Abstract
The safety and efficacy of adalimumab were evaluated over 24 weeks in Japanese patients with psoriasis in routine clinical practice. In this multicenter, observational, open‐label, postmarketing study, primary efficacy measures included the Psoriasis Area and Severity Index (PASI) and the Dermatology Life Quality Index (DLQI) in all patients with psoriasis. In patients with psoriatic arthritis (PsA), the 28‐joint Disease Activity Score (DAS28) and the visual analog scale (VAS) pain were also evaluated. Safety was assessed based on the frequency of adverse drug reactions (ADR). Among patients with psoriasis evaluated for efficacy (n = 604), significant improvements from baseline were observed in mean PASI and DLQI scores at weeks 16 and 24 (all P < 0.0001). Furthermore, in psoriasis patients without PsA, the PASI 75/90 response rates were 55.9%/28.4% at week 16 (n = 306) and 65.6%/43.3% at week 24 (n = 270), respectively. In patients with PsA evaluable for effectiveness, significant improvements from baseline were observed in PASI, DAS28 erythrocyte sedimentation rate, DAS28 C‐reactive protein and VAS pain at weeks 16 and 24 (all P < 0.0001). ADR and serious ADR were reported by 26.1% and 3.3%, respectively, of 731 safety evaluable patients with psoriasis; no unexpected safety findings were noted. The safety profile and effectiveness of adalimumab for the treatment of psoriasis in a routine clinical setting were as expected in Japanese patients.
Keywords: drug surveillance, postmarketing, psoriasis, safety, treatment outcome
Introduction
Psoriasis is a chronic, multifactorial, systemic inflammatory disease with prevalence rates that vary widely by geographic area, reflecting the complex interaction between genetic and environmental factors.1 Prevalence rates of psoriasis vary from 0.7% to 2.9% in Europe and the USA, compared with rates below 0.5% in Asia.2 The annual incidence of psoriasis in Japan was estimated to be 0.34% in 2011.3 The prevalence of psoriasis in Japanese patients in dermatology clinics has been estimated to be 4.43% in a nationwide study conducted from 2007 to 2008.4 Both psoriasis and psoriatic arthritis (PsA) markedly affect quality of life (QoL), work productivity and employment status, with PsA having a greater impact on QoL and physical function than psoriasis alone.5, 6, 7
The efficacy and safety of adalimumab, a fully human tumor necrosis factor (TNF)‐α antagonist indicated for the treatment of patients with psoriasis, were demonstrated in a randomized, placebo‐controlled, phase 2/3 trial in Japan.8 Because adalimumab is the first biologic agent to be approved in Japan to treat patients with psoriasis, the Pharmaceuticals Medical Devices Agency of Japan requires a prospective evaluation of its safety profile during the treatment of patients with psoriasis in routine clinical practice as a condition of approval. Therefore, a postmarketing study to evaluate the safety and effectiveness of adalimumab for the treatment of psoriasis was performed in Japanese patients in routine clinical practice. The main objectives of the study were to uncover any unanticipated adverse drug reactions (ADR), especially significant reactions; characterize the frequency and occurrence of ADR in clinical settings; and identify factors that may affect the safety and effectiveness of adalimumab.
Methods
Patients
All patients with psoriasis included those with both plaque‐type psoriasis vulgaris (PsV) and PsA. Psoriasis patients who were aged 18 years or older and provided written informed consent were eligible to enroll in this study. All patients were unresponsive to conventional systemic treatment including cyclosporin, retinoids and phototherapy, and had skin lesions involving 10% or more of body surface area (BSA) and/or unmanageable joint signs and symptoms. Exclusion criteria consisted of the contraindications listed in the Japanese package insert for adalimumab, which include serious infections, active tuberculosis, a history of hypersensitivity to any ingredient of adalimumab, demyelinating disease or a history of demyelinating disease, and congestive cardiac failure.
Study design
This was a 24‐week, open‐label, postmarketing observational study (ClinicalTrials.gov registration identifier: NCT01155570) evaluating the safety and effectiveness of adalimumab in adult patients living in Japan. All patients who used adalimumab were enrolled from 20 January 2010 to 20 December 2011, from a total of 202 participating facilities, and were screened for eligibility according to Japanese guidance for the use of biologics for psoriasis.9 Patients began treatment without a washout period of previous treatment (e.g. cyclosporin). Patients received a s.c. loading dose of adalimumab 80 mg, followed by a 40 mg s.c. injection every other week (EOW) from week 2. Some patients who initially did not respond to treatment were allowed to increase the dose to 80 mg. At the end of the 24‐week study period, the investigator completed a case report form for each patient, which described the clinical findings observed during the study period.
Safety assessments
Assessments of safety included the evaluation of ADR. ADR were defined as adverse events (AE) for which the causal relationship with adalimumab was “probable”, “possible” or “unclear” (i.e. anything other than “not related”). ADR were considered by system organ class (SOC) and coded with preferred terms (PT) using the bilingual, English–Japanese version of the Medical Dictionary for Regulatory Activities (MedDRA/J) version 15.0. ADR through 24 weeks were examined in terms of occurrence, date of onset, diagnosis or definite symptom, details of the symptom/course/intervention, seriousness, outcome, and possible relationship to the disease and the drug used. Before the start of the administration of the drug, screening tests for tuberculosis infection and hepatitis B virus were performed according to Japanese guidance for the use of biologics for psoriasis.9
Effectiveness assessments
Two primary effectiveness measures were evaluated in all patients: the Psoriasis Area and Severity Index (PASI), which grades erythema, infiltration and desquamation of the lesions across four anatomical sites on a scale ranging 0 (no involvement) to 72 (severest possible involvement);10 and the Dermatology Life Quality Index (DLQI) questionnaire, which measures the extent to which skin problems affect the patient's QoL on a scale from 0 (no effect) to 30 (extremely strong effect).11, 12 Mean PASI scores and the proportions of patients achieving reductions of 75% or more and 90% or more in the PASI score (PASI 75 and PASI 90) were evaluated at weeks 16 and 24. DLQI questionnaires were administered at weeks 16 and 24.
