Abstract
Background
The use of quantitative PCR (qPCR) for detection of Bordetella bronchiseptica in bronchoalveolar lavage fluid (BALF) and demonstration of bacteria adhering to ciliated epithelial cells in BALF or bronchial brushing fluid (BBF) has not been assessed in a series of affected dogs. Coinfections can worsen the clinical severity in bordetellosis, but the specific association with Mycoplasma cynos has not been evaluated.
Objectives
To assess the utility of culture, qPCR and cytologic examination of cytospin preparations in the diagnosis of bordetellosis in dogs and the influence of coinfection by M. cynos on disease severity.
Animals
Twenty‐four referred dogs with B. bronchiseptica infection and 10 healthy dogs.
Methods
Retrospective case series. qPCR (B. bronchiseptica and M. cynos) and culture results from BALF were recorded. Cytospin preparations from BALF and BBF were reviewed. qPCR on BALF from 10 healthy dogs were used as negative control.
Results
The BALF culture and qPCR detected B. bronchiseptica in 14/24 and 18/18 dogs, respectively. Coccobacilli were found adhering to ciliated epithelial cells in 20 of the 21 BALF cytologic preparations where epithelial cells were found, and 2/3 BBF cytologic preparations. Quantitative PCR detected a low level of B. bronchiseptica in one healthy dog. The frequency of detection of M. cynos was not significantly different in B. bronchiseptica (9/17 dogs) compared with healthy dogs (2/10 dogs) (P = .09).
Conclusion and Clinical Importance
Quantitative PCR detection of B. bronchiseptica in BALF appears to be a useful diagnostic tool. Cytologic examination of BALF or BBF, when positive, allows a rapid and reliable diagnosis.
Keywords: Bronchial brushing fluid, Clinical pathology, Cytology, Mycoplasma cynos, qPCR
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- BBF
bronchial brushing fluid
- CIRD
canine infectious respiratory disease
- Ct
threshold cycle
- M. cynos
Mycoplasma cynos
- qPCR
quantitative polymerase chain reaction
Bordetella bronchiseptica is a small aerobic gram‐negative rod with coccobacillary appearance, which is regarded as one of the primary causative agents of infectious respiratory disease in dogs (CIRD or « kennel cough »). Despite the widespread availability of B. bronchiseptica vaccines, the infection is still commonly seen in young dogs, especially in boarding kennels. This contagious disease is often self‐limiting, but a wide range of respiratory signs are described, from mild illness to severe pneumonia and death, depending on factors such as the presence of concomitant viral or other bacterial pathogens and immune and vaccination status.1, 2, 3 B. bronchiseptica can exist in the respiratory tract as either a commensal or pathogen.4, 5 Although the bordetellosis is a relatively common infectious disease in young dogs, large and recent descriptive clinical studies of confirmed cases, including referral cases with prior treatment, are not available.
B. bronchiseptica can be detected using bacterial culture, and more recently by PCR analysis of various samples such as pharyngeal swabs,5 bronchoalveolar lavage fluid (BALF), or transtracheal wash.6 As B. bronchiseptica has been isolated regularly in clinically healthy dogs, either by culture from the upper respiratory tracts and lungs3, 4 or by quantitative PCR (qPCR) from nasal and pharyngeal swabs,5 it is unclear how a positive qPCR result from BALF should be interpreted. Although qPCR has the potential to provide quantitative results based on the cycle threshold (Ct) value, it is unknown whether Ct values can help differentiate a carrier state from a pathological infection.
Furthermore, adherence of B. bronchiseptica to cilia via adhesion molecules, such as fimbriae and filamentous hemagglutinin, induction of ciliostasis, and damage to ciliated epithelium have been implicated in bordetellosis pathogenesis.7, 8 Accordingly, pleiomorphic cocci or coccobacilli adhering to cilia of the epithelial cells were occasionally reported as characteristic cytological features in human beings9 and anecdotally reported in dogs diagnosed with bordetellosis.10 Currently, the diagnostic accuracy of cytologic demonstration of B. bronchiseptica organisms in cytologic preparations, such as cytocentrifuged smears of BALF or bronchial brushing fluid (BBF), has not been reported. Moreover, although M. cynos was recently identified as an emerging and possibly lethal pathogen in CIRD,11, 12, 13 the role of Mycoplasma spp. as primary respiratory pathogens remains unclear.14, 15, 16, 17 In dogs with CIRD, the frequency of coinfections with Mycoplasma spp., in particular M. cynos, is unknown.
