Abstract
Background
Transsphenoidal hypophysectomy is one of the treatment strategies in the comprehensive management of dogs with pituitary‐dependent hypercortisolism (PDH).
Objectives
To describe the influence of pituitary size at time of pituitary gland surgery on long‐term outcome.
Animals
Three‐hundred–and‐six dogs with PDH.
Methods
Survival and disease‐free fractions were analyzed and related to pituitary size; dogs with and without recurrence were compared.
Results
Four weeks after surgery, 91% of dogs were alive and remission was confirmed in 92% of these dogs. The median survival time was 781 days, median disease‐free interval was 951 days. Over time, 27% of dogs developed recurrence of hypercortisolism after a median period of 555 days. Dogs with recurrence had significantly higher pituitary height/brain area (P/B) ratio and pre‐operative basal urinary corticoid‐to‐creatinine ratio (UCCR) than dogs without recurrence. Survival time and disease‐free interval of dogs with enlarged pituitary glands was significantly shorter than that of dogs with a non‐enlarged pituitary gland. Pituitary size at the time of surgery significantly increased over the 20‐year period. Although larger tumors have a less favorable prognosis, outcome in larger tumors improved over time.
Conclusions and Clinical Importance
Transsphenoidal hypophysectomy is an effective treatment for PDH in dogs, with an acceptable long‐term outcome. Survival time and disease‐free fractions are correlated negatively with pituitary gland size, making the P/B ratio an important pre‐operative prognosticator. However, with increasing experience, and for large tumors, pituitary gland surgery remains an option to control the pituitary mass and hypercortisolism.
Keywords: Canine, Cushing's disease, Kaplan‐Meier, Pituitary surgery
Abbreviations
- ACTH
adrenocorticotropic hormone
- α‐MSH
α‐melanocyte stimulating hormone
- CT
computed tomography
- MRI
magnetic resonance imaging
- P/B ratio
(pituitary height (in mm)/brain area (in mm2))
- PDH
pituitary‐dependent hypercortisolism
- UCCR
urinary corticoid to creatinine ratio
Pituitary‐dependent hypercortisolism (PDH), or Cushing's disease, is an endocrine disorder in dogs, caused by an adrenocorticotropic hormone (ACTH)‐secreting tumor in the pituitary gland. The estimated incidence is 1 or 2 cases per 1000 dogs per year.1 Clinical signs are caused by glucocorticoid excess, as a consequence of chronic stimulation of the adrenal glands by the high ACTH concentrations, and may include polyuria, polydipsia, polyphagia, muscle atrophy, central fat accumulation, alopecia and lethargy.2 Clinical signs also may be caused by compression of the brain by a mass effect of the pituitary tumor.2
Treatment can be directed at the adrenal gland, where elimination of glucocorticoid excess is achieved by destruction of adrenocortical tissue with the adrenocorticolytic drug o,p′‐DDD or by inhibition of glucocorticoid synthesis with trilostane.2 Treatment at the pituitary level mainly consists of surgical removal of the pituitary gland by transsphenoidal hypophysectomy or radiation therapy.3, 4 Pituitary gland surgery is considered the treatment of choice in human patients with Cushing's disease.5
In 1993, transsphenoidal hypophysectomy was reintroduced as treatment in dogs with PDH at the Utrecht University, the Netherlands. Short‐term (<3 years) results in 52 dogs and long‐term results in 150 and 181 dogs were reported previously.6, 7, 8 The last study reported an initial remission rate of 86% and a recurrence rate of hypercortisolism of 23%.8 The estimated 1‐year and 3‐year survival fractions were 84 and 68%, respectively.8 The main prognostic factors for long‐term remission in dogs were pituitary gland size, thickness of the sphenoid bone, plasma α‐melanocyte stimulating hormone (α‐MSH) concentration, and urinary cortisol excretion before surgery.8 Recently, the long‐term follow‐up and prognostic factors in dogs with PDH treated with trilostane were reported.9 Median follow‐up in 85 patients was 852 days with a 1‐year survival of 70% and a 3‐year survival of 29%. Age and serum phosphate concentrations were found to be prognosticators for survival time.9 The introduction of trilostane as a treatment option may have contributed to an increase in pituitary gland size of the patients that were referred for hypophysectomy over the past decade, possibly also influencing the outcome of these patients.
The aim of our study was to investigate the influence of pituitary gland size on outcome in a large cohort of dogs with PDH treated by transsphenoidal hypophysectomy. Therefore, we determined survival times, disease‐free fractions and recurrence rates and related these variables to the pituitary gland size in 306 dogs with PDH treated by transsphenoidal hypophysectomy over a 20‐year period.
Materials and Methods
Animals
Three‐hundred–and‐six dogs with PDH, referred to the Department of Clinical Sciences of Companion Animals, Utrecht University, the Netherlands, over a 20‐year period (1993–2013) underwent transsphenoidal hypophysectomy as primary treatment. Dogs of 76 different breeds and crossbred dogs were included (Table 1). There were 164 male dogs and 142 female dogs. Median age at the time of surgery was 8.9 y (range, 2.7–14.4 year) and median body weight was 17.2 kg (range, 3.7–61.0 kg).
Table 1.