In the subgroup of patients with PsA, PASI 75 was calculated at weeks 16 and 24. Two additional primary effectiveness measures were evaluated in the PsA subgroup. First, disease activity was assessed by the 28‐joint Disease Activity Score (DAS28) at weeks 4, 16 and 24. DAS28 was calculated using C‐reactive protein (DAS28‐CRP) and erythrocyte sedimentation rate (DAS28‐ESR), with a score of more than 5.1 indicating high/severe disease activity, 3.2 or more to 5.1 or less indicating moderate disease activity, 2.6 or more to less than 3.2 indicating low/minimal disease activity, and less than 2.6 indicating remission.13 Second, pain was evaluated using a visual analog scale (VAS) ranging from 0 (no pain) to 100 (pain as bad as it could be) at weeks 4, 16 and 24.
Statistical analysis
Patients who received more than one dose of adalimumab according to its labeled indication did not change hospitals after registration, and continued to make study visits after the first adalimumab dose were included in the effectiveness analysis. The changes from baseline in continuous variables (e.g. PASI, DLQI, DAS28‐ESR, DAS28‐CRP and VAS pain scores) were analyzed using a paired t‐test, with the level of significance set at 0.05. The analysis population for each effectiveness measure was based on the number of observed cases (i.e. data with “unknown” or “unreported” patient characteristics were excluded from the analyses).
Safety was assessed throughout the study by the frequency of ADR and serious adverse drug reactions (SADR) in registered, treated patients, except those who changed hospitals after registration or did not return after the first dose of adalimumab. SOC numbers and frequency of all ADR were expressed as the number of patients who experienced one or more ADR within each SOC, and PT numbers and frequency of ADR of particular interest were expressed as the number of patients who experienced one or more ADR within each PT. If the expected value was less than 5, Fisher's exact test was used for a 2 × n contingency table, with n − 1 degrees of freedom. The χ2‐test was used for other analyses. The level of significance was set at 0.05.
Results
Demographics and baseline characteristics
In total, 749 patients with psoriasis were registered in the study (Fig. 1). Two patients were registered twice, two patients made no visits after registration, seven patients transferred to other hospitals and seven patients made no visits after the first administration. The remaining 731 patients comprised the safety population and included 509 patients with PsV, 217 with PsA, and five with off‐label use including two with pustular psoriasis, two with PsV with pustular psoriasis and one with palmoplantar pustulosis. Among the excluded patients, 21 patients received only one dose of adalimumab, 79 patients were participants in an ongoing clinical trial and 22 patients were administrated adalimumab prior to the start of the study and were therefore excluded, resulting in 604 patients who were evaluable for effectiveness (414 with PsV and 190 with PsA). During the course of the study, 141 of the 731 patients discontinued treatment and 202 of the 731 patients suspended and then resumed adalimumab treatment; the most common reasons for discontinuation included insufficient effectiveness (n = 48), ADR (n = 48) and cost (n = 19).
Figure 1.
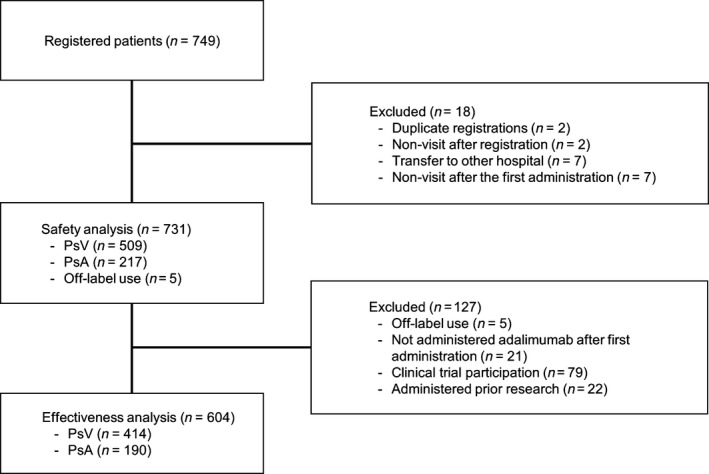
Patient disposition. PsA, psoriatic arthritis; PsV, psoriasis vulgaris.
The baseline demographics and clinical characteristics of all patients with psoriasis, including both PsV and PsA, and the subgroup of patients with PsA are shown in Table 1. More men than women were enrolled in this study. In terms of the background characteristics of the PsA population, “oral corticosteroids” and “topical and oral corticosteroid” use before and after adalimumab treatment was much more common in the PsA subgroup than all patients. Topical corticosteroids were used more frequently than oral corticosteroid in the PsA population, with the most common treatment combination being any corticosteroid with methotrexate (MTX), despite the fact that the use of MTX is off‐label in Japan. Phototherapy treatment was not used for patients in the PsA population after the administration of adalimumab.
Table 1.
