Abstract
In this article we provide an in‐depth description of a new model of informed consent called ‘meta consent’ and consider its practical implementation. We explore justifications for preferring meta consent over alternative models of consent as a solution to the problem of secondary use of health data for research. We finally argue that meta consent strikes an appropriate balance between enabling valuable research and protecting the individual.
Keywords: Informed consent, meta consent, broad consent, specific consent, health data
Introduction
The problem
The rapid increase in IT capabilities over the last 30 years have made it possible to store and analyse very large datasets, and accompanying developments in lab automation and analytic techniques have made it possible to easily extract ‘omics’ information from tissue samples. These two developments have together led to a situation in the health area where increasing amounts of personal health data is stored in an easily accessible form, and where this stored information has become increasingly valuable for research. A significant proportion of the research value is created by 1) the ability to use data that are primarily collected for administrative purposes (e.g. hospital episode data or prescription data), and 2) the ability to link data from different sources, either with other health data or with data outside the health area. There is a clear political will in many countries to try to maximize the research yield from the data under the banner of ‘Big Data’ or ‘The Learning Health Care System’.1
This, however, creates a set of ethical and regulatory problems. What regulatory structure will at the same time:
Protect the interests and autonomy of data subjects; and
Optimize the possibility to conduct valuable research
These problems are further complicated by the fact that the data sources that might have to be linked in a particular research project may have been originally collected at different times, for different original purposes, based on different legal justifications and under different consent regimes.2 Going forward we can probably achieve some degree of harmonization of legal justifications and consent regimes, but it is unlikely that all relevant diversity between data sources can be removed. A solution will therefore have to deal adequately with the historical contingencies, as well as with continuing diversity among data sources.
In the following paragraphs we will briefly outline and criticise some of the suggested ‘solutions’ to the problem, before describing and defending a new solution ‘meta consent’. Some of the critical points raised about other solutions also need to be discussed in relation to meta consent and will therefore be discussed more extensively in later sections.
Informed consent
Many of the solutions involve a consent requirement, and it is therefore necessary briefly to note that there are several distinct but overlapping justifications for such a requirement. The necessity to obtain consent from an individual before acting in ways that affect that individual can be justified by ethical considerations relating to:
Protection from harm3
Respect for autonomy4
Protection of privacy5
Property rights in data or tissue6
Protection of bodily integrity7
Maintenance of trust8
The exact shape and strength of a consent requirement will vary according to the exact way in which the requirement is justified. In this article we will assume that the justification for a consent requirement in the sphere of biomedical research is not based exclusively on considerations of protection from harm. Many of the standard consent requirements in research ethics regulation of clinical research are only intelligible if they are supposed to protect interests beyond the interest in not being harmed.
Current solutions
Current suggested solutions to the problems of protecting the interests and autonomy of data subjects at the same time as optimizing the possibility to conduct valuable research include:
The ‘no problem’ solution
Opt‐out/Right to be forgotten
Blanket/broad consent
Specific consent
Ownership of data/tissue
The ‘no problem’ solution
One suggested solution is to deny that there is a problem to be solved. This often relies on a combination of two distinct types of claim: 1) that there are no significant individual interests at stake that need to be protected, because the research process in relation to secondary use of health data adequately protects privacy and cannot cause harm; and 2) even if there were significant individual interests at stake these would in all cases of bona fide research be outweighed by the public interests in the research taking place.9 A subsidiary more technical claim is that all models involving opt‐out or consent may lead to ‘consent bias’, i.e. the phenomenon that those who agree to data use differ from those who do not agree. The existence and size of this problem in relation to secondary research use of data is disputed.10
Taken as conceptual claims both 1) and 2) are obviously false. There is nothing inherent in or to research using health data that entails that it necessarily cannot cause harm and is always in the public interest. Taken as empirical premises both are also doubtful.
Opt‐out/Right to be forgotten
It has also been suggested that the interests etc. of data subjects can be adequately protected if they are given an opportunity to opt‐out of research using their data, or if they can exercise their ‘Right to be forgotten’.11
The right‐to‐be‐forgotten suggestion is a pseudo solution. There are many types of health data, including both administrative and clinical data, where people would not be allowed to exercise a right to be forgotten and have the data deleted. And, if we per impossibile did allow people to exercise this right, it would cumulatively be very damaging to research since the deleted data could never be recovered. Persons could thus never change their minds if they had exercised the right, and if many chose to be forgotten, for instance after a public research scandal, it could lead to huge holes in the available data.
General opt‐out of research use of data is a way of protecting the interests and autonomy of data subjects, but only if the option is well publicized and easily available. It is, however a rather crude instrument in that it is all or nothing and therefore allows the data subject little or no differentiation between different kinds of research, different researchers etc.
It is possible for opt‐out to be specific for every new research use of data, but this would raise exactly the same issues as discussed below for specific informed consent.
Blanket/Broad consent
We could ask people to give an open‐ended and unspecified ‘Blanket’ or broadly specified ‘Broad’ consent to the use of their health data for research either when their data are collected, or at some specific point in time. The proposed revision of the US ‘Common Rule’, for instance, requires broad consent for all future use of tissue.12 There is a significant literature on whether blanket or broad consent equates to informed consent,13 but here, for the sake of argument, we will accept that blanket or broad consent can constitute ethically valid consent, when properly asked for and given.
There are, however good reasons to believe that many tokens of blanket or broad consent to research will not be valid, if the consent is sought in conjunction with data collection. Most collection of health data occurs at the point of care where the focus of attention of both healthcare users and providers is likely to be on the clinical problem or issue that is the occasion for the encounter with the health care system. Some users may be vulnerable, and there may be time pressures involved relating to standard lengths of consultations. It is therefore very unlikely that the user will 1) receive even the limited information necessary to give a valid blanket or broad consent, and 2) will have the time or inclination to think properly about the choice.
