens afforded protection to Rag2−/− Il2rg−/− hosts challenged with the immunizing, but not unrelated, virus (38). Similar immunization-dependent and virus-specific NK cell protective responses in the absence of adaptive immunity have been described for HSV-2, vaccinia virus and influenza virus (44-46).
Similar to the expansion of NK cells in MCMV-infected mice, human NK cells expressing the activating heterodimeric CD94/NKG2C receptor are preferentially found in the peripheral blood of healthy individuals seropositive for HCMV, compared to donors who were HCMV-seronegative or seropositive for other herpesvirus infections (47-49). This CD94/NKG2C+ subset commonly co-expresses the maturation marker CD57 and lacks expression of the inhibitory NKG2A receptor (49-52). Although the ligand driving expansion of CD94/NKG2C+ NK cells in vivo has yet to be elucidated, in vitro studies demonstrated that shRNA-mediated knockdown of HLA-E on HCMV-infected fibroblasts abrogated this expansion (53). HCMV reactivation in patients receiving allogeneic hematopoietic stem cell grafts, umbilical cord blood grafts or solid-organ transplants also precipitates expansion of CD94/NKG2C+ NK cells, followed by persistence of these cells for months to years (49, 54-57). Furthermore, the transfer of NKG2C+ NK cells in grafts from HCMV-seropositive donors resulted in enhanced target cell-induced IFN-γ production upon secondary CMV exposure in the recipient compared to grafts from HCMV-seronegative donors (50). In combination, these studies highlight the antigen-specificity, longevity and transplantability of human memory NK cell responses to HCMV. A recent study also identified a subset of CD16+CD56+ FcεRIγ− NK cells associated with prior HCMV and HSV-1 infection that persisted upwards of 9 months and was capable of mediating enhanced antibody-dependent cellular cytotoxicity (ADCC) in the presence of HCMV and HSV-1-infected cells coated by virus-specific antibodies (58, 59). Thus, human NK cells appear to have evolved redundant mechanisms to generate immunological memory.
Several studies have also demonstrated expansion of long-lived CD94/NKG2C+ human NK cells in response to HIV-1, hantavirus, chikungunya virus, hepatitis B virus (HBV) or hepatitis C virus (HCV), although it should be noted that this expansion occurred only in individuals previously infected with HCMV (60-64), suggesting that superinfection with these viruses may trigger HCMV reactivity. Interestingly, a recent study in rhesus macaques established that primate NK cells can achieve pathogen-specific memory independent of HCMV exposure (65). Infection of rhesus macaques with simian-human immunodeficiency virus (SHIV) or SIV elicited splenic and hepatic memory NK cells capable of lysing Gag- and Env-pulsed dendritic cells in an NKG2A- and NKG2C-dependent fashion for as long as 5 years post-infection (65). Vaccinating the macaques with recombinant adenovirus expressing HIV-1 Env or SIV Gag likewise produced robust, stable, and antigen-specific NK cell memory (65).
The benefit of pathogen-specific NK cell memory is clear in the case of persistent or repeated encounter with the same virus. However, there is some evidence that suggests there may be a costly trade-off associated with NK cell memory. A recent study highlighted that human NK cell receptor diversity increases with age (66). CD57+NKG2C+ NK cells that expand during HCMV infection are far from clonal, displaying substantial heterogeneity for other receptors (67). However, high NK cell receptor diversity was associated with greater risk of HIV-1 acquisition in Kenyan women (66). Given that viral challenge may enhance NK cell receptor diversity, human NK cells appear to risk unresponsiveness to novel antigens in order to better protect against previously encountered pathogens (66). The consequence of NK cell receptor diversity for human health and disease thus requires further exploration.