Baseline demographics and clinical characteristics
| All patients (n = 731) | Patients with PsA (n = 217) | |
|---|---|---|
| Age, mean (SD), years | 50.7 ± 13.4 | 47.9 ± 12.0 |
| Male sex, n (%) | 556 (76.1%) | 146 (67.3%) |
| Bodyweight (kg) | 68.9 ± 15.3 | 67.0 ± 15.7 |
| BMI, mean (SD), kg/m2 | 24.8 ± 4.7 | 24.2 ± 4.7 |
| Involved BSA, mean (SD), % | 27.4 ± 22.9 | 24.0 ± 24.6 |
| Disease duration, mean (SD), years | 15.0 ± 9.8 | 13.3 ± 9.8 |
| PASI score, mean (SD) | 15.45 ± 12.21 | 13.16 ± 13.20 |
| Complications, n (%) | 431 (59.1) | 140 (64.8) |
| Past illness, n (%) | 139 (19.1)† | 42 (19.4) |
| History of tobacco use, n (%) | 353 (53.7)* | 92 (46.5)** |
| Current smoker | 227 (34.6) | 56 (28.3) |
| Previous smoker | 126 (19.2) | 36 (18.2) |
| Pretreatment drug, n (%) | ||
| Yes | 717/730 (98.2)‡ | 211/216 (97.7)§ |
| Cyclosporin | 268/730 (36.7%) | 70/216 (32.3%) |
| Vitamin D | 541/730 (74.0%) | 142/216 (65.4%) |
| Corticosteroids | 586/730 (80.2%) | 160/216 (73.7%) |
| Topical corticosteroid | 548/730 (75.0%) | 127/216 (58.5%) |
| Oral corticosteroid | 8/730 (1.1%) | 8/216 (3.7%) |
| Topical and oral corticosteroid | 29/730 (4.0%) | 24/216 (11.1%) |
| Biologic | 144/730 (19.7%) | 45/216 (20.7%) |
| Methotrexate | 81/730 (11.1%) | 73/216 (33.6%) |
| Retinoid | 80/730 (10.9%) | 27/216 (12.4%) |
| Phototherapy | 287/730 (39.3%) | 59/216 (27.2%) |
| Concomitant drug, n (%) | ||
| Yes | 592/724 (81.8%)¶ | 180/210 (85.7%)†† |
| Cyclosporin | 36/724 (4.9%) | 15/210 (6.9%) |
| Vitamin D | 360/724 (49.2%) | 83/210 (38.2%) |
| Corticosteroids | 441/724 (60.3%) | 116/210 (53.5%) |
| Topical corticosteroid | 399/724 (54.6%) | 89/210 (41.0%) |
| Oral corticosteroid | 18/724 (2.5%) | 15/210 (6.9%) |
| Topical and oral corticosteroid | 20/724 (2.7%) | 11/210 (5.1%) |
| Methotrexate | 68/724 (9.3%) | 65/210 (30.0%) |
| Retinoid | 19/724 (2.6%) | 10/210 (4.6%) |
| Phototherapy | 10/724 (1.4%) | 0/210 (0%) |
†Unknown in five patients, so the population is n = 726. *Unknown in 74 patients, so the population is n = 657. **Unknown in 19 patients, so the population is n = 198. ‡Unknown in one patient, so the population is n = 730. §Unknown in one patient, so the population is n = 216. ¶Unknown in seven patients, so the population is n = 724. ††Unknown in seven patients, so the population is n = 210. Percentages were calculated using only observed data. BMI, body mass index; BSA, body surface area; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; SD, standard deviation; TNF, tumor necrosis factor.
Safety
The frequency of ADR and SADR classified by SOC is shown in Table 2. ADR classified by SOC were reported by 191 of the 731 patients (26.1%) and SADR were reported by 24 patients (3.3%). ADR with a reported frequency of 5% or more were “infection and infestations” (8.8%), followed by those classified into “skin and subcutaneous tissue disorders” (6.4%) and “general disorders and administration site conditions” (6.0%). SADR with a frequency 0.5% or more were “infection and Infestations” (1.1%), “Skin and subcutaneous tissue disorders” (0.5%), and “General disorders and administration site conditions” (0.5%). Serious infectious ADR were reported in three patients with PsA (one case each of diverticulitis, encephalitis herpes and herpes zoster) and five patients with PsV (one case each of facial cellulitis, herpes zoster, bacterial meningitis, pneumococcal pneumonia and pulmonary tuberculosis). The frequency of ADR and SADR by patient background is shown in Table 3. In patients with PsV, the frequency of ADR was significantly higher in patients with higher body mass index (BMI), a history of allergy, circulatory disorder and hypertension. In the prior drugs, the frequency of ADR was significantly higher in patients with any kind of corticosteroid and phototherapy. In patients with higher ADR, adalimumab was concomitantly used with any kind of corticosteroid, topical plus oral corticosteroid and retinoid. On the other hand, the frequency of ADR was significantly lower in patients previously treated with biologic agents. The frequency of SADR was significantly higher in patients previously treated by phototherapy and with MTX. In patients with PsA, the frequency of ADR was significantly higher in patients previously treated by phototherapy and significantly lower in patients who weighed more, had higher BMI, had concomitant fatty liver or were pretreated with biologic agents. The frequency of ADR in this population was also associated with smoking history at the start of the administration as was the frequency of SADR, which was significantly higher in drinkers at the start of the administration.
Table 2.
Frequency of main adverse drug reactions and serious adverse drug reactions in 1% or more of all patients (n = 731)
| ADRa | ADR | SADR | ||
|---|---|---|---|---|
| n | % | n | % | |
| All | 191 | 26.1 | 24 | 3.3 |
| ADR by SOC | ||||
| Infection and infestations | 64 | 8.8 | 8 | 1.1 |
| Skin and subcutaneous tissue disorders | 47 | 6.4 | 4 | 0.5 |
| General disorders and administration site conditions | 44 | 6.0 | 4 | 0.5 |
| Investigations | 27 | 3.7 | 1 | 0.1 |
| Respiratory, thoracic and mediastinal disorders | 19 | 2.6 | 1 | 0.1 |
| Hepatobiliary disorders | 16 | 2.2 | 2 | 0.3 |
| Musculoskeletal and connective tissue disorders | 12 | 1.6 | – | – |
| Gastrointestinal disorders | 11 | 1.5 | 2 | 0.3 |
| ADR of particular interest | ||||
| Pyrexia | 17 | 2.3 | 2 | 0.3 |
| Hepatic function abnormal | 12 | 1.6 | – | – |
| Nasopharyngitis | 12 | 1.6 | – | – |
| Herpes zoster | 9 | 1.2 | 2 | 0.3 |
| Injection‐site erythema | 9 | 1.2 | – | – |
| Folliculitis | 8 | 1.1 | – | – |
| Malaise | 8 | 1.1 | 1 | 0.1 |
| Edema peripheral | 7 | 1.0 | – | – |
| Psoriasis | 7 | 1.0 | 1 | 0.1 |
| Upper respiratory tract infection | 7 | 1.0 | – | – |
Adverse drug reactions in 1% or more of patients. ADR, adverse drug reactions; SADR, serious adverse drug reactions; SOC, system organ class.
Table 3.