If blanket or broad consent is sought for all data at one particular point in time, it is again a rather crude instrument not allowing data subjects any differentiation in their choices.
Specific informed consent
The ‘gold plated’ solution would initially seem to be to ask data subjects for specific, full informed consent for every new research use of their data.
Traditionally this solution has been rejected on the basis of pragmatic considerations, e.g. that the effort needed to locate and communicate with data subjects would be disproportionate, but just as IT developments have made research easier it has also made search and communication easier and cheaper. These pragmatic considerations have thus lost much of their force. Building on the significant IT developments a recent model of informed consent – dynamic consent – suggests that information about specific use of health data and tissue and requests for consent for this use should be put to the individual through a webbased platform.14 As part of this solution, an individual should be given the opportunity to tailor the level of information preceding consent requests.
There are, however, other reasons why the gold‐plated solution may not be optimal from an ethical point of view. The first reason is that, just like a model relying only on broad consent, a specific consent model is crude and insensitive to the preferences of data subjects in that it does not allow those who are willing to give broad consents for certain kinds of research to do so. Second, there are reasons to doubt the validity of the consents and refusals given. A resident of Denmark would, if specific consent was required before secondary research use of data, potentially receive hundreds of consent requests each year.15(28) This makes routinization of consent behaviour very likely, i.e. the phenomenon that the persons in question do not read the information and do not reflect on the choice, but simply choose habitually to consent or refuse in line with their previous choices. If we chose to rely on consents to repetitive requests we would be relying, not on real consent, but on a convenient fiction.
Ownership of data/tissue
Finally, it could be claimed that the solutions discussed above misstate the problem since they are not built on a premise of personal ownership. If people own their data and/or tissue researchers, research institutions or research data brokers would have to engage with them to arrange a (potentially commercial) transfer of ownership or rights to use. It would also mean that we would at least have to consider moving from a consent model to a contract model, i.e. shift the transaction into a quite different legal framework. A full exploration of the issues raised by this model is beyond the scope of the current paper, but it would share some of the issues raised by specific consent requirements, and create new issues of cost and burdens for the research endeavour.16(29,30)
In the following main parts of the article we will first define and describe meta consent as a new model for protecting the interests and autonomy of data subjects in the context of secondary research using health data, and then proceed to discuss how this model differs from already proposed models and why it is preferable in the specific context of secondary research use of health data.
Meta Consent – The Model
The basic idea
As outlined above, traditional accounts of informed consent make a person the locus of a request to consent to participation in research. By contrast 'meta consent’ denotes the idea that people should be asked how and when they would like to be presented with a request for consent.17 That is, people should be asked to design how they in the future would like to provide consent to the use of their personal health data and biological material. By expressing a preference for how and when to provide consent, people can be said to provide consent on a meta level. This is the defining idea in the model of meta consent.
Is the possibility of expressing a preference for how and when to provide consent relevant and valuable to the individual in practice? That is, is it likely that people will 1) have preferences for how and when to provide consent and 2) have intelligible reasons for these preferences making the option of making choices based on such preferences valuable to them? We believe it is. A preference for how and when to be asked for consent may reflect responsiveness to individual reasons related to underlying individual differences in:
Values
Vulnerabilities
Trust
People hold different values with implications for the legitimacy of purposes of research or for the conditions under which research is undertaken. Although research with potential health benefits is typically recognized as valuable, people differ widely in their views on the value of commercially driven research, dual use research (research with military and non‐military use), research that involves what people believe to be sensitive and personal data, research that may stigmatize groups in society, etc.18 If a person holds values with implications for the legitimacy of the purposes of research and for the conditions under which research is undertaken, it not only ceteris paribus gives this person reasons to provide or refuse consent in a number of cases when asked, but it also provides this person with a reason for preferring to be asked for consent when relevant. On the other hand, if a person believes that all (health) research is always of benefit to society, this not only gives this person a reason to consent when asked, but it also more generally provides this person with a reason for preferring an option to consent to as much as research as possible in one go.
Differences in peoples’ interests in how often to provide consent may also reflect emotional reactions to research on personal health data. Some may react with embarrassment in the face of knowing that others have access to their personal and, what they perhaps believe to be, sensitive information, even if the data are anonymized. Some will worry about how these data may be used, by whom and for what purposes both now and in the future. Some will be anxious that data security is poor and data may be lost. Others will be completely indifferent to all such concerns – perhaps because they do not consider health data to be sensitive. The key point here is that individual reactions may provide different individuals with different reasons for trying to control the flow of personal sensitive information. This control may be exercised through actual consent behaviour but it may also be exercised by a demand to be asked for consent more or less often.
Differences in peoples’ interests for how often to provide consent may also reflect differences in trust in researchers. Trust involves having a strong belief or expectation that others will act in a particular way. Our trust in others is inter alia influenced by the extent to which they meet our expectations in certain matters. We may trust health care professionals to offer adequate treatment and researchers to do research for the greater good, and trust both groups to protect the rights of the individual patient.19 Such expectations are likely to be based on an intricate mixture of our previous first and second hand experiences of the clinical and research settings, social norms about adequate health care and sound research, and our own beliefs and values concerning adequate care and research. When such expectations are disappointed, trust may be reduced. When they are met, trust is maintained or may be increased. The point here is, again that individual levels of trust based may provide reasons for different consent behaviour but also reasons for wanting to be asked for consent more or less often.20(38–40)
The defining idea in the model of meta consent is the idea that people should be given the opportunity to make choices based on their preferences for how and when to provide consent. We have argued that from an individual's perspective this represents a relevant and valuable option of choice, and that there are good grounds for believing that individuals will have an interest in designing future consent requests.