Mechanisms of NK cell memory generation during viral infection
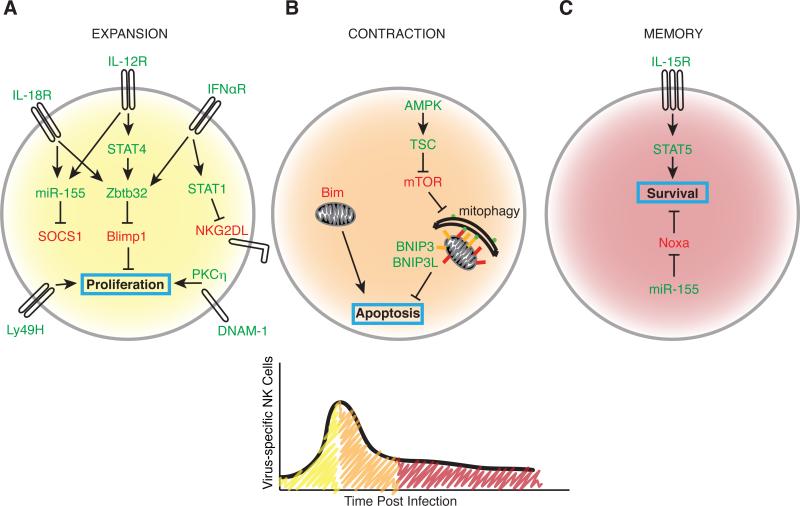
Analogous to the generation of T cell memory against pathogens (4), NK cells progress through three phases during their response to MCMV: expansion, contraction, and memory maintenance (68). During each stage, both intracellular and extracellular cues are necessary for establishing a long-lived memory NK cell pool (Figure 1). In addition to antigen engagement by activating receptors (analogous to TCR engagement for T cell activation), NK cells require proinflammatory cytokine signaling for robust expansion (4). The proinflammatory cytokine IL-12, through a STAT4-dependent, but IFN-γ-independent mechanism, is indispensable for optimal MCMV-specific NK cell clonal expansion as well as memory NK cell formation (69). IL-12 and STAT4 may be responsible for programming activated NK cells early during MCMV infection for memory formation (69). Members of the IL-1 family of cytokines, particularly IL-33 and IL-18, are similarly necessary for amplifying NK cell proliferation in response to MCMV, but are dispensable for recall responses (70, 71). A recent study also identified a role for type I interferon and downstream STAT1 signaling in shielding proliferating NK cells from fratricide (killing by other NK cells) by modulating their cell surface expression of NK group 2 member D ligands (NKG2DL) (72).
FIGURE 1.
Regulation of NK cells during each phase of the response to viral infection. A, Expansion. IL-12, IL-18, and type I interferon converge to drive Ly49H+ NK cell proliferation by inducing Zbtb32 and subsequently suppressing Blimp-1. IL-12 and IL-18 also cooperate to regulate SOCS1 in a miR-155-dependent mechanism. Signaling through IFNαR also independently contributes to memory formation by protecting against NKG2D-mediated fratricide. The costimulatory molecule DNAM-1 facilitates a PKCη-dependent proliferative burst. B, Contraction. Memory formation hinges on averting Bim-mediated mitochondrial apoptosis. Recycling dysfunctional mitochondria via mitophagy mediated by BNIP3 and BNIP3L promotes the contraction-to-memory phase transition. C, Memory maintenance. IL-15 signaling is required as NK cells contract and for the maintenance of memory cells in peripheral tissues. miR-155-mediated suppression of Noxa promotes memory NK survival. Green font represents positive regulators of memory. Red font represents negative regulators of memory. Cell colors correspond to the infection time course.
Interestingly, IL-12, IL-18, and type I interferon signaling act synergistically to drive maximal expression of the BTB-ZF family transcription factor Zbtb32, which controls the proliferative burst of virus-specific NK cells by antagonizing the anti-proliferative factor Blimp-1 (73). In parallel, IL-12- and IL-18-mediated induction of miR-155 regulates effector and memory NK cell numbers during MCMV infection by regulating targets such as Noxa and SOCS1 (74). Although the mechanism by which SOCS1 impairs the development of effector NK cells is unclear, the potent restraint that constitutive SOCS1 activity places on STAT signaling may have some influence. Lastly, akin to the necessity of costimulation for T cell activation (“signal 2”), NK cells require the costimulatory molecule DNAX accessory molecule 1 (DNAM-1) and downstream signaling through the Src-family tyrosine kinase Fyn and the serine-threonine protein kinase C isoform eta (PKCη) for optimal expansion of effector NK cells and their differentiation into memory NK cells (75).