Frequency of adverse drug reactions and serious adverse drug reactions by patient background (PsV, PsA)
| ADR† | SADR | |||||
|---|---|---|---|---|---|---|
| n | % | P | n | % | P | |
| PsV | ||||||
| BMI (kg/m2) | ||||||
| <18.5 | 1/12 | 8.3 | 1/12 | 8.3 | ||
| 18.5 to <25 | 36/176 | 20.5 | 6/176 | 3.4 | ||
| 25 to <30 | 30/101 | 29.7 | 3/101 | 3.0 | ||
| ≥30 | 12/39 | 30.8 | 0.0276‡ | 1/39 | 2.6 | 0.5122‡ |
| Allergy history | ||||||
| No | 103/465 | 22.2 | 0.0016§ | 14/465 | 3.0 | 0.3100¶ |
| Yes | 16/35 | 45.7 | 2/35 | 5.7 | ||
| Circulatory disorder | ||||||
| No | 77/367 | 21.0 | 0.0266§ | 9/367 | 2.5 | 0.0963¶ |
| Yes | 43/142 | 30.3 | 8/142 | 5.6 | ||
| Hypertension | ||||||
| No | 80/376 | 21.3 | 0.0399§ | 10/376 | 2.7 | 0.1641¶ |
| Yes | 40/133 | 30.1 | 7/133 | 5.3 | ||
| Prior drug | ||||||
| Corticosteroid | ||||||
| No | 10/88 | 11.4 | 0.0030§ | 2/88 | 2.3 | 0.7493¶ |
| Yes | 110/421 | 26.1 | 15/421 | 3.6 | ||
| Corticosteroid topical | ||||||
| No | 12/93 | 12.9 | 0.0073§ | 2/93 | 2.2 | 0.7499¶ |
| Yes | 108/416 | 26.0 | 15/416 | 3.6 | ||
| Phototherapy | ||||||
| No | 56/284 | 19.7 | 0.0213§ | 4/284 | 1.4 | 0.0064§ |
| Yes | 64/225 | 28.4 | 13/225 | 5.8 | ||
| MTX | ||||||
| No | 116/502 | 23.1 | 0.0569¶ | 14/502 | 2.8 | 0.0010¶ |
| Yes | 4/7 | 57.1 | 3/7 | 42.9 | ||
| Biologic | ||||||
| No | 111/414 | 26.8 | 0.0003§ | 14/414 | 3.4 | 1.0000¶ |
| Yes | 9/95 | 9.5 | 3/95 | 3.2 | ||
| Concomitant drug | ||||||
| Corticosteroid | ||||||
| No | 32/188 | 17.0 | 0.0077§ | 4/188 | 2.1 | 0.2441§ |
| Yes | 88/321 | 27.4 | 13/321 | 4.0 | ||
| Topical and oral corticosteroid | ||||||
| No | 114/500 | 22.8 | 0.0069¶ | 17/500 | 3.4 | 1.0000¶ |
| Yes | 6/9 | 66.7 | 0/9 | |||
| Retinoid | ||||||
| No | 114/500 | 22.8 | 0.0069¶ | 15/500 | 3.0 | 0.0330¶ |
| Yes | 6/9 | 66.7 | 2/9 | 22.2 | ||
| PsA | ||||||
| Weight (kg) | ||||||
| <60 | 25/55 | 45.5 | 3/55 | 5.5 | ||
| 60 to <80 | 27/78 | 34.6 | 2/78 | 2.6 | ||
| ≥80 | 5/31 | 16.1 | 0.0070‡ | 0/31 | 0.1463‡ | |
| BMI (kg/m2) | ||||||
| <18.5 | 6/13 | 46.2 | 1/13 | 7.7 | ||
| 18.5 to <25 | 36/84 | 42.9 | 2/84 | 2.4 | ||
| 25 to <30 | 10/40 | 25.0 | 1/40 | 2.5 | ||
| ≥30 | 4/16 | 25.0 | 0.0402‡ | 0/16 | 0.3194‡ | |
| Smoking history at start of administration | ||||||
| Current smoker | 25/56 | 44.6 | 0.0240§ | 4/56 | 7.1 | 0.0258§ |
| Previous smoker | 14/36 | 38.9 | 2/36 | 5.6 | ||
| Never smoked | 26/106 | 24.5 | 0/106 | |||
| Alcohol history at start of administration | ||||||
| Drinker | 34/107 | 31.8 | 0.9466§ | 6/107 | 5.6 | 0.0323¶ |
| Non‐drinker | 29/90 | 32.2 | 0/90 | |||
| Concurrent illnesses | ||||||
| Fatty liver | ||||||
| No | 66/198 | 33.3 | 0.0406§ | 7/198 | 3.5 | 1.0000¶ |
| Yes | 2/19 | 10.5 | 0/19 | |||
| Prior drug | ||||||
| Biologic | ||||||
| No | 61/172 | 35.5 | 0.0104§ | 7/172 | 4.1 | 0.3493¶ |
| Yes | 7/45 | 15.6 | 0/45 | |||
| Phototherapy | ||||||
| No | 43/158 | 27.2 | 0.0322§ | 5/158 | 3.2 | 1.0000¶ |
| Yes | 25/59 | 42.4 | 2/59 | 3.4 | ||
†Adverse drug reactions were defined as adverse events with a causal relationship to adalimumab that was “probable”, “possible” or “unclear”. ‡Cochran–Armitage test; §χ2‐test; ¶Fisher's exact test. ADR, adverse drug reactions; BMI, body mass index; MTX, methotrexate; PsA, psoriatic arthritis; PsV, psoriasis vulgaris; SADR, serious adverse drug reactions.
Patients were screened for tuberculosis infection by tuberculin skin tests (533/731, 72.9%), interferon‐γ release assays (IGRA) (362/731, 49.5%), chest X‐ray (663/731, 90.7%) and computed tomography (CT) examinations (436/731, 59.6%). T‐spot assay was not yet available during the investigation period. Of the 731 subjects, 133 (18.2%) underwent neither reaction testing nor IGRA, and nine (1.2%) underwent neither chest X‐ray nor CT examinations. One patient (1/731, 0.1%) with PsV was eventually found to have pulmonary tuberculosis during the study. Prophylactic administration of isoniazid was initiated 42 days before adalimumab administration because of a positive tuberculin test. However, because the medication instruction was not sufficient, isoniazid adherence was low. A positive IGRA result was obtained 98 days after administration and followed by the discontinuation of adalimumab. It resolved after treatment with a total of four antitubercular agents.
Hepatitis B virus (HBV) reactivation was observed in one patient. The patient showed negative for HBV surface (HBs) antigen before the initiation of adalimumab treatment. Thirty days after the administration of adalimumab, antibody tests were performed and both of HBs and hepatitis B core antibodies were positive without any symptoms of liver disorder. However, 102 days after administration, hepatitis appeared (aspartate aminotransferase, 40 IU/L; alanine aminotransferase, 48 IU/L) and HBV DNA was detected (<2.1 log copies/mL). Following discontinuation of adalimumab, oral entecavir hydrate 0.5 mg/day was initiated, and HBV became negative 3 weeks later. Because hepatitis had disappeared after 31 days of withdrawal of adalimumab, adalimumab was re‐administrated and hepatitis became undetectable. In order to avoid the risk of fulminant on discontinuation of biologic products, the treatment was conducted under the liver specialist consultants.