The aims of this study were: (1) to retrospectively report the signalment, clinical features, and the frequency of coinfection by M. cynos in a series of referral, previously treated dogs or both with B. bronchiseptica infection and (2) to describe performance of qPCR analysis, bacterial culture of BALF, and cytologic examination of BALF, BBF, or both in the diagnosis of B. bronchiseptica infection in dogs.
Materials and Methods
Case Selection and Data Collection
Client‐owned dogs referred to the Liège University Veterinary Small Animal Teaching Hospital between January 2006 and September 2014, and diagnosed with B. bronchiseptica infection, either by positive culture, qPCR, or both of the BALF were retrospectively included in the study. Data were collected from the medical records and included signalment, body weight, clinical signs, type and date of previous vaccination against B. bronchiseptica, hematology, thoracic radiographs results, bronchoscopy and BALF analysis results, and bronchial brushing results when available. Bronchoscopy, bronchoalveolar lavage (BAL) procedure, and BALF laboratory processing were performed as described earlier.18 Briefly, dogs were anesthetized using various anesthetic protocols, according to anesthetist's recommendations and a 5‐minute preoxygenation period was used. Five to 20 mL aliquots (depending of the body weight) of sterile saline (NaCl 0.9%) were instilled twice into a least two different lung lobes via a flexible pediatric endoscope (FUJINON© Paediatric Video‐Bronchoscope EB‐530S) under anesthesia, followed by immediate aspiration by gentle suction. The recovered BALF was immediately processed. Aliquots of naïve BALF were used for quantitative culture (Collard Laboratories, Liège, Belgium), for B. bronchiseptica and M. cynos qPCR (TDDS Laboratories), for total cell count determination using a hemocytometer, as well as for cytospin preparation (centrifugation at 221 g, for 4 minutes at 20°C, Thermo Shandon Cytospin©4). Differential cell counts calculations were established by counting a total of 300 cells at high power field on the cytospin preparation. As in previous study of the authors,19 normal cells counts were considered to be 400–600/μL; BALF cytology was considered normal if <12% of neutrophils. The remaining recovered BALF was centrifuged at 1300 × g for 15 minutes at 5°C. Supernatant and cell pellet issued from the centrifugation were stored separately at −80°C. For bronchial brushing, a 1.3‐mm sheathed cytology brush (OLYMPUS © Disposable Cytology Brush BC‐203D‐2006) was passed through the biopsy channel of the bronchoscope. The brush was extended past the end of the bronchoscope and out of the sheath, rubbed gently back and forth across the mucosal surface of a central airway (a primary or secondary bronchus, depending on the size of the dog), pulled back into the sheath, and removed from the bronchoscope. The brush was then re‐extended out of the sheath, briskly agitated in 2 mL of sterile saline in a sterile cryotube. Cytocentrifuged smears from BBF were then prepared as for BALF (Thermo Shandon Cytospin©4). Cytological preparations of BALF and BBF were stained with a May‐Grünwald‐Giemsa stain and each smear was independently examined and rereviewed by two authors (Tual C. and Ramery E.). Samples were not supposed to be contaminated with oropharyngeal material: no sample contained Simonsiella bacteria nor oropharyngeal squamous cells.
BALF Quantitative Culture
Samples were plated onto several agar plates (Chapman's, Mac Conkey's, CNA and TSS agars) at 35°C for isolation of aerobic organisms (Collard Laboratories, Liège, Belgium). Standard biochemical methods were used to identify cultured bacteria. The threshold used to define clinically relevant bacterial growth was 1.7 × 103 colony‐forming units per milliliter of BALF.19 Bacterial susceptibility testing was performed according to standards of the Belgian Microbiology Society using disk diffusion method.
BALF qPCR for B. bronchiseptica and M. Cynos
Quantitative PCR for B. bronchiseptica and M. cynos testing were performed by a commercial veterinary diagnostic laboratory. The qPCR results were expressed as Ct values. After obtaining the data, Ct values were further categorized into 5 groups, based on the B. bronchiseptica DNA load, as a helping interpretation guide for clinicians: very high load (Ct < 20), high load (20.1–24), moderate load (24.1–28), low load (Ct 28.1–32), and very low load (>32.1).