Breeds of 306 dogs with pituitary‐dependent hypercortisolism included in the study
| Breed | No |
|---|---|
| Dachshund | 23 |
| Maltese | 16 |
| Beagle | 14 |
| Labrador Retriever | 14 |
| Miniature Poodle | 13 |
| Jack Russell Terrier | 10 |
| Boxer | 9 |
| Yorkshire Terrier | 9 |
| Golden Retriever | 8 |
| English Cocker Spaniel | 6 |
| German Pointer | 6 |
| Hovawart | 5 |
| Stabyhoun | 5 |
| Bouvier des Flandres | 4 |
| Cavelier King Charles Spaniel | 4 |
| French Bulldog | 4 |
| German Shepherd | 4 |
| Poodle | 4 |
| Shih Tzu | 4 |
| Crossbred | 66 |
| Other Breedsa | 78 |
Other breeds include 5 breeds with 3 dogs each, 11 breeds with 2 dogs each and 41 breeds with a single dog in the study.
Diagnosis of PDH was based on clinical signs, results of routine blood tests and measurement of increased urinary corticoid‐to‐creatinine ratios (UCCRs) in combination with an oral high‐dose dexamethasone suppression test, as described previously.10 Median suppression of the UCCR was 80%. In 36 dogs (12%) with <50% suppression of the UCCR, dexamethasone‐resistant PDH was identified by measurement of plasma ACTH concentration, visualization of the adrenal glands by ultrasonography, and pituitary gland imaging using computed tomography (CT) or magnetic resonance imaging (MRI).11, 12 In dogs with PDH, enlarged pituitariy glands were distinguished from non‐enlarged pituitariy glands by their P/B ratio (pituitary height [mm]/brain area [mm2]), as described previously.13 Enlarged pituitary glands have P/B ratio >0.31 and non‐enlarged pituitary glands have ratio ≤0.31.
Surgery and Follow‐Up
All dogs were treated by transsphenoidal hypophysectomy performed by the same neurosurgeon, and peri‐operative treatment followed previously described protocols.6 Dogs were treated with life‐long hormone substitution therapy using cortisone acetate1 at a dosage of 0.25 mg/kg q12h, and thyroxine2 at a dosage of 15 μg/kg q12h. Desmopressin3, 1 drop in the conjunctival sac q8h, was administered for 2 weeks routinely and continued if polyuria caused by central diabetes insipidus persisted.
The first revisit for clinical examination of the dogs usually was scheduled for 8 weeks after surgery at the endocrinology outpatient clinic. After surgery, UCCR measurements were performed at 2 weeks, 8 weeks, 6 months and once a year thereafter, or more frequently in dogs suspected of recurrence of hypercortisolism. All morning urine samples for UCCR measurements were collected at home by the owner when the dog had not received cortisone acetate in the previous 24 hours, and samples were sent to our laboratory by mail or brought in on follow‐up visits. Follow‐up reports were obtained from the routine follow‐up examinations in the clinic, or during telephone conversations with the owner, referring veterinarian or both. Postoperative mortality was defined as death within 4 weeks after surgery, regardless of the cause of death. Remission was defined as UCCR <10 × 10−6 and resolution of clinical signs of hypercortisolism. Residual disease was defined as early postoperative (<8 weeks) UCCR >10 × 10−6. Recurrence was defined as UCCR ≥10 × 10−6 and return of clinical signs of hypercortisolism after initial remission.
Statistical Analysis
All analyses were made using IBM® SPSS®Statistics for Windows, Version 20.04 . Survival and disease‐free fractions were analyzed by the Kaplan‐Meier estimation procedure. Dogs that died of unrelated causes or were alive at last follow‐up were counted as censored cases. Differences between Kaplan‐Meier curves were compared with the Log Rank test. Comparisons between dogs with and without recurrence were done using the Mann Whitney‐U test for non‐parametric data. The P/B ratio over time were analyzed using the Kruskal‐Wallis test and Mann Whitney‐U tests. Significance was set at P < .05.
Univariate Cox's proportional hazard analysis was used to assess the prognostic value of pituitary gland size represented by the P/B ratio on survival time and disease‐free interval. The median end‐point ratio was calculated by dividing the median follow‐up of dogs with a non‐enlarged pituitary gland by the median follow‐up of dogs with an enlarged pituitary gland.
Results
Postoperative Mortality
Twenty‐seven dogs (8.8%) died within 4 weeks after surgery (Fig 1). The median P/B ratio of this subgroup was 0.54 (range, 0.21–1.40) whereas the median P/B ratio of the dogs alive at 4 weeks after surgery was 0.38 (range, 0.13–1.38). The P/B ratio of dogs that died within 4 weeks after surgery was significantly higher than the P/B ratio of dogs that were alive at 4 weeks (P = .004, Fig 2). Twelve of these cases have already been described; 2 dogs died as a result of arterial hemorrhage during surgery, 2 dogs died within 6 hours after surgery and in 1 of these necropsy findings included thrombo‐endocarditis of the right atrium, concentric myocardial hypertrophy of the left ventricle, and lung edema due to circulatory failure. Two dogs died of dyspnea of unknown cause, 1 dog had glucocorticoid‐associated myotonia and 1 dog had hypernatremia as a result of insufficient oral fluid intake. One dog died 5 days after surgery caused by accidental IV injection of potassium solution meant for PO use. Two dogs had a prolonged stay in the intensive care unit for 2 weeks because of severe hypernatremia and diabetic ketoacidosis and were eventually euthanized and 1 dog died of severe bronchopneumonia.7 Of the others dogs, 2 dogs were euthanized shortly after surgery because they did not recover from anesthesia. Three dogs remained comatose for several days and were euthanized in the intensive care unit within 5 days. One dog was euthanized after 3 days because of severe hypernatremia. Five dogs were euthanized or died spontaneously because of postoperative dyspnea; in 1 of these dogs pulmonary thromboembolism was found during necropsy. One dog died at home, 4 days after surgery for unknown reasons. Two dogs were euthanized because of persistent vomiting after release from the hospital. One dog developed septic peritonitis associated with an intestinal foreign body 2 weeks after surgery and was euthanized during emergency surgery.