Following viral infection, contraction of effector lymphocytes serves to eliminate activated CD8+ T and NK cells to stave off immunopathology (76, 77). Mitochondrial apoptosis mediated by the proapoptotic factor Bim shapes the size, maturity, and functionality of the memory NK cell pool in response to MCMV (78), similar to CD8+ T cells (79). The accumulation of depolarized mitochondria and mitochondrial-released reactive oxygen species (ROS) in effector NK cells after virus-driven expansion results in either cell death, or clearance of damaged mitochondria, resulting in NK cell survival (80). Analogous to the autophagy-dependent survival and memory formation of virus-specific effector CD8+ T cells (81-83), surviving NK cells undergo the self-catabolic process of mitophagy during the contraction-to-memory phase transition, requiring the autophagosome machinery component Atg3 and the mitophagy-specific receptors BCL2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3) and BNIP3-like (BNIP3L or Nix) to promote their survival (80). Mitophagy in contracting NK cells is induced by mechanistic target of rapamycin (mTOR) inhibition or AMP-activated protein kinase (AMPK) activation (80), suggesting that mitophagy may also link other cellular metabolic processes, similar to the catabolic processes memory T cells employ to fuel oxidative phosphorylation during non-proliferative states (84).
In addition to intracellular mechanisms, extracellular cues can also promote the maintenance of NK cell memory. Adoptive transfer of Ly49H+ NK cells isolated from MCMV-infected hosts at day 7 or day 21 post-infection into IL-15-deficient recipients led to decreased persistence of the transferred cells, supporting a role for IL-15-dependent maintenance of NK cells during contraction (85). IL-15 was previously shown to promote NK cell survival via Mcl-1 (86). miR-155-mediated suppression of Noxa may also aid long-term survival of memory NK cells (74). Thus, generating long-lived, MCMV-specific memory NK cells requires a complex combination of intracellular and extracellular signals during both the early and late phases of the antiviral response.
Identifying memory NK cell precursors
During viral infection, two subsets of effector CD8+ T cells have been described to develop: terminally differentiated KLRG1hi short-lived effector cells (SLECs) that die after infection, and KLRG1lo memory precursor effector cells (MPECs) that are long-lived and participate in secondary responses (87). Analogous to CD8+ T cells, recent evidence supports the idea of heterogeneity within antiviral NK cell populations that can dictate memory potential. KLRG1lo Ly49H+ NK cells preferentially generate memory NK cells compared to KLRG1hi cells, which have a limited capacity for MCMV-driven expansion (88). KLRG1 is associated with NK cell maturation, as indicated by the greater percentage of KLRG1hi NK cells that are also of the most mature CD11b+ CD27− phenotype, suggesting that NK cell maturation is antagonistic to their memory potential (88). However, because memory NK cells are themselves KLRG1hi CD11b+ CD27−, yet still competent to undergo robust secondary expansion following MCMV challenge, KLRG1 alone does not dictate proliferative potential (40, 88).
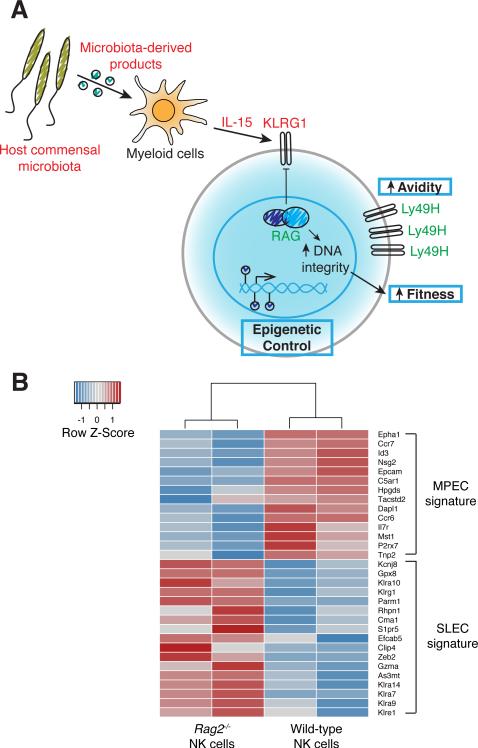
Interestingly, KLRG1 expression on naïve NK cells may be dictated during development by the activity of the RAG recombinase. NK cells with a history of RAG activity during ontogeny preferentially expand and persist as memory cells following MCMV infection (Figure 2A), due to an enhanced overall cellular fitness measured by the ability to repair DNA breaks which can occur during stresses such as rapid proliferation or exposure to ionizing radiation (30). In contrast, the absence of RAG expression in developing NK cells results in diminished expression of the DNA damage repair machinery and a subsequent impairment in DNA double-strand break resolution following DNA damage (30). Thus, although the underlying mechanisms remain to be elucidated, RAG activity during ontogeny shields NK cells from global genomic instability and apoptosis. Furthermore, Rag2−/− NK cells display a transcriptional signature markedly similar to that observed in SLECs rather than MPECs (SLEC and MPEC signatures published in (89)) (Figure 2B), supporting the hypothesis that RAG dictates functional heterogeneity within the NK cell compartment. It will be of interest to determine whether the degree of genomic integrity similarly specifies SLEC versus MPEC fate.