Control Group and Blank Lavages
To investigate whether a positive qPCR result for B. bronchiseptica might possibly either indicate a carrier state or result from contamination during the procedure, qPCR analysis was retrospectively performed on BALF samples collected from 10 healthy dogs. Bronchoalveolar lavage fluid samples collected from those healthy dogs were available from previous studies for which ethical approval had been previously obtained from the Liège University Local Ethical Committee. Ten control urban or suburban dogs belonging to veterinary staff or students (8) or from shelters (2), with neither history nor clinical signs of respiratory problems, were used. Bronchoscopy, BAL procedure, and BALF laboratory processing had been performed as for B. bronchiseptica dogs, and cytologic examination of cytocentrifuged preparations of BALF was within normal limits in each dog. For the retrospective qPCR analysis, the frozen pelleted cells were thawed and resuspended in a 0.5 mL volume of sterile 0.9% NaCl at the time of qPCR testing.
To further evaluate the risk of samples contamination during the endoscopic procedure and subsequent false positive results, 15 blank lavages of the channel of the bronchoscope were performed randomly over the study period after standardized cleaning and disinfection by a trained technician. Briefly, once the endoscope was disinfected, 5 mL of sterile saline was flushed into the endoscopic channel used for BALF collection and recovered in a sterile cryotube for qPCR assessment. Two blank samples were obtained after endoscopic cleaning subsequently to BALF collection in dogs finally diagnosed with B. bronchiseptica infection.
Statistical Analysis
Statistical analyses were performed with a commercially available software (XLstat software). Data were expressed as median and range for continuous variables and as proportion for categorical variables.
Chi‐square test (for n > 5) and exact Fisher's test (for n ≤ 5) were used to compare the frequency of qPCR detection of M. cynos between healthy and B. bronchiseptica dogs.
Values of P ≤ .05 were considered significant.
Results
Signalment and Clinical Findings
Clinical records from 24 dogs diagnosed with B. bronchiseptica infection between 2006 and 2014 were retrospectively reviewed. Median age was 0.5 years (range 0.2–3.8) and median weight was 4.8 kg (range 1.3–30). french bulldogs, cavalier king charles spaniels, and yorkshire terriers accounted for 15/24 cases. Vaccination status was recorded for 22 dogs. Seven of them had been vaccinated against B. bronchiseptica infection: 2 had received intranasal vaccine (IN) [Nobivac BbPi©, Intervet International] (No 4, 10) and 5 subcutaneous (SC) vaccine [Pneumodog©, Merial Sanofi] (No 3, 5, 15, 17, 19). Five dogs were vaccinated within 3–6 weeks before onset of clinical sign (No 4, 5, 15, 17, 19) and in the last 2 dogs, a mild cough was already reported at the time of vaccination (No 3, 10).
Chronic productive harsh and hacking daily cough of at least 1 week to 2 year's duration (median = 2 months) was present in all cases. In all but 2 dogs, cough was reported since acquisition. Laryngo‐tracheal reflex (tracheal sensitivity) was positive in all but three dogs. Thirteen dogs were moderately to severely dyspneic; in 7 of these 13 dogs, hospitalization and oxygen supplementation were required. A variable degree of lethargy and mild fever (39.1° to 39.6°C) were observed in 12 and 4 cases, respectively. A mild serous or sero‐mucous nasal discharge was reported in 4 dogs. Twenty dogs had been unsuccessfully treated with oral antibiotics including amoxicillin or amoxicillin/clavulanic acid (n = 17), doxycycline (n = 6), marbofloxacin or enrofloxacin (n = 6), cephalexin (n = 2) and lincomycin (n = 2). Among these dogs, at the time of diagnosis, antibiotics had been stopped for less than 48 hours in 6 dogs and at least 1 week before BALF collection in the 14 remaining dogs.
Mild to moderate neutrophilic leukocytosis was present in 14 dogs (for dogs with leukocytosis, median value = 23.4 × 103/μL, range 15.9–45.5]). A mild leukopenia was observed in 1 dog (No 7, 5.8 × 103/μL). Radiographic features included generalized or caudo‐dorsal bronchial or bronchointerstitial pattern (n = 14), peri‐bronchial pattern (n = 4), and alveolar changes (n = 11). In almost all dogs with alveolar pattern, lesions were considered severe and predominantly presented as multifocal patches, often more pronounced in ventro‐cranial lung areas. Bronchoscopy revealed hyperemic and edematous mucosa, thickened bronchi or thick and white‐yellow or hemorrhagic material, in moderate to large amounts, within the bronchial and sometimes the tracheal lumens in all dogs (Fig 1).