Figure 1.
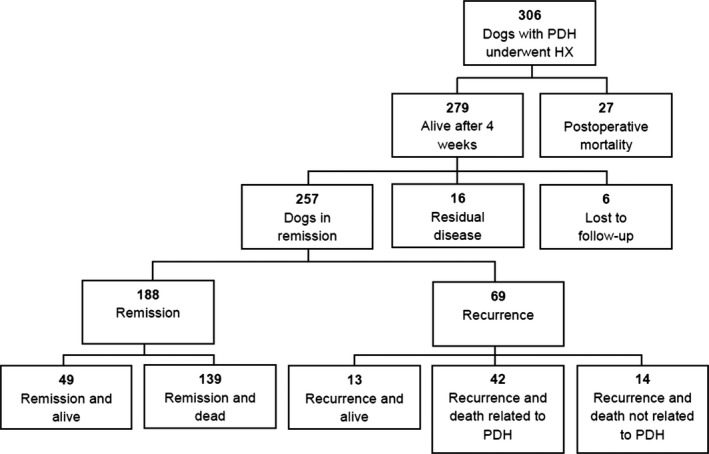
Flowchart of 306 dogs with pituitary‐dependent hypercortisolism (PDH) that underwent transsphenoidal hypophysectomy (Hx) in the period 1993–2013.
Figure 2.
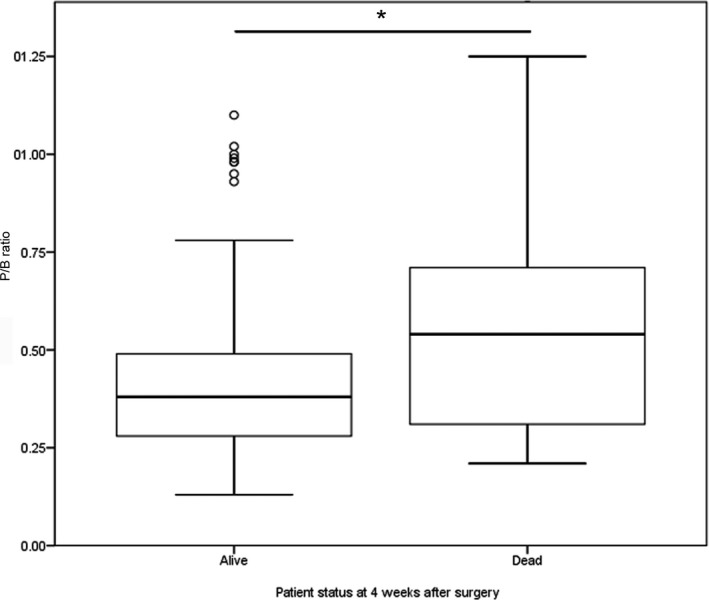
Boxplots displaying the pituitary height/brain area (P/B) ratio of dogs alive (n = 275) and dead (n = 26) at 4 weeks after transsphenoidal hypophysectomy as treatment for pituitary‐dependent hypercortisolism. The P/B ratio of dogs that died within 4 weeks after surgery is significantly higher (*, P = .004). The box represents the interquartile range (i.e. from 1st to 3rd quartile), the line represents the median, the whiskers represent the highest and lowest value within 1.5× the interquartile range, ◦ indicates outlier.
Long‐Term Survival
Two‐hundred‐seventy‐nine dogs were alive 4 weeks after surgery (Fig 1). Of these dogs, 6 were lost to follow‐up and therefore no disease status was available. Remission was confirmed in 257 dogs (84% of the total patient group, 92% of dogs that were alive at 4 weeks) with UCCR <10 × 10−6 and resolution of clinical signs of hypercortisolism at 8 weeks after surgery. Residual disease (UCCR >10 × 10−6 at 8 weeks) was present in 16 dogs (5.7% of dogs that were alive at 4 weeks). Seven of these dogs were euthanized within 5 months for reasons associated with hypercortisolism. One dog was treated by bilateral adrenalectomy, and survived for 34 months. One dog was diagnosed with ectopic ACTH production, as described previously.14 Four dogs were treated with o,p′‐DDD5 , follow‐up times of these dogs were 160 days, 237 days, 594 days and 783 days at last follow‐up. Two dogs were treated with trilostane6 with a follow‐up of 594 and 1967 days. One dog with residual disease had no clinical signs and was euthanized after 783 days because of a liver tumor.