The six elements of a model of meta consent
The model of meta consent may be described both formally and substantively. In a formal description meta consent is any model of consent that allows an individual to express a preference for how and when to provide consent, i.e. to design future consent requests, and it has the following six elements:
-
1)
A limited number of categories for designing consent requests
-
2)
A key for the prioritization of consent requests
-
3)
A definition of the time for providing meta consent
-
4)
A default setting if meta consent is not provided
-
5)
A scheme for redesigning meta consent
-
6)
A method and infrastructure for requesting and recording meta consent
For each of these formal constituents, an actual implementation of a model of meta consent will involve choosing one option among competing alternatives. Both the formal elements and the choices involved in any substantive account of meta consent may obviously be subject to discussion. In the following we shall briefly elaborate on each of these elements and in particular the implementation of meta consent we favour.
The categories for designing consent requests
The first essential component in the model of meta consent is the idea that a person should be provided with a predefined set of types of consent, a set of types of data, and a set of types of research contexts with which to design future consent requests. That is, a person should be able to choose how and when to provide consent based on predefined categories of consent, data and contexts of research. We suggest values for each of the three variables in Figure 1.
Figure 1.
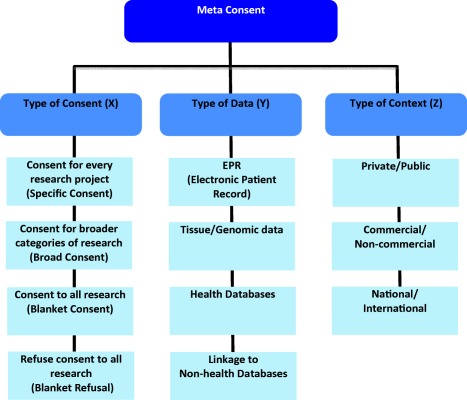
Possible values of meta consent
The values to be implemented in a specific implementation of meta consent should, to some extent, be defined according to the preferences of the population in which the implementation is to be used, and according to the specific legal situation. The values suggested here are thus not fixed but we take them to be reasonable suggestions.
Specific consent is – as indicated – consent requests not for each and every use of data but for each new specific project using data. Broad consent is consent for broader categories of research such as, for instance, cancer‐related research. A person choosing the broad consent option would be asked for broad consent the first time his or her data was being used in a project within a given category. These categories could be predefined or they could be derived from the purposes of research projects submitted to the system. Blanket consent and blanket refusal are one‐off decisions concerning participation or non‐participation in research. Concerning types of data, it is worth mentioning that we are here making no distinction between tissue and already sequenced genomic data. Neither do we distinguish between anonymized, pseudonymized and person‐identifiable data in health databases. We believe that none of these distinctions make a fundamental philosophical difference in relation to whether or not these categories of data should be subject to consent procedures. It is clear that, for instance proper anonymization removes some risks relating to privacy and harm, but it is not clear that it removes all risks, or that it is relevant to other reasons people may have for controlling their health data. A decision concerning whether or not to include this distinction in an implementation of meta consent should therefore take into account the views in the population regarding the importance of this distinction.
We do, however, distinguish between health and non‐health related data simply because we believe that people may have different consent preferences across these categories of data. Finally, in terms of contexts, they may in some countries be partly overlapping. Thus, in many European countries, private research will be commercial and public research will be non‐commercial. This is not the case in all countries, and again we believe that actual consent preferences will to some extent depend on these features of the research context.
On the basis of the categories found in Table 1 a person may now design future consent requests by pairing a type of consent (X) with either a type of data (Y) or a type of context (Z). This could be done using a simple form or tick‐box as in Table 1, or as an interactive web‐site or app.
Table 1.
Meta consent form
| Meta consent | Types of consent (X) | ||||
|---|---|---|---|---|---|
| Types of Data (Y) | Specific | Broad | Blanket | Refusal | |
| EPR (Electronic Patient Record) | |||||
| Tissue/Genomic data | |||||
| Health Databases | |||||
| Linkage to non‐health data | |||||
| Types of Context (Z) | Specific | Broad | Blanket | Refusal | |
|---|---|---|---|---|---|
| Private | Public | ||||
| Commmercial | Non‐commercial | ||||
| National | International | ||||
A completed form of this kind makes up a meta consent. Restated in natural language a completed form in this example corresponds to seven statements of the form:
-
1)
‘In relation to the future research use of my Y, I wish to be asked for/wish to X’
-
2)
‘In relation to the future use of my data for Z research, I wish to be asked for/wish to X’
Thus if a person ticks off the box in the left hand corner it reads 'In relation to the future research use of my Electronic Patient Record, I wish to be asked for specific consent’. It means that the person requires to be asked for consent for every research project in which data from his or her electronic patient record is used.
After a person has filled in the form the meta consent system can then provide feedback on the implications of the choices in relation to 1) the likely number of consent requests the person will receive in the future, and 2) the likely extent of use of the person's data for research. After having received this feedback the person can then either confirm, or modify the choices.
The prioritisation of consent requests
All research projects for which meta consent applies always both involve a type of data (Y) and are conducted in a research context (Z) that may be characterized by one or the other of the predicates listed for each of the three types of context. A particular research project may involve tissue and be conducted as a public, non‐commercial project in a national setting. Several entries in the meta consent form is therefore of relevance for such research projects. The third component of the model of meta consent is the choice of how to interpret or balance a person's potentially different consent requirements related to the type of data used in a research project and related to the various contexts of a research project. We suggest that a person's strongest consent requirement should dominate all other consent requirements with the following order of dominance, where ‘X > Y’ means that ‘X’ dominates ‘Y’: Blanket refusal > Specific consent > Broad consent > Blanket consent. To illustrate, if a person has required specific consent requests for research on his or her tissue, and broad consent requests for public research, then public research on tissue would have to request consent for each research project, and so on. Our choice of how to prioritize a person's consent requirements is based on the view that it should be left to the individual to the largest extent possible to decide if they want make to make their data and tissue available for research projects to which different individual consent requirements apply. In this way we protect individual preferences.