Figure 1.
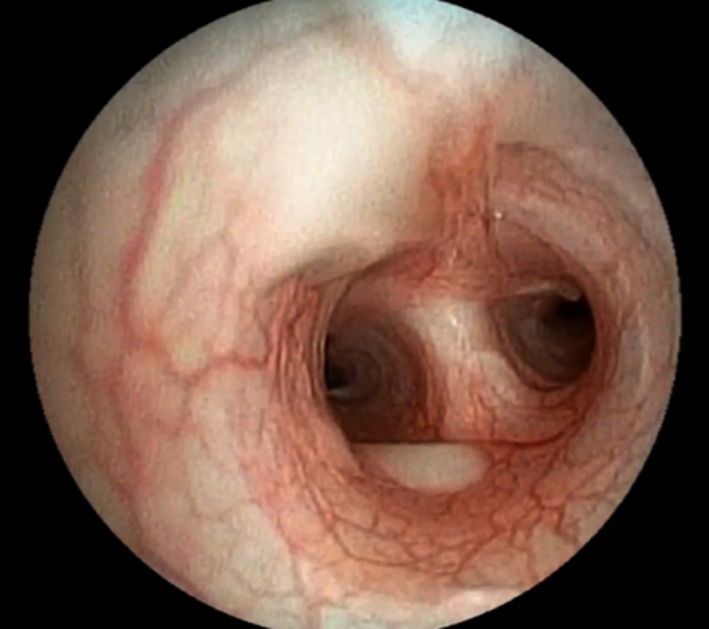
Bronchoscopic view of tracheobronchic bifurcation showing congested mucosa and thick and white‐yellow material (Dog n°3, sharpei, female, 6 months old).
Total cell count of BALF varied from 500 to 39,000 cells/μL (median = 2030 cells/μL) with predominantly neutrophilic inflammation in all but 1 case (dog No 3, cytology showing predominance of macrophages). For 22 dogs, the accurate percentage of BALF neutrophils was available (median 89%, range 11–98%). Neutrophils appeared highly segmented with frequent degenerative changes and sometimes a pyknotic aspect of the nucleus. Ciliated epithelial cells were present in cytologic preparations from 21 BALF samples.
B. bronchiseptica Diagnostic Test Results
Bronchoalveolar lavage fluid bacterial culture was positive for B. bronchiseptica in 14/24 dogs (58%). Available antibiotic susceptibility was available in 13 of them, and revealed that all strains were susceptible to gentamicin and fluoroquinolones; 10/13 and 9/13 strains were susceptible to doxycycline and amoxicillin/clavulanic acid, respectively. Only 2/13 strains showed susceptibility to cephalexin and 3rd generation cephalosporins. Among the 13 dogs for which antibiotic susceptibility was available, 11 dogs were unsuccessfully treated with antibiotic drugs prior BALF collection; 9 of these 11 dogs received 1 (1 dog) or 2 (8 dogs) appropriate drugs, based on the susceptibility patterns of the isolated organisms.
Pleiomorphic cocci or coccobacilli were found adhering to the cilia of the epithelial cells in 18/24 cytocentrifuged BALF preparations (75%) and in two cytocentrifuged BBF preparations (Fig 2). In three of the four dogs for which cytological examination of BALF was negative (No 2, 6, 13), no ciliated epithelial cells were found on the cytospin preparation; BBF was not performed and bacterial culture was also negative. In the fourth dog (No 14), bacterial culture yielded B. bronchiseptica growth and identification but both cytological examination of BALF and BBF failed to detect coccobacilli even though ciliated epithelial cells were observed on the cytological preparations.
Figure 2.