Median survival time of the 300 dogs for which follow‐up information was available was 781 days (range, 0–3808 days; Fig 3A). Estimated 1‐year survival rate was 86% (95% confidence interval [CI], 82–90%), estimated 2‐year survival rate was 79% (95% CI, 73–85%), estimated 3‐year survival rate was 74% (95% CI, 68–80%), estimated 4‐year survival rate was 72% (95% CI, 66–78%) and estimated 5‐year survival rate was 64% (95% CI, 56–72%).
Figure 3.
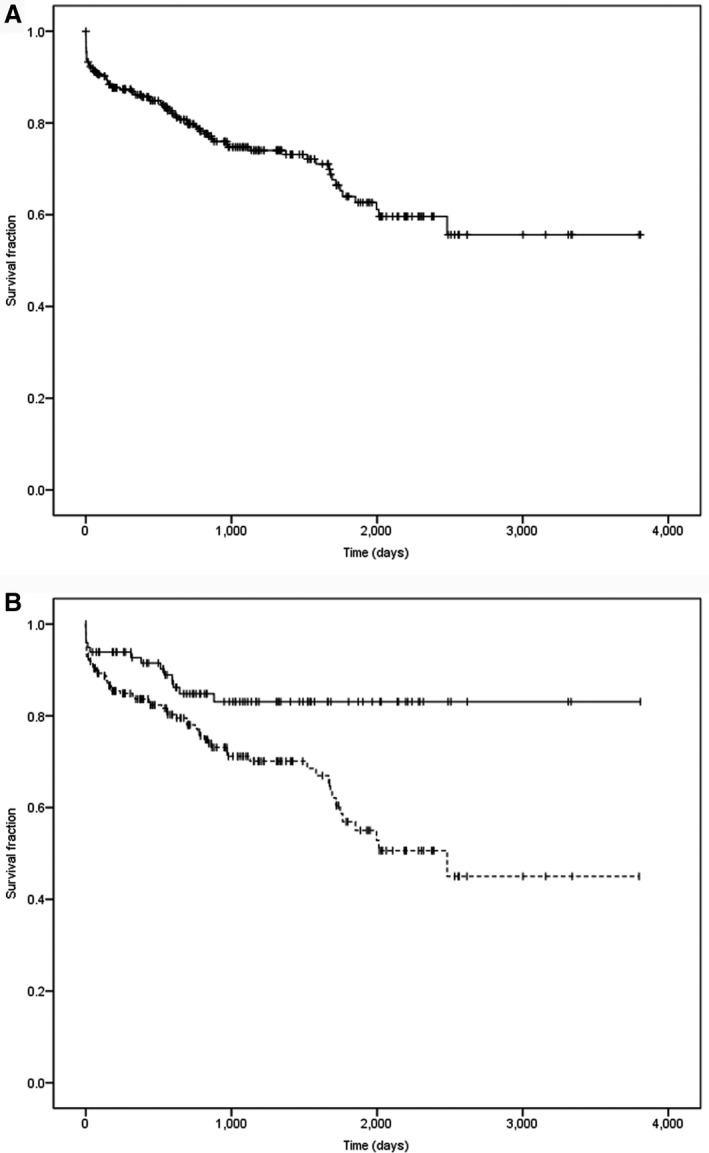
(A) Survival curve of 300 dogs after transsphenoidal hypophysectomy as treatment for pituitary‐dependent hypercortisolism. Censored cases (i.e. dogs that died from unrelated causes or were still alive at last follow‐up) are represented with vertical bars. (B) Survival curves for dogs with an enlarged pituitary (n = 197, P/B > 0.31; dotted line) and non‐enlarged pituitary (n = 99, P/B ≤ 0.31; continuous line). Censored cases (i.e. dogs that died from unrelated causes or were still alive at last follow‐up) are represented with vertical bars. P = .003.
Disease‐free interval was analyzed for 257 dogs with confirmed remission of hypercortisolism after surgery. Median disease‐free interval was 951 days (range, 31–3808 days; Fig 4A). Estimated 1‐year, 2‐year, 3‐year, 4‐year and 5‐year disease‐free fractions were 89% (95% CI, 85–93%), 79% (95% CI, 73–85%), 74% (95% CI, 68–80%), 64% (95% CI, 56–72%) and 57% (95% CI, 49–65%), respectively.
Figure 4.