FIGURE 2.
Functional heterogeneity within the effector NK cell pool. A, Multiple mechanisms regulate memory precursor identity in NK cells. KLRG1 expression inversely correlates with NK cell memory potential. RAG expression during ontogeny not only negatively regulates KLRG1 expression but also promotes enhanced NK cell fitness by supporting optimal expression of DNA damage repair enzymes to maintain DNA integrity. Host commensal microbiota-derived products and IL-15 signaling, the availability of which is determined by competition with conventional T cells, drive NK cell expression of KLRG1. Greater avidity for ligand via higher cell surface concentration of Ly49H and epigenetic programs that drive a particular suite of memory genes may also converge to dictate NK cell memory precursor identity. Green font represents positive regulators of memory potential. Red font represents negative regulators of memory potential. B, Comparison of the gene expression profile of Rag2−/− and wild-type NK cells with that of MPECs and SLECs. Rag2−/− and wild-type NK cells were purified from mixed bone marrow chimeric mice and RNA-sequencing performed. Heat map shows the relative mRNA expression in Rag2−/− and wild-type NK cells of the top differentially expressed genes between MPEC and SLEC populations, as previously described (89). The transcriptional signature of Rag2−/− NK cells resembles that of SLECs whereas wild-type NK cells exhibit an MPEC-like signature.
Although RAG appears to determine KLRG1 expression levels in a cell-intrinsic manner, KLRG1 expression can also be influenced in a cell-extrinsic manner. One study demonstrated that T cells can restrain NK cell maturation by limiting the availability of IL-15, driving the preferential generation of KLRG1lo memory progenitors at the expense of KLRG1hi NK cells (Figure 2A) (88). Signals derived from the host commensal microbiota were also shown to control expression of KLRG1 in NK cells (Figure 2A) (88). Treatment with broad-spectrum antibiotics in the drinking water diminished KLRG1 expression and boosted the frequency of memory NK cells compared with untreated wild-type mice, suggesting that the host microbiota regulates the NK cell pool containing memory potential (88). Thus, the foundation for NK cell memory formation may be laid even prior to encountering virus.
MCMV-specific memory NK cells display greater cell surface expression of not only KLRG1 but also Ly49H compared with naïve NK cells, and expression of these receptors is further enhanced in secondary memory NK cells (90). In contrast, naïve, memory and secondary memory NK cells express comparable levels of the activating receptors NK1.1 and Ly49D (90). These data together imply that there may be selection for NK cells with the greatest avidity for ligand during successive rounds of MCMV infection (Figure 2A). T cells undergo a process of affinity maturation, whereby T cells bearing TCRs with highest affinity for peptide rise to clonal dominance during the response to a pathogen (91-94). A similar process may occur with NK cells responding against infection. There is currently no evidence to support the idea that NK cells undergo somatic mutation of their antigen receptor genes (i.e. the affinity of each Ly49H receptor for m157 is equivalent), indicating that NK cell avidity for viral antigen is determined solely by the number of Ly49H receptors on the surface of a given NK cell. However, it remains to be determined at what stage of the antiviral response activating signals through Ly49H select for memory precursors to constitute the long-lived pool.
Emerging evidence also illustrates the contribution of the activating receptor Ly49D and the inhibitory receptor Ly49A, both of which recognize the MHC class I molecule H-2Dd, during the NK cell memory response to MCMV (95). Adoptive transfer of B10.D2 (H-2Dd-sufficient background) NK cells into MCMV-infected syngeneic Ly49H-deficient recipients demonstrated that Ly49D+Ly49A−Ly49H+ NK cells preferentially differentiated into memory NK cells compared with Ly49D−Ly49A+Ly49H+ NK cells (95). Similar to how MCMV infection can break the anergic state of unlicensed NK cells in B6 mice, it appears that acute viral infection can also breach tolerance of activating receptors for self-MHC class I, a phenomenon that has functional consequences for host protection and NK cell memory (95-98).