The timing and presentation of meta consent
The model of meta consent requires the individual to design future consent requests. When should this happen? Since many societies endow the individual with full legal capacity around the age of 18 years, this may be the appropriate time for collecting an initial meta consent. People who do not provide a meta‐consent can then be reminded of the possibility in various ways, e.g. when they use national health portals, visit their GP or renew their driving licence or passport etc. When and where reminders are most appropriately generated and issued will depend on the local context. It will, however rarely be appropriate to require people to complete a meta‐consent as part of a clinical encounter, for reasons discussed in the section on broad consent in the Introduction.
The presentation of the meta consent choice should make it clear that this is an important choice with significant future implications. There have to be ongoing public information campaigns and issues relating to consent for research could be included in the school curriculum. The actual meta consent system could also promote reflective decision‐making by providing feedback on the implications of choices and by requiring endorsement of the choices made after a ‘cooling off’ period.
The types of consent, data, and contexts (see Figure 1) have to be clearly described and further information should be easily available, for instance in ‘pop‐ups’. It has to be noted that there is a significant difference between the meta consent choices and the ‘standard’ choice of participating in a clinical trial. The amount and specificity of the information needed to make a fully informed choice differs significantly. Persons who have strong preferences for knowing every single detail about a research project before deciding to participate or not can easily be provided with enough information about the meta choices to make it clear that they should choose ‘consent for every research project’ in the meta consent system. Given the importance of the meta consent choice, the information should be designed to maximize understanding and should be tested in the relevant population before the implementation of the system.
A default setting if meta consent is not provided
A meta consent system need to define a default that is implemented if a person does not provide meta consent despite repeated reminders, or does not provide a complete meta consent (i.e. leaving empty boxes in the consent form). This default has to be communicated clearly in invitations to provide meta consent. Meta consent represents an important opportunity for a person to influence the future use of his or her data and tissue in various contexts. It provides the individual with a strong right to control the use of personal health data. However, as a right it may be waived. What should the default be if people choose not to exercise their meta consent rights? As we have already stated there are significant benefits that flow from research using health data and tissue and every one of us therefore has good general reasons to contribute to research. We therefore think that setting a default of broad or blanket consent strikes the right balance between the different interests at stake. Individuals will have been presented with the possibility to make a choice, and the fact that they have not made one provides at least a partial indication that they do not consider the choice to be important. In this case it seems acceptable to let general interest in scientific research decide the default. However, the exact specification of the default must, like other aspects of a concrete implementation of the model take account of both the preferences of the population and the prevailing legal regulation. It may, for instance, make sense to specify different defaults for particularly sensitive types of data or types of research. The model allows for this. The defaults may thus in the end differ between different implementations of the model.
The redesigning of meta consent
A person may over time come to have strong reasons for changing his or her views concerning when and how to provide consent in the light of personal experiences, scientific developments, or events at a societal level. A meta consent system should therefore allow people to modify the meta consent choices if and when they wish to do so. Such changes would then govern the generation of consent requests in relation to future projects using their data.
The system could also actively prompt people to re‐state or reconfirm their choices at regular intervals, e.g. every five years in order to ensure that the consent preferences recorded in and used by the system are in accordance with the persons’ current preferences.
The recording of meta consent and the generation of consent requests
The sixth component concerns the practical implementation of the model of meta consent. A meta consent system can be implemented as an integrated IT platform that generates the meta consent requests and reminders, allows persons to record and change their meta consent, and generates consent requests based on the individuals meta consent choices. This could most easily be implemented in contexts where citizens are uniquely identifiable (e.g. through a unique social security number), where this identifier is in general use, and where they already have an official e‐mail address or electronic mail box that is used in communications with public authorities. But even in such contexts, there will be a small group of people who are exempted from the use of electronic communications for a variety of reasons and a meta consent system must include a mechanism by which they can easily register their meta consent preferences.
Researchers would interact with ‘the other side’ of the meta consent platform. At the same time as they are submitting their data requests to the relevant holders of the data they would submit a specification of the position of their project in relation to the values included in the meta consent model and a specific information sheet and consent form to the meta consent platform. The platform would then 1) issue specific consent requests to those data subjects who have made this choice and predefined broad consent forms to data subjects who have this preference and have not previously given broad consent for the relevant type of research, 2) collect consents and refusals, and 3) provide data providers with a list of those data subjects who allow their data to be used, i.e. those who have provided either blanket, broad or specific consent covering the specific research project, and those who have not stated a preference but who are covered by a default.
In addition to the functions that are necessary for meta consent the system could also easily be designed to produce individualized feedback to users on when, and for what purposes their data had been used, if they wanted to have such feedback. And it could produce general information about the overall use of health data for research. The system would already contain the information necessary to produce both individual and general feedback, and it would be easy to extract. Adding a feedback functionality would incorporate an important aspect of the dynamic consent model into the meta consent system.21(25–27)
The six elements of meta consent – Formal conditions and substantial choices
We started this section by listing the six formal conditions of the model of meta consent. Subsequently we have suggested how each of the conditions should be should be met in a specific, substantive implementation of meta consent. We have in this process only briefly sketched our reasons for making the particular choices. All of these choices may be subjected to further analysis and criticism, and they may eventually be revised without compromising the formal conditions of the model of meta consent. That is, there are many alternative possible implementations of meta consent that satisfy the formal conditions, and which one to choose depends on the context of implementation. In the remainder of this article, however, we shall be arguing that any model of meta consent satisfying the formal conditions ceteris paribus will improve the conditions for individual decision‐making.
Meta Consent – Improving Conditions of Decision‐Making
As already argued, meta consent provides an individual with an opportunity to express a preference that is likely to be relevant to and valuable for the individual. This is both part of the rationale behind the model of meta consent and also, clearly, a strength in comparison with alternative models of consent. We believe, however, that in the particular context of secondary use of health data, the model of meta consent is also preferable to competing alternative models of consent because of improving the conditions of individual decision‐making concerning participation in research. More specifically, the possibility of providing meta consent enables preferences that are 1) more informed and deliberated, and 2) more consistent to a greater degree than alternative models of consent. In the following we aim to show 1) and 2) in turn.