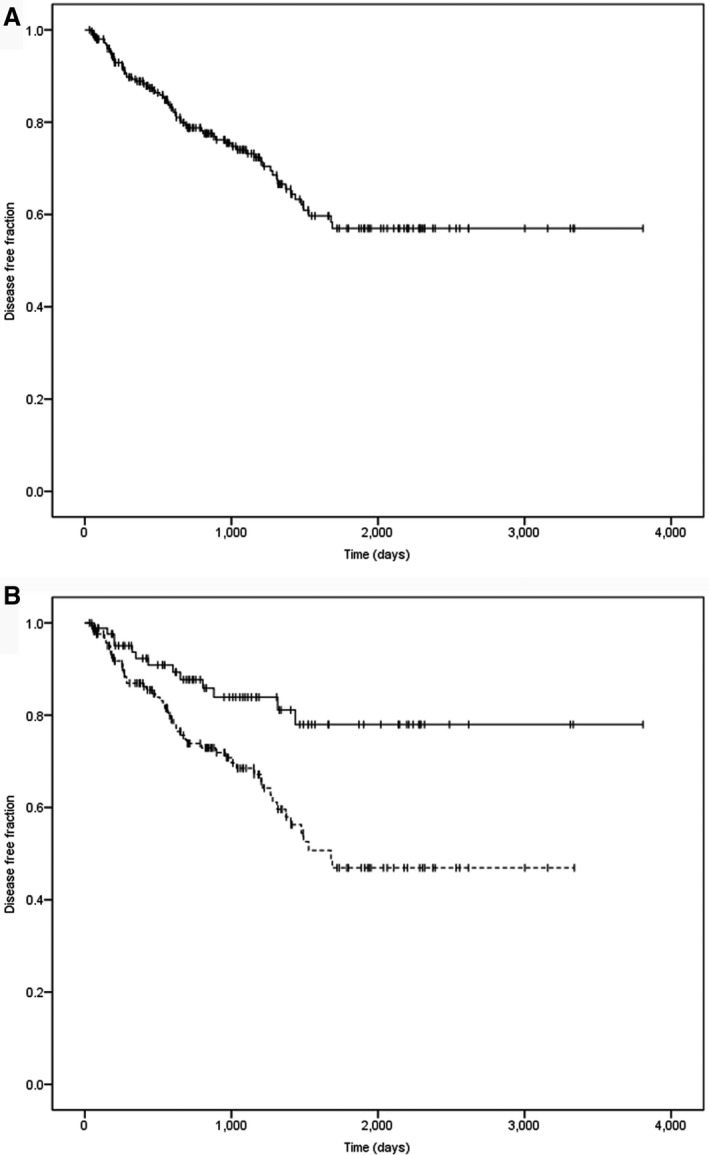
(A) Disease‐free fraction curve of 257 dogs with post‐operative remission after transsphenoidal hypophysectomy as treatment for pituitary‐dependent hypercortisolism. Censored cases (i.e. dogs that died from unrelated causes or were still alive at last follow‐up) are represented with vertical bars. (B) Disease‐free fraction curves for dogs with an enlarged pituitary (n = 169, P/B > 0.31; dotted line) and non‐enlarged (n = 86, P/B ≤ 0.31; continuous line). Censored cases (i.e. dogs that died from unrelated causes or were still alive at last follow‐up) are represented with vertical bars. P = .002.
Recurrences
In 188 of 257 dogs (73%), hypercortisolism was in remission at the time of analysis. Of these dogs, 139 had died or were euthanized because of non‐Cushing's‐related causes, after a median period of 935 days after hypophysectomy (range, 31–3808 days). Over time, 69 of 257 (27%) dogs had recurrence of hypercortisolism after a median period of 555 days (range, 44–1688 days).
At last follow‐up, 13 of these dogs were alive, 42 had died or were euthanized because of recurrent signs of hypercortisolism and 14 died of non‐related causes (Fig 1). Dogs with recurrence of hypercortisolism had a significantly higher P/B ratio (P < .001) and pre‐operative basal UCCR (P = .009) than did dogs without recurrence (Fig 5).
Figure 5.
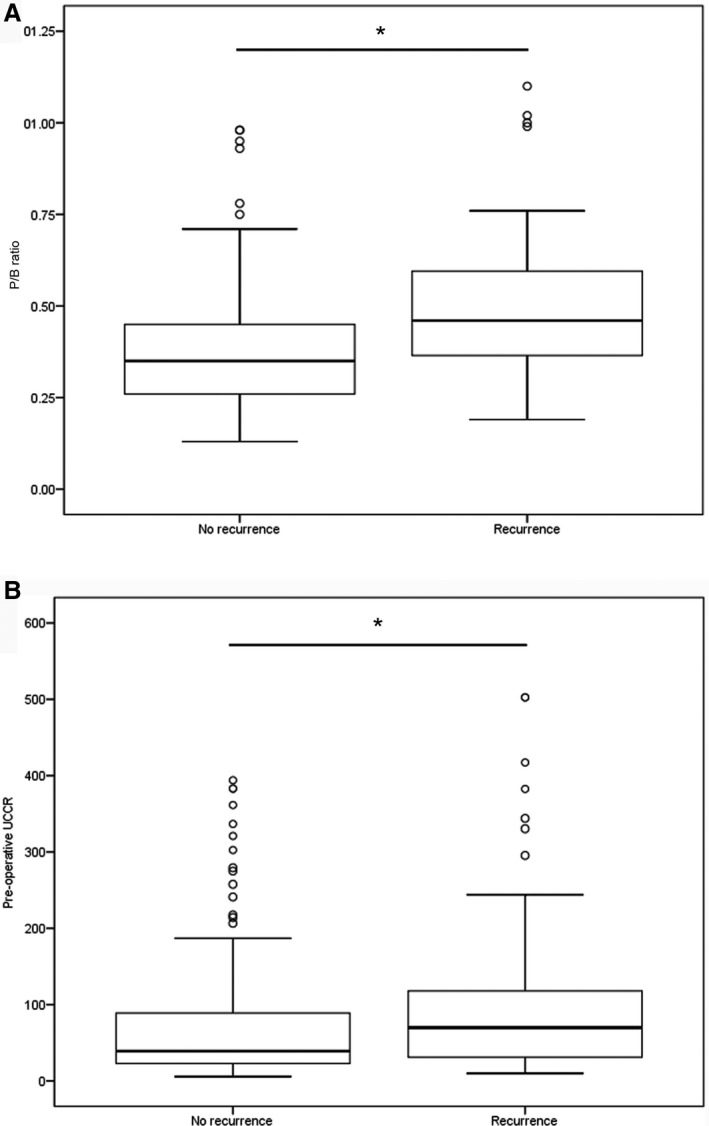
(A) Boxplots of the P/B ratio of dogs with (n = 67) and without (n = 188) recurrence after transsphenoidal hypophysectomy as treatment for pituitary‐dependent hypercortisolism (*, P < .001); (B) Boxplots of the pre‐operative urinary corticoid‐to‐creatinine ratio (UCCR, ×10−6) of dogs with (n = 69) and without (n = 181) recurrence (*, P = .009). The box represents the interquartile range (i.e. from 1st to 3rd quartile), the line represents the median, the whiskers represent the highest and lowest value within 1.5× the interquartile range, ◦ indicates outlier.