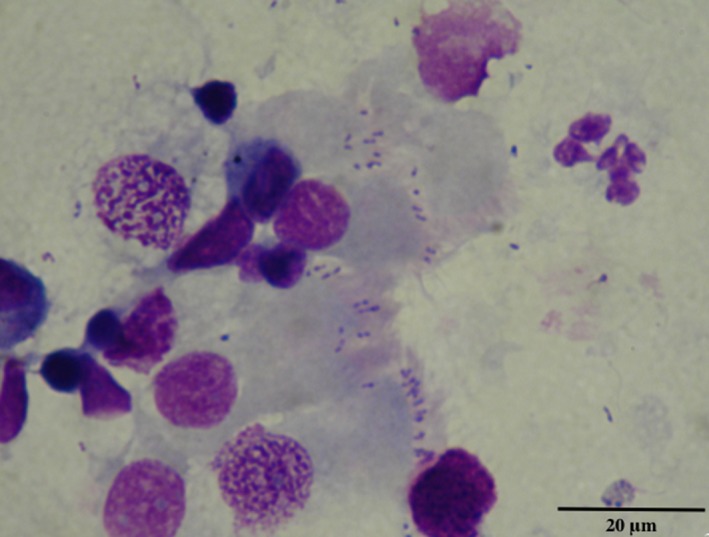
Cytological detection of pleiomorphic cocci or coccobacilli adhering to the cilia of the epithelial cells of BALF (May‐Grünwald‐Giemsa stain, ×400 magnification, Dog n°8, Yorkshire Terrier, female, 5 months old).
The qPCR was positive for B. bronchiseptica in 18/18 dogs tested; a qPCR Ct‐value data for B. bronchiseptica was available in all but one of them.
In dogs with high and very high B. bronchiseptica DNA loads (ie, with Ct values lower than 24) (n = 9), cytologic examination of BALF was always positive, whereas culture was positive in 6 of them. In dogs with moderate B. bronchiseptica DNA load (ie, Ct values between 24.1 and 28) (n = 7), cytology of BALF or BBF and culture were positive in three and two cases, respectively. One BALF showed a low B. bronchiseptica DNA load (ie, Ct value higher than 28.1) and cytology of BALF from this dog was positive. Dogs with positive cytology had higher B. bronchiseptica DNA load (mean Ct = 21.2) compared with dogs with negative cytology (mean Ct = 26.1) (P = .009).
Among 17 dogs in which qPCR data for M. cynos were available, 9 dogs (53%) were positive.
Other common bacteria were also cultured in 8/24 (33%).
Control Group and Blank Lavages
The control group was composed of 10 dogs (6 females, 4 males) of various breeds aged from 2 to 11 years (median = 6 years). Bronchoalveolar lavage fluid culture and cytologic examination of cytocentrifuged smears were negative for B. bronchiseptica in all control dogs. In one of them, qPCR detected a very low B. bronchiseptica DNA load (Ct = 33.44).
M. cynos was detected by qPCR assay in 2 of the control dogs. The frequency of detection of M. cynos was not significantly different in B. bronchiseptica (9/17 dogs) compared with healthy dogs (2/10 dogs) (P = .09).
Among the 15 blank lavages, B. bronchiseptica qPCR was positive at very low grade (Ct = 35.6) in only one case. This blank lavage was performed after cleaning of the endoscope subsequently to bronchoscopical examination in a dog ultimately diagnosed with B. bronchiseptica.
Discussion
This study suggests that, in this specific population of affected dogs, referred with mostly chronic clinical signs, a qPCR detection of B. bronchiseptica in BALF is a useful diagnostic test. Indeed, qPCR was positive, with indication of moderate to very high DNA content, in all suspected animals tested, even when bacterial culture or cytologic detection were negative. This study also shows that cytologic examination of BALF and BBF cytospin preparations allows rapid and reliable suspicion of B. bronchiseptica infection in the great majority of dogs; this method seems to be more sensitive than BALF culture.