Maximally informed and deliberated preferences
It seems that regardless of the justification of a requirement of informed consent it is desirable that any implementation of such a requirement promotes informed and deliberated preferences regarding participation in research. In slightly different words, a model of informed consent should aim to provide the individual with conditions of decision‐making that furthers informed and deliberated preferences concerning participation in research. Although different models of informed consent may lead to informed and deliberated preferences concerning participation in research passing a threshold of acceptability, it seems that we should, all other things being equal, prefer a model of informed consent that lead to more informed and deliberated preferences than alternative models of consent. That is, we should prefer a model that under the given circumstances maximizes the level of information and deliberation on which decision to consent or refuse consent is based, without sacrificing other important considerations
Recent research suggest that there is an intricate balance between the number of consent requests, the information provided, and the level of information and deliberation a decision to consent or refuse consent is actually based on. Thus the provision or refusal of consent may become routinized. Routinization occurs when the provision or refusal of consent becomes an act of routine, i.e. a habitual, unreflective act.22 In a recent study on consent behaviour in relation to the use of webportal containing personal health data, we have provided evidence of routinization.23 The users of the portal are required to consent to the terms and conditions of use and the data protection policy of the site before use on the basis of being informed about these. Taking a low degree of reading of relevant information material to be indicative of routinization, the study finds that 79% of respondents read half or less of the relevant information materials before using the portal, indicating a significant degree of routinization among the users. Among the main reasons for not reading are the high general frequency of having to read such material, the length of the material and the time it takes to read the material. Some also provide as reason for not reading that the text is incomprehensible and that the consent process is just 'a hurdle to be overcome’. None of these reasons indicate that the users have reflected on the conditions of use and the data protection policy without having read the relevant information material, and therefore they are fully consistent with the routinization indicated by their failure to read. Although there are differences between the context of a health related website and the research context, this study certainly provides evidence to the effect that consent may become routinized. The possibility of routinization shows that an individual's preferences concerning whether or not to participate in research cannot simply be thought of as being based on information and reflection, or lack of it. The provision or refusal of consent will to very different degrees be based on information and deliberations on this information. Note that routinized choices may still reflect an individual's preferences but, except in cases where people have blanket preferences, the fit between choice and preference is merely coincidental. That is, the person making the routinized choice does not know whether it fits his or her preferences.
The evidence of routinization has implications for the ideal of maximizing the level of information and deliberation underlying an individual's decision to consent or refuse consent. Thus it suggests that there is an inverse proportionality such that the more information that is provided, including the more often information is provided and consent requested, the more often consent decisions are made without the consenter being truly informed and lacking the basis of reflection. In the debate on existing models of consent, proponents of specific consent have argued that broad consent cannot be informed consent since only at the time of requesting consent can it provide information in broad, abstract categories about future research project for which an individual's data and biological material may be used.24 For a person to be truly informed in the sense of being able to completely determine if all aspects of future research are something to which they can consent requires a great deal of information about the specific details of a research project. Even if we accept this view of what informed consent should enable the individual to evaluate, the evidence of routinization suggests that this will in practice not lead to maximally informed and reflected decisions, but rather to uninformed and unreflected decisions. On the contrary, if the individual is approached often with consent requests and information about the specific details about a research project – and it is difficult to see how a model of specific consent should not lead to many consent requests – it is likely to cause the routinization of consent. The risk of routinization can be modified by limiting the amount of information provided each time, but routinization is not sector specific and consent may become routinized because of practices in a different sector, e.g. in relation to internet shopping, downloading of software and apps etc. If routinization is already present, the length of information provided is likely to have minor effect on whether a routinized decision will be made. A model of broad consent is not, it seems, faced with this problem since it limits the number of requests to the individual by asking for consent for broad categories of research. However, a model of broad consent will not lead to maximally informed and deliberated preferences for consent either. Thus a model of broad consent limits an individual's access to specific information about a research project even if the individual has a strong preference for making a consent decision on the basis of specific information.
We argue that meta consent enables maximally informed and deliberated preferences to a greater degree than both the models of specific and broad consent. On the one hand, meta consent empowers the individual to limit the consent requests – and the informational material to process – by providing broad consent, blanket consent or refuse consent. These types of consent will, to different degrees, limit the number of future consent requests – the limiting case being no future consent requests – and this will obviously reduce the threat of routinization. On the other hand, it also empowers the individual to require information and consent requests for each individual research project. In this way meta consent balances between the threats of the consent being uninformed and unreflected as a result of being based on too little specific information and the threat of consent being uninformed and unreflected as a result of too much information through too many repeated consent requests, leading to the routinization of consent. Meta consent allows persons to choose this balance for themselves, based on their own preferences and their own knowledge about their personality and cognitive style.
Note also, very importantly, that there is a potential secondary benefit of meta consent for the attempt to protect and further informed and reflected consent decisions. Thus there seems to be a significant difference between the situation in which a consent request – as in the models of specific and broad consent – is forced upon an individual, and the situation in which consent request is actively and voluntarily wished for by the individual through a previously provided meta consent. Reflection on the nature and purposes of research seems more likely to happen if an individual has previously expressed an explicit interest in providing consent to a given type of research.
Maximally consistent preferences
Parallel to our considerations about the desirability of models of informed consent that further informed and deliberated preferences, it seems equally desirable that a model of consent promotes consistency in preferences concerning consent. Consistency in preferences may be defined generally as a matter of entertaining identical preferences in relevantly identical situations. If a person's choice in a given situation is based on the person's own preferences, failure to make the same choice in relevantly identical situations will imply a failure by the person to act on his or her own, true preferences. Ultimately it follows that a model of informed consent that enhances a person's ability to exhibit consistency in preferences concerning participation in research enhances that person's ability to act on his or her true preferences.