Pituitary Gland Size
In this study, median P/B ratio was 0.39 (range, 0.13–1.40). The pituitary gland was enlarged in 201 dogs (median, 0.47, range 0.32–1.4) and not enlarged in 100 dogs (median, 0.25, range, 0.13–0.31). The P/B ratio was not available in 5 dogs. The pre‐operative UCCR was significantly higher in dogs with enlarged pituitary glands, compared to dogs with non‐enlarged pituitary glands (P = .01). Survival time (P = .003) and disease‐free interval (P = .002) of dogs with enlarged pituitary glands was significantly shorter than those in dogs with non‐enlarged pituitary glands (Figs 3B, 4B).
A P/B ratio >0.31 was associated with significantly shorter survival (hazard ratio, 2.34; P = .004) and a shorter disease‐free interval (hazard ratio, 2.40; P = .004). The median endpoint survival was 1.4, indicating that dogs suffering from PDH with a P/B ratio >0.31 have twice the chance of shorter survival than dogs with a P/B ratio <0.31, with a median survival ratio of 1.4 between dogs with enlarged and non‐enlarged pituitary glands.
Pituitary gland size, reflected by the P/B ratio, significantly increased over the time period 1993–2013 with a median P/B ratio of dogs operated between 1993 and 1997 (n = 74) of 0.32, 0.34 for those operated between 1997 and 2003 (n = 75), 0.4 for those operated between 2003 and 2007 (n = 75), and 0.5 for those operated between 2007 and 2013 (n = 77; Fig 6, P < .001). The postoperative remission rate increased over the time period 1993–2013, but not significantly (P = .08). It was 79% in the period 1993–1997, 87% in 1997–2003, 87% in 2003–2007 and 83% in 2007–2013. The recurrence rate in these 4 time periods did not differ significantly (P = .14). It was 19% in the period 1993–1997, 37% in 1997–2003, 24% in 2003–2007 and 26% in 2007–2013.
Figure 6.
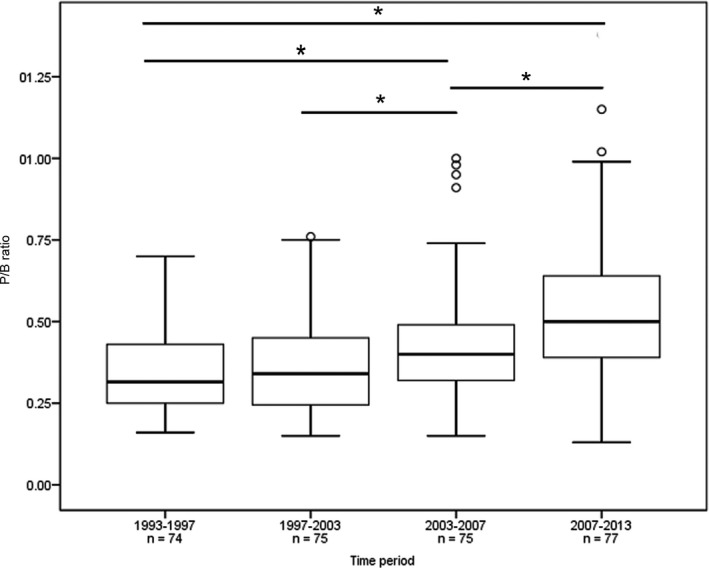
Boxplots of P/B ratio of 301 dogs that underwent transsphenoidal hypophysectomy as treatment for pituitary‐dependent hypercortisolism. The dogs were divided in four quartiles of 74–77 dogs based on the time of surgery over a 20‐year period (1993–2013). A significant difference in P/B ratio was found between the 1st and 3rd quartile (*, P < .001), 1st and 4th quartile (*, P < .001), 2nd and 3rd quartile (*, P = .03) and 3rd and 4th quartile (*, P = .004). The box represents the interquartile range (i.e. from 1st to 3rd quartile), the line represents the median, the whiskers represent the highest and lowest ratio within 1.5× the interquartile range, ◦ indicates outlier.
Discussion
Since our first report in 1998 on the results of transsphenoidal hypophysectomy in 52 dogs,6 the number of operated dogs has steadily increased to a total of 306 dogs. With a median follow‐up of >2 years and longest follow‐up of >10 years after surgery, our present study provides long‐term results and confirms that transsphenoidal hypophysectomy is an effective treatment for dogs with PDH.