The present case series confirms that B. bronchiseptica infection mainly affects young dogs from small breeds and that clinical presentation can be quite severe. Almost all dogs from the present case series were presented with chronic cough (median = 2 months), while CIRD is classically associated with a shorter duration of respiratory signs (less than 2 weeks).3 Of interest, cavalier king charles spaniels, yorkshire terriers, and french bulldogs represented more than a half of the study population. Since the majority of the dogs affected in this case series were very young, and since three young french bulldogs developed concomitant generalized demodicosis, an inadequate or impaired immune response can be strongly suspected but was not investigated. In cavalier king charles spaniels, previous descriptions of Pneumocystosis jiroveci infection, some of them with concomitant demodicosis, have suggested that immune incompetence underlies the susceptibility of this breed to infectious diseases.20
Pleiomorphic cocci or coccobacilli were found adhering to the ciliary apexes and interciliary spaces of the epithelial cells in 20/24 cytocentrifuged BALF, BBF, or both, preparations. In comparison, only 14/24 dogs had positive culture and only 1 dog had negative cytology despite a positive culture. The pathogenesis of bordetellosis involves adherence of the B. bronchiseptica to the cilia of the respiratory tract.7 In a previous experimental study using tracheal cell culture models in dogs, electron microscopy allowed observation of bacteria adhering to cilia of tracheal epithelial cells, although optic microscopy was not investigated.8 Until the present study, demonstration of bacteria adhering to ciliated epithelial cells obtained by BAL or per‐endoscopic bronchial brushing from dogs with spontaneous B. bronchiseptica infection has not been assessed in the diagnosis of bordetellosis in dogs. Adhered bacteria were observed by standard cytology in all dogs with high and very high B. bronchiseptica DNA loads. These results suggest that standard cytologic examination of cytocentrifuged preparations of BALF and BBF allows reliable suspicion of B. bronchiseptica in the vast majority of dogs. Besides, cytologic preparations can be immediately assessed, before results of bacterial culture or qPCR analysis are obtained. Although in some cases, B. bronchiseptica can be formally identified, based on cytological findings alone, its strict identification might be dependent on the quality of the cytologic preparations as well as on the expertise of the cytologist. In theory, any coccobacillus (such as Pasteurella sp. or Acinetobacter sp.) superimposed on the apical side of ciliated cells would be falsely diagnosed as B. bronchiseptica. In such cases, confirmation is strongly advised, and in this respect, qPCR on BALF appears particularly useful. Furthermore, effects of delay of BALF processing or speed and time of cytocentrifugation on the number of intact ciliated epithelial cells were not anticipated in this study; any factor affecting the integrity of ciliated epithelial cells would reasonably decrease the relative sensibility of the BALF cytology in the diagnosis of B. bronchiseptica infection.
In a recent study, BBF was shown to be inferior to BALF for the diagnosis of septic inflammation in dogs.21 However, among the 6 dogs with negative BALF cytology, BBF cytology was positive in 2 of the 3 dogs in which this procedure was performed. This suggests that BBF could be the sample of choice for cytologic investigation of B. bronchiseptica. This is logical since higher numbers of ciliated tracheobronchial cells are recovered by brushing procedures, compared to BALF, which only contains a low percentage of ciliated epithelial cells (<1%).22, 23, 24
In this study, bacterial culture was negative in 10/24 dogs while qPCR confirmed the presence of B. bronchiseptica in 18/18 tested dogs. This is not surprising since sensitivity of bacterial culture for the diagnosis of Bordetella spp. infection (B. bronchiseptica, B. pertussis, and parapertussis) has been reported to be lower than PCR in a human study.25 Currently available qPCR methods are able to detect as few as 10 genome copies of B. bronchiseptica/μL.26 However, the great majority of infected dogs had received various antibiotic drugs before diagnosis and a non‐negligible proportion of them had received antimicrobial treatment within the 48 hours before the bronchoalveolar lavage, which is susceptible to affect culture results. Because infection and aerosol shedding can persist for weeks,3 positive results of both culture and PCR must be interpreted cautiously. In a recent study, B. bronchiseptica was detected in up to 45% of healthy dogs using qPCR analysis on nasal and pharyngeal swabs5, but bronchial samples were not assessed. Comparative assessment of qPCR results in affected dogs, healthy dogs, and blank lavages of the endoscope led us to consider the Ct values, as well as their interpretation into B. bronchiseptica DNA loads, as a useful and accurate tool to distinguish true infection from carrier state or environmental contamination. Indeed, moderate to very high loads were found in all but 1 affected dog while the load was very low in both the single blank and the single control sample, which were positive. We feel confident that the case with low B. bronchiseptica DNA load (high Ct value) (No 16) did suffer from bordetellosis since cytology was positive.
Despite appropriate cleaning and disinfection of the endoscopic material, a very low B. bronchiseptica DNA load positive qPCR was obtained in 1 blank lavage sample; this observation highlights the possibility of false positive results because of contamination during the BALF procedure from previously sampled cases. The question of appropriate disinfection should thus clearly be considered before interpretation of qPCR results of BALF. Therefore, it is unclear how the positive qPCR result from the healthy dog in this study should be interpreted. Whether it potentially indicates a carrier state or simply results from a contamination is unknown.