Thirty‐nine patients with PsV (5.3%) discontinued therapy with adalimumab solely because of an AE, and an additional nine other patients with PsV (1.2%) discontinued because of an AE and other reasons. Among patients with PsA, 14 (6.5%) discontinued therapy with adalimumab because of an AE. With respect to malignant tumors, 12 patients had a history of malignant tumor and two had a concurrent malignant tumor, although there was no reported recurrence or worsening of malignancy.
Four deaths occurred during the study, and none of these deaths were related to treatment with adalimumab.
Efficacy
Mean PASI scores improved significantly from baseline in patients with PsV and PsA at weeks 16 and 24 (Fig. 2). At week 16, approximately 56% (171/306) of patients with PsV achieved PASI 75 response, and its response rates improved to 65.6% (177/270) at week 24. PASI 90 response was achieved by 28.4% (87/306) and 43.3% (117/270) of patients with PsV at weeks 16 and 24, respectively. The mean PASI score improved significantly from baseline in the subgroups of patients with PsA at weeks 16 and 24; the mean scores in these subgroups at weeks 16 and 24 were similar to those of patients with PsV. PASI 75 response was achieved by approximately half of patients with PsA at weeks 16 and 24. As shown in Figure 3, mean DAS28‐ESR and DAS28‐CRP values at baseline were 4.4 and 3.5, respectively, which represent moderate disease activity (≥3.2 to ≤5.1) according to the American College of Rheumatology (ACR) thresholds for DAS28.13 By 4 weeks of treatment, DAS28‐ESR and DAS28‐CRP values had decreased to below the ACR cut‐off for low disease activity (3.2), and by 16 and 24 weeks, mean DAS28 values had decreased to below the ACR cut‐off for disease remission (<2.6). Compared with baseline, VAS pain scores decreased significantly at weeks 4, 16 and 24. In patients with PsV, the mean DLQI scores improved from 9.1 ± 6.2 at baseline to 3.0 ± 4.4 at week 16 and 2.4 ± 3.8 at week 24. In patients with PsA, mean DLQI scores improved from 8.6 ± 6.7 at baseline to 3.2 ± 4.7 at week 16 and 2.6 ± 4.1 at week 24 (P < 0.0001).
Figure 2.
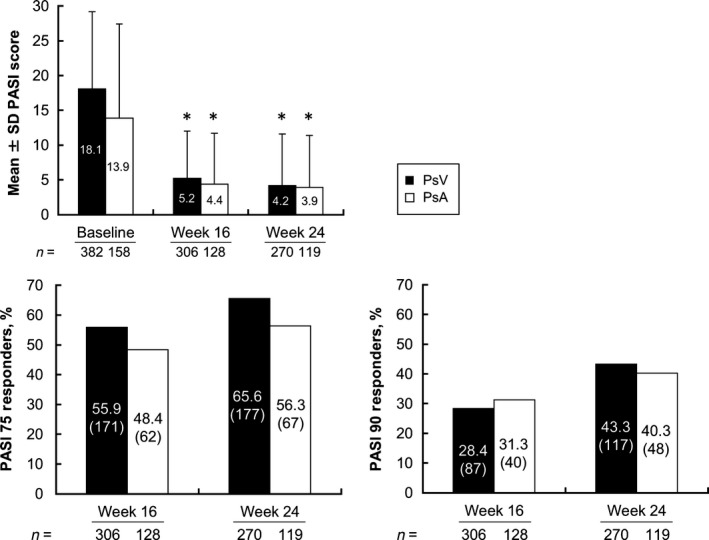
PASI scores for patients with PsV and patients with PsA. Percentage of patients with PASI 75 response and PASI 90 response. PASI score values are means ± SD. Parentheses indicates the number of responders in PASI 75 and PASI 90. *P < 0.0001 for all patients and patients with PsA at weeks 16 and 24 versus baseline by observed case analysis (paired t‐test). PASI, Psoriasis Area and Severity Index; PASI 75, 75% improvement from baseline in the PASI score; PASI 90, 90% improvement from baseline in the PASI score; PsA, psoriatic arthritis; PsV, psoriasis vulgaris; SD, standard deviation.
Figure 3.
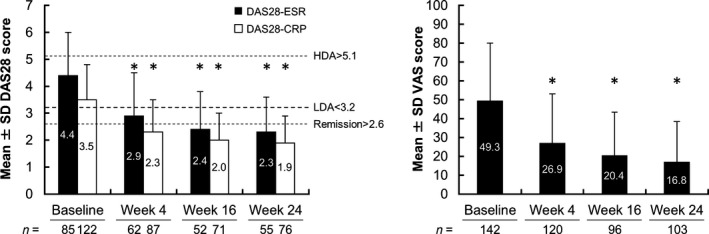
DAS28‐ESR and DAS28‐CRP and VAS pain score for patients with PsA. DAS28‐ESR, DAS28‐CRP and VAS values are means ± SD. *P < 0.0001 for DAS28‐ESR and DAS28‐CRP at weeks 4, 16 and 24 versus baseline by observed case analysis (paired t‐test). CRP, C‐reactive protein; DAS28, 28‐joint disease activity score; ESR, erythrocyte sedimentation rate; HDA, high disease activity; LDA, low disease activity; PsA, psoriatic arthritis; SD, standard deviation; VAS, visual analog scale.
Univariate analyses of patients with PsV revealed that PASI 75 response rate at week 16 was significantly higher in younger patients, patients with higher proportion of BSA involvement, and significantly lower in patients with liver disorder, patients who were previously treated with cyclosporin and patients with concomitant drugs for PsV (Table 4). In terms of concomitant drugs, the PASI 75 response rate was significantly lower in patients using topical corticosteroid and those using any kind of corticosteroid. In the same population, the PASI 75 response rate at week 24 was significantly lower in patients with liver disorder, those with fatty liver, those with kidney disorder, those who were previously treated with cyclosporin and those with concomitant drugs for PsV. The PASI 75 response rate was significantly lower in patients using topical vitamin D3, topical corticosteroid and any kind of corticosteroid as concomitant drugs.
Table 4.