Recent human studies have suggested that epigenetic heterogeneity is also associated with differential capacity for NK cell longevity (Figure 2A). The aforementioned CD16+ CD56+ FcεRIγ− NK cells are a subset of memory-like NK cells that can be isolated from HCMV-seropositive individuals, although they can be characterized by either the absence or presence of CD94/NKG2C (58, 59, 99, 100). Interestingly, this population lacks expression of the tyrosine kinase SYK, the signaling adaptors DAB2 and EAT-2, and the transcription factors promyelocytic leukemia zinc finger protein (PLZF) and Helios due to promoter hypermethylation at several of these loci (99, 100). Another study has identified epigenetic imprinting at the Ifng conserved non-coding sequence (CNS) 1 in NKG2C+ NK cells from HCMV-seropositive individuals that is critical for Ifng transcriptional activity in response to stimulation through NKG2C (101). It is unclear whether this epigenetic heterogeneity is a cause or consequence of NK cell memory. Nevertheless, receptor heterogeneity, the microbiota, and history of RAG expression during ontogeny precipitate differential fitness of cells within the naïve NK pool. During viral infection, selective pressure may then be acting on the effector pool to preferentially select memory precursors to establish the memory pool.
Antigen-independent NK cell memory
Although proinflammatory cytokine signaling is critical in driving the clonal expansion and maintenance of MCMV-specific memory NK cells, proinflammatory cytokines alone were found to be capable of supporting NK cell memory properties in the absence of antigen. Splenic NK cells pre-activated with a cocktail of IL-12, IL-15, and IL-18, and adoptively transferred into Rag1−/− mice become long-lived and can be identified in these recipients up to 3 weeks following transfer (102). These cytokine-induced “memory-like” NK cells retained a cell-intrinsic capacity for enhanced IFN-γ production, but not cytotoxicity, when re-stimulated with cytokine, plate-bound antibody or target cells (102, 103). Human NK cells pre-activated with cytokines also displayed the same properties (104). The progeny of cytokine-induced memory-like NK cells similarly exhibited enhanced effector functions (102, 104), suggesting that NK cell memory properties may be epigenetically inherited. These data may have implications for the antigen-independent self-renewal of memory NK cells following clearance of pathogens, although future studies are necessary to dissect the contribution of cytokine-induced memory-like NK cells during a recall response to a pathogen against which pathogen-specific memory NK cells already exist. Given that antigen-specific NK cells demonstrate diminished bystander activation to heterologous infection (105), cytokine-induced memory-like NK cells may represent a strategy for the host to nonspecifically respond to a new proinflammatory stimulus. Recent studies have shown that NK cells pre-activated with IL-12, IL-15, and IL-18 demonstrate enhanced persistence and antitumor activity against established, irradiated mouse tumors compared with naïve NK cells, suggesting that harnessing the sustained effector functions of cytokine-induced memory-like NK cells may represent a potential enhancement to adoptive NK cell immunotherapy (106). ILCs are thought to respond exclusively to cytokine cues (107), and it will be of interest to determine whether cytokines can similarly support longevity in other innate lymphocytes.
Lastly, in an NK cell-deficient lymphopenic host, adoptively transferred NK cells undergo a rapid, antigen-independent homeostatic proliferation to fill the empty niche, a process that requires common-γ-chain-dependent cytokine signaling (108-110). These same cytokines, as well as TCR-mediated self-peptide/MHC interactions, are known to support the acquisition of memory-like characteristics in naïve T cells undergoing lymphopenia-induced proliferation (111). Similarly, following homeostatic proliferation in Rag2−/− Il2rg−/− or sublethally irradiated recipients, adoptively transferred NK cells contract to form a long-lived population that persists in both lymphoid and nonlymphoid organs for at least 6 months (112). These NK cells display an enhanced capacity for IFN-γ production and degranulation when stimulated ex vivo 10 days after transfer, but the functionality of these homeostatically-driven NK cells following contraction is unclear (112). Nevertheless, homeostatically-expanded NK cells self-renew and are capable of mounting a robust proliferative response when challenged with MCMV 6 months after transfer, thus sharing some properties with MCMV-specific memory NK cells (112). Emerging evidence demonstrates that Atg5-mediated autophagy is critical for the survival of NK cells during homeostatic proliferation, suggesting that this is one mechanism by which NK cells acquire memory properties following lymphopenia-driven proliferation (113).