According to the results of this study, positive culture or positive cytology or moderate to very high B. bronchiseptica DNA loads can be proposed as criteria to establish a definitive diagnosis of bordetellosis in dogs with compatible clinical signs. However, such findings do not rule out the possibility of concurrent infections contributing to clinical presentation. Moreover, this study included a challenging population of dogs being referred, frequently with prior unsuccessful treatment that can have interfered with culture or cytology more than PCR; in such dogs, qPCR on BALF could be assumed to be more sensitive than culture or cytology. However, BALF analysis should always include semiquantitative bacterial culture in case of clinical suspicion of B. bronchiseptica infection, in order to identify common bacterial coinfections and to obtain antimicrobial susceptibility patterns.
This study had some limitations. The study group is comprised of dogs that were referred, and do not represent the majority of dogs with canine infectious respiratory disease complex. Nearly all had received prior antibiotic treatment and the duration of signs was not typical (chronic cough), so these dogs might not reflect typical cases. The second major issue concerns the lack of well‐recognized gold standard for the diagnostic of clinical bordetellosis. Besides, because of retrospective nature of the study, prior antibiotic treatment in some dogs may have affected the proportion of dogs with positive quantitative culture for B. bronchiseptica or other concomitant bacteria, proportion of dogs with positive qPCR for M. cynos, and results from all complementary examinations were not available for all included dogs. Among B. bronchiseptica dogs, BBF was performed in only a limited number of them, preventing an adequate comparison and definitive conclusion concerning the relative benefit of the BBF procedure, compared to the BALF one, for cytologic detection of B. bronchiseptica. A prospective study including larger groups of infected and healthy dogs in which BALF, BBF, culture, and qPCR are performed is warranted. Such a study would allow the assessment of the sensitivity and the specificity of each diagnostic test, assuming that qPCR detection of B. bronchiseptica at moderate to very high DNA load in the BALF of a dog with compatible clinical signs might be considered as a gold standard for the definitive diagnosis of canine bordetellosis.
Conclusion
This retrospective clinical case series highlights that B. bronchiseptica infection can have chronic and sometimes severe and fatal clinical presentations and it commonly affects very young dogs of small breeds. Standard cytologic examination of cytocentrifuged preparations of BALF, BBF, or both can be considered as a rapid and inexpensive diagnostic modality. Providing that the sample is adequately collected and processed, qPCR detection of B. bronchiseptica on BALF, with consideration of the B. bronchiseptica DNA load, appears to be the most sensitive method for B. bronchiseptica confirmation in the studied population since qPCR detection of B. bronchiseptica on BALF was positive in all cases tested, including those with negative cytology and bacteriology results.
Acknowledgments
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Preliminary results were presented as poster at ECVIM Congresses 2013, Liverpool, and 2014, Mainz.
References
- 1. Bemis DA. Bordetella and Mycoplasma respiratory infections in dogs and cats. Vet Clin North Am Small Anim Pract 1992;22:1173–1186. [DOI] [PubMed] [Google Scholar]
- 2. Keil DJ, Fenwick B. Role of Bordetella bronchiseptica in infectious tracheobronchitis in dogs. J Am Vet Med Assoc 1998;212:200–207. [PubMed] [Google Scholar]
- 3. Ford R. Canine infectious tracheobronchitis In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 3rd ed Saint Louis: Saunders Elsevier; 2006:54–61. [Google Scholar]