In our present study, postoperative mortality was 9% and postoperative remission rate was 84%, which were the same as reported previously.7 The remission rate increased over time, from 79% in 1993–1997 to 83% in 2007–2013. Remission rates in humans after pituitary gland surgery for Cushing's disease vary from 42 to 93%, depending on the definition used.5 The medical treatment most commonly used in dogs with Cushing's disease is trilostane, a competitive inhibitor of the 3β‐hydroxysteroid dehydrogenase/isomerase system which is essential for the synthesis of cortisol.2 Reported remission rates of clinical signs in dogs after treatment with trilostane are 70–86%.15, 16 Large case series such as ours are not available for medical treatment, but reported follow‐up is approximately 900 days.9, 15, 16 Because medical treatment is not directed at the pituitary tumor itself, expansion of the pituitary lesion may lead to neurological signs by a mass effect. For these patients, hypophysectomy or radiotherapy are the only options left for treatment. Yearly re‐evaluations, including CT scan of the pituitary gland to monitor tumor size are advised for patients with non‐enlarged pituitary glands treated with trilostane.
The estimated survival and disease‐free intervals slightly improved compared to our previous study. Recurrence rate in the present study was 27%, a small increase from the previously reported 25%.7 This difference may have been caused by increase in pituitary gland size of patients referred for hypophysectomy over the past decade. With the introduction of trilostane treatment, dogs with a non‐enlarged pituitary gland tend to be treated medically first, whereas dogs with an enlarged pituitary gland frequently are referred for surgery. The median P/B ratio in our present study increased significantly over time indicating a tendency of veterinarians to refer primarily dogs with enlarged pituitary glands for surgery. Both survival time and disease‐free interval were significantly lower in dogs with larger pituitary glands. Dogs suffering from PDH with a P/B ratio >0.31 have twice the chance of early death. Also, dogs that died within 4 weeks of surgery had a significantly higher P/B ratio. This finding was also shown in studies of human patients, where pituitary gland size was the main prognosticator.17, 18 However, overall long‐term results were not very different from the results reported in our previous case series, and recurrence rate was not significantly different among the 4 time periods. Apparently, the negative effect of larger pituitary gland size on survival and disease‐free fractions was compensated for by an increase of experience of the neurosurgeon in removing larger pituitary tumors and improvement in peri‐ and post‐operative care.
We also found that the pre‐operative UCCR of dogs that developed recurrence was higher than that of dogs without recurrence. This finding was also shown in human patients, in whom high urinary cortisol concentrations predicted recurrence.19 The secretion of ACTH and thus cortisol may be is related to pituitary gland size and therefore may result in a less favorable prognosis.13 Indeed, in our present study, a significant difference was found in pre‐operative UCCR between dogs with an enlarged as compared to a non‐enlarged pituitary gland.
If patients with a higher risk of recurrence are identified at an earlier time point, preferably before surgery, they can be monitored more closely or they can be treated with adjuvant therapy, such as radiation therapy of the pituitary fossa.4, 20 In human medicine, stereotactic radiation therapy and proton therapy have been introduced in postoperative treatment of patients with pituitary adenomas.21, 22 Both treatments are effective, but recurrence of hypercortisolism also occurs after such treatments. In veterinary medicine, the additional costs of these treatment options might be a barrier for owners. Recurrences in dogs with PDH are thought to occur because of regrowth of the pituitary adenoma from microscopic islets of tumor cells left behind during surgery or normal pituitary cells that transform into adenoma cells. Recently, a telescope and high definition camera system with 16× magnification have been used for better visualization during transsphenoidal hypophysectomy in dogs.23 With this technique, the fossa can be inspected for macroscopic pituitary tissue, that otherwise might be left behind, possibly improving postoperative remission in some patients. However, this technique probably will not influence recurrence rates, if indeed microscopic tissue plays a role. The risk of leaving pituitary cells behind may be greater in dogs with larger pituitary tumors, causing an increased risk of recurrence.
Transsphenoidal hypophysectomy is an effective treatment for PDH in dogs, with an acceptable long‐term outcome. Survival time and disease‐free fractions decrease with increasing pituitary gland size, which indicates that the P/B ratio is an important pre‐operative prognosticator. However, with increasing experience, especially for large tumors, pituitary gland surgery remains an option to control the pituitary mass and hypercortisolism.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands.