The model of meta consent aids a person in exhibiting consistency in preferences concerning participation in research. It aids a person in exhibiting consistency by presenting a comprehensive number of areas or fields in which consistency may be exhibited. Thus those variables of meta consent that allow for a person to make a fine‐grained choice concerning how and when to provide consent, are in effect also variables that may be of relevance for showing consistency in preferences. Thus a person may exhibit consistency in his or her consent‐choices concerning:
Types of consent
Types of data, and
Types of research institutions, and
The possibility of changes.
A person may hold values, vulnerabilities and trust dictating that he or she should always be asked for consent for a specific research project regardless of the type of data or research institution. Through meta consent this person may consistently express this preference for research on different types of data by asking for specific consent requests. A person may hold values, vulnerabilities and trust that dictate that there are relevant differences between kinds of data or kinds of research institutions such that specific consent is only required in particular for particular combinations of these variables. Meta consent allows for the consistent expression of such preferences. And, perhaps most importantly, meta consent allows for consistency across time. Thus meta consent will continuously serve as a reminder to a person of how that person has balanced values, vulnerabilities and trust in the past in relation to research.
Alternative models of consent – specific or broad consent – does not allow the individual to have different preferences concerning future consent requests, and therefore obviously cannot provide the individual with an option to be consistent in relation to this specific type of preference.
Meta Consent – Objections and Responses
There are two main objections to the model of meta consent:
-
1)
It impedes research
-
2)
It does not provide adequate protection of a person's interests etc.
We shall deal with each of these objections in turn.
Protection of research
It has been claimed in relation to our initial statement of the meta consent idea that if meta consent is implemented it will impede valuable research.25(31) It will a) create new burdens and costs for researchers, and an unnecessary layer of bureaucracy,26 and b) produce consent bias.27 Note that this objection can only be made in a context where researchers already have easy access to data and tissue, and where there are no or very limited requirements of consent. Note also that this objection is not specific to meta consent. It applies equally to any other model introducing a substantive requirement of consent for the secondary use of data.
It is an open question whether a regulatory system for the secondary use of health data and tissue could be justified without any requirements of consent or possibilities of opt‐out. We believe that a proper balancing of public and individual interests makes some form of consent and/or opt‐out necessary. To prove this conclusively is, however, outside the scope of this article. It is worth noting that few countries have implemented 'consent‐free’ regulations in this area.
If the need for some requirement of consent and/or opt‐out is accepted, the issue becomes comparative: Is meta consent more or less likely to impede research than alternative models of consent and/or the possibility of opt‐out? Let us begin with opt‐out. Meta consent will actually enhance the possibility of doing research and reduce the risk of consent bias if some of the people who would opt‐out of research completely if offered that option only, 'opt‐in’ to some forms of research under conditions of meta consent. Persons may have so strong objections against one particular kind of research that they choose to opt‐out of all research if that is the only way in which they can ensure that their data is not used for research they find objectionable. Meta consent allows them to specifically opt‐out of such research while at the same time making their data and tissue available for other kinds of research.
The issues around consent are more complicated. All consent models impose some burdens and costs on researchers but these have been considerably reduced by the introduction of information technology and its widespread use in society. It is difficult to estimate the relative burden and costs associated with different models of consent except that a model of specific consent is likely to be the most expensive because it will generate the most consent requests. In terms of the problem of consent bias a model of meta consent seems likely to be a lesser evil than models of specific and broad consent for two reasons: 1) it allows people to provide broad and blanket consent if they wish to, and 2) it operates on a ‘liberal’ default setting of broad or blanket consent in the cases where people have not made any choices.
Protection of individual interests
An almost diametrically opposite objection to the model of meta consent is that it does not provide adequate protection of an individual and his or her interests. Meta consent includes the options of broad and blanket consent. Such forms of consent do not adequately protect individuals because a) they are not and cannot be provided with sufficient information to make a truly informed choice, and b) by choosing one of these options individuals may come to participate in research which they would have refused to participate in if asked about the specific project.
With regard to a). First, it is not at all clear that an adequate protection of a person through consent requires information on a very specific level, e.g. the level of information specified for informed consent in the Helsinki Declaration.28 If a person holds few and general preferences of relevance for research, then information about future research projects in general terms may be sufficient for the individual to determine if participation or non‐participation is to be preferred. If, for instance, the only relevant interest of a person is a preference for health‐related research, then information in general terms about the character of future research projects may be sufficient for this person to express an informed preference for participation in this research. Second, and more importantly, the problem may be overridden by deeper problems for any model of informed consent. If routinization, as previously argued, is a real threat to the ability of informed consent to protect individual interests and preferences, and if routinization is in part triggered by the number of consent requests directed to a person, then we have a strong reason to limit the number of such requests. Broad and blanket consent limit the number of requests to the individual, and by incorporating these in the model of meta consent we leave it to the individual to balance the interest in specific information about research projects against trust in researchers and the interest in not being overloaded by consent requests and other related interests and preferences. Thereby the model of meta consent allows individuals to make what may – given their individual psychology and interests – be an inescapable trade‐off between high levels of detailed information and a limited number of consent requests.29
With regard to b), it may be reasonable for individuals to act on their preferences in relation to frequency and specificity of consent (see above” The basic idea”) even in the knowledge that this restricts future choices. We generally accept that people can bind their future selves in a variety of ways that may come into conflict with their later preferences and interests. It is difficult to see why secondary research use of data should be an exception. That I later come to regret a decision does not in itself show that the decision was not reasonable or right, when I first made it!
Having considered these objections we might in conclusion note that their diametrically opposed nature could indicate that meta consent is located somewhere in the region of the golden mean.
Biographies
Thomas Ploug is Professor of Applied Ethics and director of the Centre for Applied Ethics and Philosophy of Science at Aalborg University Copenhagen. His research centres around issues of informed consent and personal autonomy, including models of consent, consent behaviour and the tensions between the application of ‘nudging strategies’ and informed consent.