Univariate analysis of factors contributing to the PASI 75 response rate at weeks 16 and 24 in (a) PsV patients (b) PsA patients
| PASI 75 achieved | ||||||
|---|---|---|---|---|---|---|
| Week 16 | Week 24 | |||||
| n | % | P | n | % | P | |
| (a) PsV | ||||||
| Age (years) | ||||||
| <15 | 0 | 0.0124† | 0 | 0.0722† | ||
| 15–29 | 6/8 | 75.0 | 5/7 | 71.4 | ||
| 30–44 | 62/94 | 66.0 | 65/89 | 73.0 | ||
| 45–64 | 70/137 | 51.1 | 75/120 | 62.5 | ||
| ≥65 | 33/67 | 49.3 | 32/54 | 59.3 | ||
| Proportion of BSA (%) | ||||||
| <10 | 9/23 | 39.1 | 0.0011† | 10/22 | 45.5 | 0.1310† |
| 10 to <30 | 66/132 | 50.0 | 82/120 | 68.3 | ||
| ≥30 | 95/143 | 66.4 | 84/122 | 68.9 | ||
| Liver disorder | ||||||
| No | 157/270 | 58.1 | 0.0288‡ | 163/235 | 69.4 | 0.0006‡ |
| Yes | 14/36 | 38.9 | 14/35 | 40.0 | ||
| Fatty liver | ||||||
| No | 162/285 | 56.8 | 0.2129‡ | 169/249 | 67.9 | 0.0058‡ |
| Yes | 9/21 | 42.9 | 8/21 | 38.1 | ||
| Kidney disorder | ||||||
| No | 160/283 | 56.5 | 0.4184‡ | 167/248 | 67.3 | 0.0384‡ |
| Yes | 11/23 | 47.8 | 10/22 | 45.5 | ||
| Cyclosporin (prior drug) | ||||||
| No | 108/169 | 63.9 | 0.0017‡ | 111/150 | 74.0 | 0.0011‡ |
| Yes | 63/137 | 46.0 | 66/120 | 55.0 | ||
| Concomitant drugs for psoriasis | ||||||
| No | 42/51 | 82.4 | <0.0001‡ | 48/53 | 90.6 | <0.0001‡ |
| Yes | 129/255 | 50.6 | 129/217 | 59.4 | ||
| Vitamin D3(concomitant drug) topical | ||||||
| No | 83/129 | 64.3 | 0.0110‡ | 93/120 | 77.5 | 0.0002‡ |
| Yes | 88/177 | 49.7 | 84/150 | 56.0 | ||
| Corticosteroid (concomitant drug) topical | ||||||
| No | 74/103 | 71.8 | 0.0001‡ | 85/102 | 83.3 | <0.0001‡ |
| Yes | 97/203 | 47.8 | 92/168 | 54.8 | ||
| Corticosteroid (concomitant drug) | ||||||
| No | 71/96 | 74.0 | <0.0001‡ | 81/97 | 83.5 | <0.0001‡ |
| Yes | 100/210 | 47.6 | 96/173 | 55.5 | ||
| (b) PsA | ||||||
| Weight (kg) | ||||||
| <60 | 11/29 | 37.9 | 0.2518† | 7/24 | 29.2 | 0.0098† |
| 60 to <80 | 27/52 | 51.9 | 37/55 | 67.3 | ||
| ≥80 | 8/15 | 53.3 | 7/11 | 63.6 | ||
| Unknown | 16/32 | 16/29 | ||||
| Smoking history at start of administration | ||||||
| Current | 14/33 | 42.4 | 0.0203‡ | 14/31 | 45.2 | 0.0007‡ |
| Previous | 7/25 | 28.0 | 8/26 | 30.8 | ||
| Never | 37/62 | 59.7 | 42/58 | 72.4 | ||
| Unknown | 4/8 | 3/4 | ||||
| Cyclosporin (prior drug) | ||||||
| No | 49/85 | 57.6 | 0.0034‡ | 53/81 | 65.4 | 0.0034‡ |
| Yes | 13/43 | 30.2 | 14/38 | 36.8 | ||
| Corticosteroid (prior drug) topical | ||||||
| No | 28/46 | 60.9 | 0.0350‡ | 28/42 | 66.7 | 0.0923‡ |
| Yes | 34/82 | 41.5 | 39/77 | 50.6 | ||
| Corticosteroid (prior drug) topical and oral | ||||||
| No | 47/109 | 43.1 | 0.0039‡ | 54/101 | 53.5 | 0.1394‡ |
| Yes | 15/19 | 78.9 | 13/18 | 72.2 | ||
| Biologic agent prior treatment | ||||||
| No | 59/109 | 54.1 | 0.0020‡ | 62/101 | 61.4 | 0.0081‡ |
| Yes | 3/19 | 15.8 | 5/18 | 27.8 | ||
| Corticosteroid (concomitant drug) topical and oral | ||||||
| No | 55/120 | 45.8 | 0.0291§ | 62/112 | 55.4 | 0.4661§ |
| Yes | 7/8 | 87.5 | 5/7 | 71.4 | ||
| Corticosteroid (concurrent drug) topical | ||||||
| No | 39/71 | 54.9 | 0.1009‡ | 43/66 | 65.2 | 0.0299‡ |
| Yes | 23/57 | 40.4 | 24/53 | 45.3 | ||
†Cochran–Armitage test; ‡χ‐square test; §Fisher's exact test. BSA, body surface area; PASI, Psoriasis Area and Severity Index; PASI 75, 75% improvement from baseline in the PASI score; PsA, psoriatic arthritis; PsV, psoriasis vulgaris.
In the subgroup of patients with PsA, the PASI 75 response rate at week 16 was significantly associated with smoking history at the start of administration and was significantly lower in patients previously treated with cyclosporin, topical corticosteroid or biologic agents, and significantly higher in patients previously treated with topical and oral corticosteroid or patients concomitantly using topical and oral corticosteroid (Table 4). In the same subgroup, the rate at week 24 was significantly associated with weight and smoking history at the start of administration and significantly lower in patients previously treated with cyclosporin or biologic agents, or concomitantly using topical corticosteroid.
Discussion
The present study was the largest clinical trial to evaluate adalimumab for the treatment of psoriasis in a Japanese population and the only published trial of adalimumab that specifically examined Japanese patients with PsV and PsA.