Conclusions
Immunological memory represents just one example among the adaptive features NK cells exhibit during their dynamic life span. Mounting evidence in both mice and humans points to the remarkable capacity of NK cells to generate memory responses in both infectious and noninfectious settings. Virus infection models are allowing us to uncover the molecular mechanisms necessary for NK cell memory formation and maintenance, yet the pathways that govern cytokine- and lymphopenia-induced memory-like NK cells remain poorly understood. NK cells have expanded the classical definition of immunological memory found in textbooks. It will now be of interest to determine whether a similar capacity for longevity, self-renewal, and robust recall responses exists within the newly described ILC lineages, which generally respond to proinflammatory cytokine cues. NK cells have a diverse repertoire of cell surface activating receptors, and future studies are necessary to address whether other activating receptors are sufficient to drive NK cell memory when exposed to cognate ligands. Resolving these questions will facilitate NK cell vaccination strategies and adoptive NK cell immunotherapies for viral infection and malignancy.
Acknowledgements
We thank members of the Sun laboratory for helpful discussions and review of this manuscript. We apologize to those whose work we were unable to discuss due to space limitations.
N.M.A. was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. T.E.O was supported by a fellowship from the American Cancer Society. J.C.S. was supported by the Ludwig Cancer Center, the Cancer Research Institute, and the National Institutes of Health grants AI085034 and AI100874. Our laboratory is also supported by National Institutes of Health/National Cancer Institute Cancer Center Support Grant (CCSG) P30CA008748.
Abbreviations used in this article
- MCMV
mouse CMV
- ILC
innate lymphoid cell
- DNFB
2,4-dinitro-1-fluorobenzene
- HCMV
human CMV
- SOCS1
suppressor of cytokine signaling 1
- KLRG1
killer cell lectin-like receptor G1
References
- 1.Lanier LL. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 5.Finstad J, Good RA. The evolution of the immune response. III. Immunologic responses in the lamprey. J. Exp. Med. 1964;120:1151–1168. doi: 10.1084/jem.120.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehm T, McCurley N, Sutoh Y, Schorpp M, Kasahara M, Cooper MD. VLR-based adaptive immunity. Annu. Rev. Immunol. 2012;30:203–220. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flajnik MF. Re-evaluation of the immunological Big Bang. Curr. Biol. 2014;24:R1060–R1065. doi: 10.1016/j.cub.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faulhaber LM, Karp RD. A diphasic immune response against bacteria in the American cockroach. Immunology. 1992;75:378–381. [PMC free article] [PubMed] [Google Scholar]
- 9.Kurtz J, Franz K. Innate defence: Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 10.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLos Pathog. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth O, Kurtz J. Phagocytosis mediates specificity in the immune defence of an invertebrate, the woodlouse Porcellio scaber (Crustacea: Isopoda). Dev. Comp. Immunol. 2009;33:1151–1155. doi: 10.1016/j.dci.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. Biol. Sci. 2009;276:145–151. doi: 10.1098/rspb.2008.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsive-mode prophylaxis in the mealworm beetle, Tenebrio molitor. Proc. Biol. Sci. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006;8:2105–2112. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Sadd BM, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 18.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 19.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 20.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 21.Anegón I, Cuturi MC, Trinchieri G, Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J. Exp. Med. 1988;167:452–472. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason LH, Anderson SK, Yokoyama WM, Smith HR, Winkler-Pickett R, Ortaldo JR. The Ly-49D receptor activates murine natural killer cells. J. Exp. Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith HR, Chuang HH, Wang LL, Salcedo M, Heusel JW, Yokoyama WM. Nonstochastic coexpression of activation receptors on murine natural killer cells. J. Exp. Med. 2000;191:1341–1354. doi: 10.1084/jem.191.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho EL, Carayannopoulos LN, Poursine-Laurent J, Kinder J, Plougastel B, Smith HR, Yokoyama WM. Costimulation of multiple NK cell activation receptors by NKG2D. J. Immunol. 2002;169:3667–3675. doi: 10.4049/jimmunol.169.7.3667. [DOI] [PubMed] [Google Scholar]
- 26.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 27.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 30.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell. 2014;159:94–107. doi: 10.1016/j.cell.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichii M, Shimazu T, Welner RS, Garrett KP, Zhang Q, Esplin BL, Kincade PW. Functional diversity of stem and progenitor cells with B-lymphopoietic potential. Immunol. Rev. 2010;237:10–21. doi: 10.1111/j.1600-065X.2010.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 34.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cudkowicz G, Stimpfling JH. Induction of immunity and of unresponsiveness to parental marrow grafts in adult F-1 hybrid mice. Nature. 1964;204:450–453. doi: 10.1038/204450a0. [DOI] [PubMed] [Google Scholar]