Søren Holm is a Danish medical doctor and philosopher. He is Professor of Bioethics at the Centre for Social Ethics and Policy, University of Manchester, UK, and holds part‐time Chairs in Medical Ethics at the University of Oslo, Norway and the University of Aalborg, Denmark. He has a long standing research interest in informed consent.
Footnotes
Information Commisioner's Office. 2014. Big Data and Data Protection [Internet]. Information Commisioner's Office; Available from: https://ico.org.uk/media/for-organisations/documents/1541/big-data-and-data-protection.pdf; Institute of Medicine; 2012 Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington DC: The Institute of Medicine.
This problem is further exacerbated when old data sources are digitized.
Faden R.R. & Beauchamp T.L.. 1986. A history and theory of informed consent. Oxford: Oxford University Press.
G. Dworkin 1988. The Theory and Practice of Autonomy. Cambridge: Cambridge University Press; N.C. Manson & O. O'Neill. 2007. Rethinking informed consent in bioethics Vol. 1. Cambridge University Press Cambridge; Available from: http://www.langtoninfo.co.uk/web_content/9780521697477_frontmatter.pdf [Accessed 24 Oct 2014].
T.L. Beauchamp & J. F. Childress. 2001. Principles of Biomedical Ethics 5th edn. USA, Oxford University Press, p.472; F.G. Miller. Research on Medical Records Without Informed Consent. J Law Med Ethics. 2008 Sep 1;36(3):560–6.
B. Godard, J. Schmidtke, J‐J. Cassiman & S. Aymé. Data storage and DNA banking for biomedical research: informed consent, confidentiality, quality issues, ownership, return of benefits. A professional perspective. Eur J Hum Genet 2003;11:S88–122; C. Safran, M. Bloomrosen, W.E. Hammond, S. Labkoff, S, Markel‐Fox, P.C. Tang, et al. Toward a national framework for the secondary use of health data: an American Medical Informatics Association White Paper. J Am Med Inform Assoc. 2007;14(1):1–9.
F.G. Miller. op, cit. note 5.
O. O'Neill. 2002. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press;.
Miller, op, cit. note 5.
M.A. Rothstein & A.B. Shoben. Does Consent Bias Research? Am J Bioeth 2013;13(4):27–37; M.A. Rothstein. Currents in Contemporary Ethics. J Law Med Ethics 2009 Sep 1;37(3):507–12; M.E. Kho, M Duffett, D.J. Willison D.J. Cook & M.C. Brouwers. Written informed consent and selection bias in observational studies using medical records: systematic review. BMJ. 2009; 338:866.
J. lsen. Meta consent – A workable procedure in the area of Big Data? BMJ online. Available at: http://www.bmj.com/content/350/bmj.h2146/rr [accessed 10 Dec 2015]
K.L. Hudson & F.S. Collins. Bringing the Common Rule into the 21st Century. N Engl J Med. 2015; 10;373(24):2293–6.
V. Arnason. Coding and Consent: Moral Challenges of the Database Project in Iceland. Bioethics 2004;18(1):27–49; M.G. Hansson, J. Dillner, C.R. Bartram, J.A. Carlson & G. Helgesson. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol 2006;7:266–9; M.F.A. Otlowski. 2009. Developing an Appropriate Consent Model for Biobanks: In Defence of “Broad” Consent. In: Principles and Practice in Biobank Governance [Internet]. J. Kaye J, & M. Stranger, eds. Farnham, Ashgate Publishing; 79–92. [cited 2014 Sep 24] Available from: http://ecite.utas.edu.au/60622; B. Hofmann. Broadening Consent: And Diluting Ethics? J Med Ethics 2009; 35(2):125–9; B. Hofmann, J.H. Solbakk & S. Holm. 2009. Consent to Biobank Research: One Size Fits All? In: The Ethics of Research Biobanking. D.H.H. Solbakk, D.S. Holm & D.B. Hofmann, eds. USA, Springer: 3–23. [cited 2014 Sep 24]. Available from: http://link.springer.com/chapter/10.1007/978-0-387-93872-1_1; J.R. Karlsen, J.H. Solbakk & S. Holm. Ethical Endgames: Broad Consent for Narrow Interests; Open Consent for Closed Minds. Camb Q Healthc Ethics 2011; 20:572–83; G. Helgesson In Defense of Broad Consent. Camb Q Healthc Ethics 2012; 21:40–50; M. Sheehan. Can Broad Consent be Informed Consent? Public Health Ethics 2011 Aug 3;phr020; K.S. Steinsbekk, B. Kåre Myskja & B. Solberg. Broad consent versus dynamic consent in biobank research: Is passive participation an ethical problem? Eur J Hum Genet. 2013;21(9):897–902.
J. Kaye, L. Curren, N. Anderson, K. Edwards, S.M. Fullerton, N. Kanellopoulou et al. From patients to partners: participant‐centric initiatives in biomedical research. Nat Rev Genet 2012; 13(5): 371–376; N.K. Kanelloupoulou, J. Kaye, E. Whitley, S. Creese, D. Lund, K. Hughes. Dynamic consent–a solution to a perennial problem. BMJ 2011; J. Kaye, E.A. Whitley, D. Lund, M. Morrison, H. Teare, K. Melham. Dynamic consent: a patient interface for twenty‐first century research networks. Eur J Hum Genet [Internet]. 2014 May 7 Available from: http://www.nature.com/ejhg/journal/vaop/ncurrent/full/ejhg201471a.html [accessed 2 Jun 2014].