In this observational study, the ADR results during the 24 weeks of open‐label adalimumab treatment were consistent with the safety profile that was reported in previous clinical trials and postmarketing surveillance. Significant improvements in skin assessments, VAS pain and QoL were observed after 16 weeks of treatment, and additional improvements were noted at 24 weeks. PASI scores improved to a similar extent in patients with PsV compared with the subgroup of patients with PsA. Among the patients with PsA, joint evaluations were significantly improved at week 4 and later time points. The mean scores of DAS28‐ESR and DAS28‐CRP were significantly improved from baseline at weeks 4, 16 and 24 in patients with PsA; the mean DAS28 scores at these time points were at or below levels indicating low disease activity or remission. In addition, DLQI scores improved significantly from baseline at both weeks 16 and 24 in overall patients and in the PsA subgroup.
Tuberculosis is more frequently reported in Asian than Caucasian patients.14 According to World Health Organization data from 2013, the prevalence and incidence of tuberculosis in Japan are 23 and 18 per 100 000 population, respectively.15 Despite the fact that tuberculosis screening was carefully conducted before the study, a high incidence of tuberculosis could be expected among patients in any anti‐TNF study involving Japanese patients. In the present study, one patient developed tuberculosis after treatment with adalimumab despite the screening procedure. As this study was conducted in an Asian country with a relatively high incidence of tuberculosis (per 100 000 population from 2013 WHO data is 70 in China, 97 in Korea and 400 in Cambodia), these findings demonstrate the safety of using adalimumab with preventive medication and screening.
Adalimumab has also been evaluated for the treatment of PsV in Japanese patients in a randomized, placebo‐controlled trial. In the phase 2/3, 24‐week study (n = 169), treatment with adalimumab at three different dosing regimens resulted in dose‐dependent improvements in PASI 75.8 At weeks 16 and 24, 62.8% and 69.8% of patients receiving adalimumab 40 mg EOW plus a loading dose of 80 mg had achieved PASI 75 response, respectively. Furthermore, patients in the same dose group had mean ± standard deviation changes from baseline in DLQI scores of −5.1 ± 5.73 at week 16 and −5.5 ± 6.06 at week 24. Rates of serious AE, severe AE, AE leading to premature discontinuation and infectious AE were not significantly different between the placebo and adalimumab treatment groups. The most common AE in the present study was abnormal hepatic function; similarly, hepatic events in the phase 2/3 study were more frequent in the adalimumab arms than in the placebo arm. Despite the differences in study design, the phase 2/3 efficacy results were similar to the efficacy outcomes observed in the current study for PASI 75 response rate and DLQI score.
Two small observational studies have been reported in the published work for the treatment of plaque psoriasis with adalimumab in Japanese patients.16, 17 In one study, nine of 11 patients (81.8%) who received treatment with adalimumab 40 mg EOW following an 80‐mg loading dose achieved PASI 75 response at week 16.16 In the other study, 17 patients with PsV treated with 24 weeks of induction therapy with adalimumab (40 mg EOW following a loading dose of 80 mg) were assigned to maintenance therapy with adalimumab 40 mg EOW (n = 7) or every month (n = 10). Among the 15 patients (88.2%) who achieved PASI 75 response at week 24 of induction therapy, responses were maintained with either maintenance regimen for up to 60 weeks.17 The PASI 75 response rates observed in the present study appear relatively low compared with these two observational studies, but this is not unexpected given the variability inherent with their small sample sizes. Because the current study did not determine the washout period before treatment with adalimumab and there is also a variety in patient backgrounds, these factors might have contributed to relative inferiority compared with the clinical trial with respect to PASI 75 response.
Our analysis of factors affecting the PASI 75 response rate revealed that concomitant use of other drugs and corticosteroid was associated with lower rate of achievement of PASI 75. This association was observed both in patients with PsV and PsA at week 24. However, in patients with PsA, the response rate at week 16 was significantly higher in patients previously or concomitantly treated with topical plus oral corticosteroid. Because our analysis was univariate, it is not clear whether the response rate was influenced by the characteristics of patients treated with corticosteroid or use of corticosteroid affected the response rate.
From a safety perspective, serious infections, tuberculosis, reactivation of HBV and malignant tumor were reported during the use of adalimumab; therefore, careful attention should be paid to these conditions. There were no deaths related to the study drug and only a few cases of serious infectious ADR or tuberculosis were observed. Comprehensive screening for tuberculosis with the tuberculin skin test, IGRA, and chest X‐ray and CT examinations showed that only one patient with PsV eventually developed pulmonary tuberculosis; this patient resolved with discontinuation of the study drug and appropriate medical therapy. With respect to malignant tumors, although 12 patients had a history of malignant tumor and two had a concomitant malignant tumor, there were no ADR related to malignancy.
Our analysis of the frequency of ADR by patient background showed that previous or concomitant use of corticosteroid is associated with higher incidence of ADR in patients with PsV. In PsV and PsA, previous use of phototherapy was associated with higher frequency of ADR, whereas previous use of biologic agents was associated with lower incidence of ADR. Phototherapy might have been selected due to the difficulty in applying other treatment modalities because of complications, and PsV patients with higher BMI are likely to have such complications. Interestingly, BMI was positively correlated with the frequency of ADR in patients with PsV, but negatively correlated with the incidence of ADR in patients with PsA. These results are consistent with a previous report on the analysis of the impact of BMI in patients with moderate to severe PsV in Spain, which showed that overweight to obesity was associated with a 17% increased risk of having an adverse event (hazard ratio, 1.17; 95% confidence interval, 1.02–1.36).18 On the other hand, it is possible that PsA patients with lower BMI have a greater risk of adverse events, as previously reported in rheumatoid arthritis patients.19 Further analysis is necessary to determine the causal relationship between ADR and these factors.