- 4. Chalker VJ, Toomey C, Opperman S, et al. Respiratory disease in kennelled dogs: Serological responses to Bordetella bronchiseptica lipopolysaccharide do not correlate with bacterial isolation or clinical respiratory symptoms. Clin Diagn Lab Immunol 2003;10:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulz BS, Kurz S, Weber K, et al. Detection of respiratory viruses and Bordetella bronchiseptica in dogs with acute respiratory tract infections. Vet J 2014;201:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viitanen SJ, Lappalainen A, Rajamäki MM. Co‐infections with respiratory viruses in dogs with bacterial pneumonia. J Vet Intern Med 2015;29:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bemis DA, Greisen HA, Appel MJ. Pathogenesis of canine bordetellosis. J Infect Dis 1977;135:753–762. [DOI] [PubMed] [Google Scholar]
- 8. Anderton TL, Maskell DJ, Preston A. Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica . Microbiology 2004;150:2843–2855. [DOI] [PubMed] [Google Scholar]
- 9. Tuomanen EI, Hendley JO. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infect Dis 1983;148:125–130. [DOI] [PubMed] [Google Scholar]
- 10. Burkhard M, Millward L. Chapter 5: Respiratory tract In: Raskin RE, Meyer DJ, eds. Canine and Feline Cytology: A Color Atlas and Interpretation Guide, 2nd ed Saunders: Elsevier Inc; 2001:123–170. [Google Scholar]
- 11. Priestnall SL, Mitchell JA, Walker CA, et al. New and emerging pathogens in canine infectious respiratory disease. Vet Pathol 2014;51:492–504. [DOI] [PubMed] [Google Scholar]
- 12. Rycroft AN, Tsounakou E, Chalker V. Serological evidence of Mycoplasma cynos infection in canine infectious respiratory disease. Vet Microbiol 2007;120:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeugswetter F, Weissenböck H, Shibly S, et al. Lethal bronchopneumonia caused by Mycoplasma cynos in a litter of golden retriever puppies. Vet Rec 2007;161:626–627. [DOI] [PubMed] [Google Scholar]
- 14. Chan CM, Ridgway MD, Mitchell MA, et al. Association between Mycoplasma‐specific polymerase chain reaction assay results and oral bacterial contamination of bronchoalveolar lavage fluid samples from dogs with respiratory tract disease: 121 cases (2005–2012). J Am Vet Med Assoc 2013;243:1573–1579. [DOI] [PubMed] [Google Scholar]
- 15. Chandler JC, Lappin MR. Mycoplasmal respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc 2002;38:111–119. [DOI] [PubMed] [Google Scholar]
- 16. Chalker VJ, Owen WMA, Paterson C, et al. Mycoplasma associated with canine infectious respiratory disease. Microbiology 2004;150:3491–3497. [DOI] [PubMed] [Google Scholar]
- 17. Johnson LR, Queen EV, Vernau W, et al. Microbiologic and cytologic assessment of bronchoalveolar lavage fluid from dogs with lower respiratory tract infection: 105 cases (2001–2011). J Vet Intern Med 2013;27:259–267. [DOI] [PubMed] [Google Scholar]
- 18. Dehard S, Bernaerts F, Peeters D, et al. Comparison of bronchoalveolar lavage cytospins and smears in dogs and cats. J Am Anim Hosp Assoc 2008;44:285–294. [DOI] [PubMed] [Google Scholar]
- 19. Peeters DE, McKiernan BC, Weisiger RM, et al. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens in dogs. J Vet Intern Med 2000;14:534–541. [DOI] [PubMed] [Google Scholar]
- 20. Watson PJ, Wotton P, Eastwood J, et al. Immunoglobulin deficiency in Cavalier King Charles Spaniels with Pneumocystis pneumonia. J Vet Intern Med 2006;20:523–527. [DOI] [PubMed] [Google Scholar]
- 21. Zhu BY, Johnson LR, Vernau W. Tracheobronchial brush cytology and bronchoalveolar lavage in dogs and cats with chronic cough: 45 cases (2012–2014). J Vet Intern Med 2015;29:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawkins EC, DeNicola DB, Kuehn NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat State of the art. J Vet Intern Med 1990;4:267–274. [DOI] [PubMed] [Google Scholar]
- 23. Vail D, Mahler P, Soergel S. Differential cell analysis and phenotypic subtyping of lymphocytes in bronchoalveolar lavage fluid from clinically normal dogs. Am J Vet Research 1995;56:282–285. [PubMed] [Google Scholar]
- 24. Rajamäki M, Järvinen A, Saari S, et al. Effect of repetitive bronchoalveolar lavage on cytologic findings in healthy dogs. Am J Vet Research 2001;62:13–16. [DOI] [PubMed] [Google Scholar]
- 25. Reizenstein E, Johansson B, Mardin L, et al. Diagnostic evaluation of polymerase chain reaction discriminative for Bordetella pertussis, B. parapertussis, and B. bronchiseptica . Diagn Microbiol Infect Dis 1993;17:185–191. [DOI] [PubMed] [Google Scholar]
- 26. Koidl C, Bozic M, Burmeister A, et al. Detection and differentiation of Bordetella spp. by real‐time PCR. J Clin Microbiol 2007;45:347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]