- 36.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 37.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 38.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2016;43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 42.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 43.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdul-Careem MF, Lee AJ, Pek EA, Gill N, Gillgrass AE, Chew MV, Reid S, Ashkar AA. Genital HSV-2 infection induces short-term NK cell memory. PLoS One. 2012;7:e32821. doi: 10.1371/journal.pone.0032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillard GO, Bivas-Benita M, Hovav AH, Grandpre LE, Panas MW, Seaman MS, Haynes BF, Letvin NL. Thy1+ NK cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7:e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Helden MJ, de Graaf N, Boog CJ, Topham DJ, Zaiss DM, Sijts AJ. The bone marrow functions as the central site of proliferation for long-lived NK cells. J. Immunol. 2012;189:2333–2337. doi: 10.4049/jimmunol.1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 48.Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 2012 doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendricks DW, Balfour HH, Jr., Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol. 2014;192:4492–4496. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rölle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, Cerwenka A. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J. Clin. Invest. 2014;124:5305–5316. doi: 10.1172/JCI77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Vergès S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Della Chiesa M, Falco M, Podestà M, Locatelli F, Moretta L, Frassoni F, Moretta A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119:399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 56.Muccio L, Bertaina A, Falco M, Pende D, Meazza R, Lopez-Botet M, Moretta L, Locatelli F, Moretta A, Chiesa MD. Analysis of memory-like natural killer cells in human cytomegalovirus-infected children undergoing αβ+ T and B cell-depleted hematopoietic stem cell transplantation for hematological malignancies. Haematologica. 2016;101:371–381. doi: 10.3324/haematol.2015.134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horowitz A, Guethlein LA, Nemat-Gorgani N, Norman PJ, Cooley S, Miller JS, Parham P. Regulation of adaptive NK cells and CD8 T cells by HLA-C correlates with allogeneic hematopoietic cell transplantation and with cytomegalovirus reactivation. J. Immunol. 2015;195:4524–4536. doi: 10.4049/jimmunol.1401990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int. Immunol. 2012;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J. Immunol. 2013;190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petitdemange C, Becquart P, Wauquier N, Béziat V, Debré P, Leroy EM, Vieillard V. Unconventional repertoire profile is imprinted during chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gumá M, Cabrera C, Erkizia I, Bofill M, Clotet B, Ruiz L, López-Botet M. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J. Infect. Dis. 2006;194:38–41. doi: 10.1086/504719. [DOI] [PubMed] [Google Scholar]
- 62.Björkström NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaëlsson J, Malmberg KJ, Klingström J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 64.Béziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A, Hervier B, Theodorou I, Martinot M, Debré P, Björkström NK, Malmberg KJ, Marcellin P, Vieillard V. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 2012;42:447–457. doi: 10.1002/eji.201141826. [DOI] [PubMed] [Google Scholar]
- 65.Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, Barouch DH. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 2015;16:927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, Blish CA. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl. Med. 7: 297ra115. 2015 doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horowitz A, Strauss-Albee DM, Leipold M, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 5: 208ra145. 2013 doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat. Rev. Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nabekura T, Girard JP, Lanier LL. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. J. Immunol. 2015;194:5948–5952. doi: 10.4049/jimmunol.1500424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madera S, Sun JC. Cutting edge: stage-specific requirement of IL-18 for antiviral NK cell expansion. J. Immunol. 2015;194:1408–1412. doi: 10.4049/jimmunol.1402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madera S, Rapp M, Firth MA, Beilke JN, L Lanier L, Sun JC. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 2016;213:225–233. doi: 10.1084/jem.20150712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaulieu AM, Zawislak CL, Nakayama T, Sun JC. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat. Immunol. 