Infliximab has also been studied as a treatment for PsV and PsA in Japanese individuals.20, 21, 22 Compared with these previous findings, the incidence of serious ADR for adalimumab (3.3%) in the present study was numerically lower than that for infliximab (6.94%) in a postmarketing surveillance study.20 Although the postmarketing surveillance study included both patients with pustular psoriasis and those with erythrodermic psoriasis, the higher incidence of serious ADR is attributable to the higher incidence of serious infections with infliximab compared with adalimumab (2.49% vs 1.1%).20 In a long‐term study of infliximab, the incidence of serious ADR was 3.1%,21 which is similar to the present results. The present study also found similar results in terms of the efficacy of adalimumab compared with infliximab. After 6 months of administration, PASI 75 was achieved by 65.6% of patients with PsV who received adalimumab, but only 60.7% of patients who received infliximab.20 Furthermore, in patients with PsA, PASI 75 was achieved by 56.3% of patients who received adalimumab, which is comparable with that of patients who received infliximab (59.4%).20
The limitations of the current study include the open‐label design and the lack of a placebo or comparator group; however, the efficacy and safety of adalimumab were already established in previous randomized, placebo‐controlled studies. Additionally, the relatively short duration of treatment (24 weeks) did not allow for the observation of safety concerns that may arise with longer treatment durations. Finally, the findings regarding the PASI 75/90 response rates must be considered in light of the fact that data from 48 patients who discontinued adalimumab due to insufficient effectiveness were excluded from the analysis.
In conclusion, treatment with adalimumab in the clinical setting was found to improve the signs and symptoms of plaque psoriasis and PsA in Japanese patients. Additionally, there were no unexpected findings for the types or frequency of ADR observed in the present study compared with the ADR reported in previous studies in patients from Japan or elsewhere.
Conflict of Interest
The authors and AbbVie scientists designed the study and analyzed and interpreted the data. All authors contributed to the development of the content; all authors and AbbVie reviewed and approved the manuscript; the authors maintained control over the final content. A. Asahina, H. Torii, M. Ohtsuki and H. Nakagawa received consultant and speaker fees from AbbVie Japan, Mitsubishi Tanabe Pharma, Eisai and Janssen Pharmaceuticals. T. Tokimoto, H. Hase, T. Tsuchiya, Y. Shinmura and O. Reyes Servin received salaries as employees of AbbVie and also received AbbVie stock and stock options.
Acknowledgments
Initial medical writing support was provided by Susan Hogan, Ph.D., Jennifer Han, M.S., and Michael Theisen, Ph.D., of Complete Publication Solutions, LLC; this support was funded by AbbVie. In addition, further editorial support was provided by FORTE Science Communications (Tokyo, Japan); this support was also funded by AbbVie. All authors contributed to the writing or critical review of the manuscript, and approved the final version for submission. The standard operation procedures manual for data management and transformation was developed by the Postmarketing Surveillance group in AbbVie Japan. We thank Dr Jungo Sawa, Ph.D., a contracted biostatistician of AbbVie, for his support in the development of the statistics plan and interpretation of the results.
References
- 1. Basko‐Plluska JL, Petronic‐Rosic V. Psoriasis: epidemiology, natural history, and differential diagnosis. Psoriasis: Targets Ther 2012; 2: 67–76. [Google Scholar]
- 2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 3. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5(1): e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furue M, Yamazaki S, Jimbow K et al Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol 2011; 38: 310–320. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003‐2011. PLoS ONE 2012; 7: e52935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krueger G, Koo J, Lebwohl M et al The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient‐membership survey. Arch Dermatol 2001; 137: 280–284. [PubMed] [Google Scholar]
- 7. Rosen CF, Mussani F, Chandran V et al Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012; 51: 571–576. [DOI] [PubMed] [Google Scholar]
- 8. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab M04‐688 Study Group. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010; 37: 299–310. [DOI] [PubMed] [Google Scholar]
- 9. Ohtsuki M, Terui T, Ozawa A et al Japanese guidance for use of biologics for psoriasis (the 2013 version). Dermatological Association. J Dermatol 2013; 40: 683–695. [DOI] [PubMed] [Google Scholar]
- 10. Fredriksson T, Pettersson U. Severe psoriasis ‐ oral therapy with a new retinoid. Dermatology 1978; 157: 238–244. [DOI] [PubMed] [Google Scholar]
- 11. Finlay AY. Quality of life indices. Indian J Dermatol Venereol Leprol 2004; 70: 143–148. [PubMed] [Google Scholar]
- 12. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 13. Prevoo MLL, van‘t Hof MA, Kuper HH et al Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 14. WHO Global TB Report 2014. [Cited 15 March 2015.] Available from URL: http://www.who.int/tb/publications/en/.
- 15. WHO Tuberculosis country profiles. [Cited 20 August 2015.] Available from URL: http://www.who.int/tb/country/data/profiles/en/.
- 16. Noda S, Mizuno K, Adachi M. Treatment effect of adalimumab and infliximab in Japanese psoriasis patients: results in a single community‐based hospital. J Dermatol 2012; 39: 265–268. [DOI] [PubMed] [Google Scholar]
- 17. Taniguchi T, Noda S, Takahashi N et al An observational, prospective study of monthly adalimumab therapy for disease maintenance in psoriasis patients: a possible new therapeutic option for good responders to the initial induction treatment. J Eur Acad Dermatol Venereol 2013; 27: 1444–1447. [DOI] [PubMed] [Google Scholar]
- 18. Carrascosa JM, Vilavella M, Garcia‐Doval I et al Body mass index in patients with moderate‐to‐severe psoriasis in Spain and its impact as an independent risk factor for therapy withdrawal: results of the Biobadaderm Registry. J Eur Acad Dermatol Venereol 2014; 28: 907–914. [DOI] [PubMed] [Google Scholar]
- 19. Verstappen SM, Bakker MF, Heurkens AH et al Adverse events and factors associated with toxicity in patients with early rheumatoid arthritis treated with methotrexate tight control therapy: the CAMERA study. Ann Rheum Dis 2010; 69: 1044–1148. [DOI] [PubMed] [Google Scholar]
- 20. Torii H, Terui T, Matsukawa M et al Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: Results from the prospective post‐marketing surveillance. J Dermatol 2015; 42: 1–12. [DOI] [PubMed] [Google Scholar]
- 21. Torii H, Nakagawa H, The Japanese Infliximab Study Investigators . Long‐term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol 2011; 38: 321–334. [DOI] [PubMed] [Google Scholar]
- 22. Torii H, Nakagawa H, The Japanese Infliximab Study Investigators . Infliximab monotherapy in Japanese patients with moderate‐to‐severe plaque psoriasis and psoriatic arthritis. A randomized, double‐blind, placebo‐controlled multicenter trial. J Dermatol Sci 2010; 59: 40–49. [DOI] [PubMed] [Google Scholar]