Footnotes
Cortisoni acetas, Genfarma, Maarssen, the Netherlands
L‐thyroxine, Aesculaap, Boxtel, the Netherlands
Minrin, Ferring, Hoofddorp, the Netherlands
IBM® SPSS®Statistics for Windows, Version 20.0, Armonk, NY
Lysodren, Bristol‐Myers Squibb, Utrecht, the Netherlands
Vetoryl, Dechra, Shrewsbury, UK
References
- 1. Willeberg P, Priester W. Epidemiological aspects of clinical hyperadrenocorticism in dogs (canine Cushing's syndrome). J Am Anim Hosp Assoc 1982;18:717–724. [Google Scholar]
- 2. Galac S, Reusch CE, Kooistra HS, Rijnberk A. Adrenals In: Rijnberk A, Kooistra HS, eds. Clinical endocrinology of dogs and cats, 2nd ed Hannover: Schlütersche; 2010:93–154. [Google Scholar]
- 3. Meij BP. Hypophysectomy as a treatment for canine and feline Cushing's disease. Vet Clin North Am Small Anim Pract 2001;31:1015–1041. [DOI] [PubMed] [Google Scholar]
- 4. De Fornel P, Delisle F, Devauchelle P, Rosenberg D. Effects of radiotherapy on pituitary corticotroph macrotumors in dogs: A retrospective study of 12 cases. Can Vet J 2007;48:481–486. [PMC free article] [PubMed] [Google Scholar]
- 5. Rees D, Hanna F, Davies J, et al. Long‐term follow‐up results of transsphenoidal surgery for Cushing's disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 2002;56:541–551. [DOI] [PubMed] [Google Scholar]
- 6. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Results of transsphenoidal hypophysectomy in 52 dogs with pituitary‐dependent hyperadrenocorticism. Vet Surg 1998;27:246–261. [DOI] [PubMed] [Google Scholar]
- 7. Hanson JM, Hoofd MM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2005;19:687–694. [DOI] [PubMed] [Google Scholar]
- 8. Hanson JM, Teske E, Voorhout G, et al. Prognostic factors for outcome after transsphenoidal hypophysectomy in dogs with pituitary‐dependent hyperadrenocorticism. J Neurosurg 2007;107:830–840. [DOI] [PubMed] [Google Scholar]
- 9. Fracassi F, Corradini S, Floriano D, et al. Prognostic factors for survival in dogs with pituitary‐dependent hypercortisolism treated with trilostane. Vet Rec 2015;176:49. [DOI] [PubMed] [Google Scholar]
- 10. Galac S, Kooistra H, Teske E, Rijnberk A. Urinary corticoid/creatinine ratios in the differentiation between pituitary‐dependent hyperadrenocorticism and hyperadrenocorticism due to adrenocortical tumour in the dog. Vet Q 1997;19:17–20. [DOI] [PubMed] [Google Scholar]
- 11. Bosje J, Rijnberk A, Mol J, et al. Plasma concentrations of ACTH precursors correlate with pituitary size and resistance to dexamethasone in dogs with pituitary‐dependent hyperadrenocorticism. Domest Anim Endocrinol 2002;22:201–210. [DOI] [PubMed] [Google Scholar]
- 12. Van der Vlugt‐Meijer RH, Voorhout G, Meij BP. Imaging of the pituitary gland in dogs with pituitary‐dependent hyperadrenocorticism. Mol Cell Endocrinol 2002;197:81–87. [DOI] [PubMed] [Google Scholar]
- 13. Kooistra H, Voorhout G, Mol J, Rijnberk A. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary‐dependent hyperadrenocorticism. J Endocrinol 1997;152:387–394. [DOI] [PubMed] [Google Scholar]
- 14. Galac S, Kooistra H, Voorhout G, et al. Hyperadrenocorticism in a dog due to ectopic secretion of adrenocorticotropic hormone. Domest Anim Endocrinol 2005;28:338–348. [DOI] [PubMed] [Google Scholar]
- 15. Alenza DP, Arenas C, Lopez ML, Melian C. Long‐term efficacy of trilostane administered twice daily in dogs with pituitary‐dependent hyperadrenocorticism. J Am Anim Hosp Assoc 2006;42:269–276. [DOI] [PubMed] [Google Scholar]
- 16. Reine NJ. Medical management of pituitary‐dependent hyperadrenocorticism: mitotane versus trilostane. Top Companion Anim Med 2012;27:25–30. [DOI] [PubMed] [Google Scholar]
- 17. Bochicchio D, Losa M, Buchfelder M, et al. Factors influencing the immediate and late outcome of Cushing‐disease treated by transsphenoidal surgery: A retrospective study by the European Cushings‐disease survey group. J Clin Endocrinol Metab 1995;80:3114–3120. [DOI] [PubMed] [Google Scholar]
- 18. Meij BP, Lopes MS, Ellegala DB, et al. The long‐term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg 2002;96:195–208. [DOI] [PubMed] [Google Scholar]
- 19. Sonino N, Zielezny M, Fava G, et al. Risk factors and long‐term outcome in pituitary‐dependent Cushing's disease. J Clin Endocrinol Metab 1996;81:2647–2652. [DOI] [PubMed] [Google Scholar]
- 20. Kent MS, Bommarito D, Feldman E, Theon AP. Survival, neurologic response, and prognostic factors in dogs with pituitary masses treated with radiation therapy and untreated dogs. J Vet Intern Med 2007;21:1027–1033. [DOI] [PubMed] [Google Scholar]
- 21. Tritos NA, Biller BM. Update on radiation therapy in patients with Cushing's disease. Pituitary 2015;18:263–268. [DOI] [PubMed] [Google Scholar]
- 22. Wattson DA, Tanguturi SK, Spiegel DY, et al. Outcomes of proton therapy for patients with functional pituitary adenomas. Int J Radiat Oncol Biol Phys 2014;90:532–539. [DOI] [PubMed] [Google Scholar]
- 23. Mamelak AN, Owen TJ, Bruyette D. Transsphenoidal surgery using a high definition video telescope for pituitary adenomas in dogs with pituitary dependent hypercortisolism: methods and results. Vet Surg 2014;43:369–379. [DOI] [PubMed] [Google Scholar]