The Danish Council of Ethics. Research with health data and biological material in Denmark [Internet]. Copenhagen; 2015 p. 42. Available from: http://old.etiskraad.dk/en/Udgivelser/~/media/bibliotek/udtalelser/2015/Research-with-health-data-and-biological-material-in-Denmark-Statement.pdf
P.M. Schwartz. Property, Privacy, and Personal Data. Harv Law Rev 2004;117(7):2056–1283; R. Rao. Genes and Spleens: Property, Contract, or Privacy Rights in the Human Body? J Law Med Ethics. 2007 Sep 1;35(3):371–82.
T. Ploug & S. Holm. Meta consent: a flexible and autonomous way of obtaining informed consent for secondary research. BMJ 2015 May 7;350(may07 31):h2146–h2146; T.Ploug & S. Holm. Going Beyond the False Dichotomy of Broad or Specific Consent: A Meta‐Perspective on Participant Choice in Research Using Human Tissue. Am J Bioeth. 2015;15(9):44–6.
G. Barrett, J.A. Cassell, J.L. Peacock, & M.P. Coleman. National survey of British public's views on use of identifiable medical data by the National Cancer Registry. BMJ 2006 4;332(7549):1068–72; B.S. Buckley, A.W. Murphy & A.E. MacFarlane. Public attitudes to the use in research of personal health information from general practitioners’ records: a survey of the Irish general public. J Med Ethics 2010 Nov 11;jme.2010.03790; B. Campbell, H. Thomson, J. Slater, C. Coward, K. Wyatt & K. Sweeney. Extracting information from hospital records: what patients think about consent. Qual Saf Health Care 2007;16(6):404–8; M.M. Al‐Qadire, M.M. Hammami, H.M. Abdulhameed & E.A.A. Gaai. Saudi views on consenting for research on medical records and leftover tissue samples. BMC Med Ethics. 2010 Oct 18;11(1):18.
O. O'Neill. 2002. Autonomy and Trust in Bioethics Cambridge, Cambridge University Press; 22; O. O'Neill. Accountability, trust and informed consent in medical practice and research. Clin Med. 2004 May 1;4(3):269–76.
H. Busby. Consent, trust and ethics: reflections on the findings of an interview based study with people donating blood for genetic research for research within the NHS. Clin Ethics 2006 Dec 1;1(4):211–5; C.S. Molyneux, N. Peshu & K. Marsh. Trust and informed consent: insights from community members on the Kenyan coast. Soc Sci Med 2005;61(7):1463–73; E. Sala, J. Burto & G. Knies. Correlates of Obtaining Informed Consent to Data Linkage Respondent, Interview, and Interviewer Characteristics. Sociol Methods Res. 2012 Aug 1;41(3):414–39.
J. Kaye, L. Curren, N. Anderson, K. Edwards, S.M. Fullerton, N. Kanellopoulou, et al. From patients to partners: participant‐centric initiatives in biomedical research. Nat Rev Genet 2012;13(5):371–6; N.K. Kanelloupoulou, J. Kaye, E. Whitley, S. Creese, D. Lund & K. Hughes. Dynamic consent – a solution to a perennial problem. BMJ 2011; Kaye et al. op. cit. note 14.
T. Ploug & S. Holm. Informed consent and routinisation. J Med Ethics 2012 2013;39(4):214–8; T. Ploug & S. Holm. Agreeing in Ignorance: Mapping the Routinisation of Consent in ICT‐Services. Sci Eng Ethics. 2013 Oct 30.
T. Ploug & S. Holm. Routinisation of Informed Consent in Online Health Care Systems. Int J Med Inf. 2015;
B. Hofmann, J.H. Solbakk & S. Holm. 2009. Consent to Biobank Research: One Size Fits All? In: D.J.H. Solbakk, D.S. Holm & D.B. Hofmann, eds.The Ethics of Research Biobanking [Internet]. USA, Springer US; 3–23. Available from: http://link.springer.com/chapter/10.1007/978-0-387-93872-1_1 [accessed 24 Sep 2014]; J.R. Karlsen, J.H. Solbakk & S. Holm. Ethical Endgames: Broad Consent for Narrow Interests; Open Consent for Closed Minds. Camb Q Healthc Ethics. 2011;20(04):572–83.
T. Ploug & S. Holm. Meta consent: a flexible and autonomous way of obtaining informed consent for secondary research. BMJ. 2015;7:350: 2146.
F.G. Miller. Research on Medical Records Without Informed Consent. J Law Med Ethics 2008;36(3):560–6; M.A. Rothstein. Currents in Contemporary Ethics. J Law Med Ethics. 2009;37(3):507–12.
Kho et al., op. cit. note 10.
E. Sala, j. Burton & G. Knies. Correlates of Obtaining Informed Consent to Data Linkage Respondent, Interview, and Interviewer Characteristics. Sociol Methods Res. 2012;41(3):414–39; Article 26 in the 2013 version of the Helsinki Declaration lists 11 specific items of information that should be communicated and which the participant should understand, as well as the catch all “any other relevant aspects of the study”.
The meta consent model has a number of similarities to the latest development of ‘dynamic consent’. (Kaye et al. op. cit. note 14.) Both models rely on modern IT‐technology, both allow individuals to state their preferences concerning the future use of their health data and samples, and both enable these preferences to be communicated to researchers. There are also, however, important differences between the two models. The starting point for the development of the two models is different. Dynamic consent was initially developed to solve consent issues in biobanking, whereas meta consent was developed with the aim of handling the consent preferences for a whole population for all kinds of data and biological samples, new or old. It is thus an integral part of the meta consent model that it should engage with each and every citizen in their role as potential participants in ‘big data’ research, since medical research is likely to involve whole population datasets in the future. Because of the linkage possibilities it is also important that a definitive answer can be given to whether researchers can use a particular piece of data in a dataset not previously used for research. The meta consent model is designed to provide a definitive answer by letting individuals design future consent requests on the basis of predefined types of consent, data, and contexts. The proponents of dynamic consent could incorporate this ‘meta aspect’ of the meta consent model, and thereby make their model substantially identical to the meta consent model; but unless and until they do so, the two models are distinct.