2014;15:546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zawislak CL, Beaulieu AM, Loeb GB, Karo J, Canner D, Bezman NA, Lanier LL, Rudensky AY, Sun JC. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc. Natl. Acad. Sci. USA. 2013;110:6967–6972. doi: 10.1073/pnas.1304410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier LL. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 77.Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr. Opin. Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Min-Oo G, Bezman NA, Madera S, Sun JC, Lanier LL. Proapoptotic Bim regulates antigen-specific NK cell contration and the generation of the memory NK cell pool after cytomegalovirus infection. J. Exp. Med. 2014;211:1289–1296. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc. Natl. Acad. Sci. USA. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Sullivan TE, Johnson LR, Kang HH, Sun JC. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity. 2015;43:331–342. doi: 10.1016/j.immuni.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu X, Araki K, Li S, Han JH, Ye L, Tan WG, Konieczny BT, Bruinsma MW, Martinez J, Pearce EL, Green DR, Jones DP, Virgin HW, Ahmed R. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat. Immunol. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puleston DJ, Zhang H, Powell TJ, Lipina E, Sims S, Panse I, Watson AS, Cerundolo V, Townsend AR, Klenerman P, Simon AK. Autophagy is a critical regulator of memory CD8(+) T cell formation. eLife. 2014;3:e03706. doi: 10.7554/eLife.03706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlie K, Westerback A, DeVorkin L, Hughson LR, Brandon JM, MacPherson S, Gadawski I, Townsend KN, Poon VI, Elrick MA, Côté HC, Abraham N, Wherry EJ, Mizushima N, Lum JJ. Survival of effector CD8+ T cells during influenza infection is dependent on autophagy. J. Immunol. 2015;194:4277–4286. doi: 10.4049/jimmunol.1402571. [DOI] [PubMed] [Google Scholar]
- 84.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J. Exp. Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J. Exp. Med. 2013;210:2981–2990. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, Motoyama N, Huang DC, Nutt SL, Tarlinton DM, Strasser A. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamimura Y, Lanier LL. Homeostatic control of memory cell progenitors in the natural killer cell lineage. Cell Rep. 2015;10:280–291. doi: 10.1016/j.celrep.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dominguez CX, Amezquita RA, Guan T, Marshall HD, Joshi NS, Kleinstein SH, Kaech SM. The transcription factors ZEB2 and T-bet cooperate to program cytotoxic T cell terminal differentiation in response to LCMV viral infection. J. Exp. Med. 2015;212:2041–2056. doi: 10.1084/jem.20150186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun JC, Beilke JN, Lanier LL. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol. Rev. 2010;236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Busch DH, Pilip I, Pamer EG. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J. Exp. Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 93.Bachmann MF, Speiser DE, Ohashi PS. Functional management of an antiviral cytotoxic T-cell response. J. Virol. 1997;71:5764–5768. doi: 10.1128/jvi.71.8.5764-5768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nabekura T, Lanier LL. Activating receptors for self-MHC class I modulate differentiation of natural killer cells during mouse cytomegalovirus infection. Immunity. 2016 doi: 10.1016/j.immuni.2016.06.024. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun JC, Lanier LL. Cutting edge: viral infection breaks NK cell tolerance to “missing self”. J. Immunol. 2008;181:7453–7457. doi: 10.4049/jimmunol.181.11.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tay CH, Welsh RM, Brutkiewicz RR. NK cell response to viral infection in beta 2-microglobulin-deficient mice. J. Immunol. 1995;154:780–789. [PubMed] [Google Scholar]
- 98.Orr MT, Murphy WJ, Lanier LL. 'Unlicensed' natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 2010;11:321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K, Larsson S, Schaffer M, Malmberg KJ, Ljunggren HG, Miller JS, Bryceson YT. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luetke-Eversloh M, Hammer Q, Durek P, Nordström K, Gasparoni G, Pink M, Hamann A, Walter J, Chang HD, Dong J, Romagnani C. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10:e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keppel MP, Yang L, Cooper MA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J. Immunol. 2013;190:4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min-Oo G, Lanier LL. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J. Exp. Med. 2014;211:2669–2680. doi: 10.1084/jem.20141172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J. Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 110.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Müller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 111.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'Sullivan TE, Geary CD, Weizman OE, Geiger TL, Rapp M, Dorn II GW, Overholtzer M, Sun JC. Atg5 is essential for the development and survival of innate lymphocytes. Cell Rep. 2016;15:1910–1919. doi: 10.1016/j.celrep.2016.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]