ABSTRACT
Intervertebral disc degeneration proceeds with age and is one of the major causes of lumbar pain and degenerative lumbar spine diseases. However, studies in the field of intervertebral disc biology have been hampered by the lack of reliable cell lines that can be used for in vitro assays. In this study, we show that a chordoma‐derived cell line U‐CH1‐N cells highly express the nucleus pulposus (NP) marker genes, including T (encodes T brachyury transcription factor), KRT19, and CD24. These observations were further confirmed by immunocytochemistry and flow cytometry. Reporter analyses showed that transcriptional activity of T was enhanced in U‐CH1‐N cells. Chondrogenic capacity of U‐CH1‐N cells was verified by evaluating the expression of extracellular matrix (ECM) genes and Alcian blue staining. Of note, we found that proliferation and synthesis of chondrogenic ECM proteins were largely dependent on T in U‐CH1‐N cells. In accordance, knockdown of the T transcripts suppressed the expression of PCNA, a gene essential for DNA replication, and SOX5 and SOX6, the master regulators of chondrogenesis. On the other hand, the CD24‐silenced cells showed no reduction in the mRNA expression level of the chondrogenic ECM genes. These results suggest that U‐CH1‐N shares important biological properties with notochordal NP cells and that T plays crucial roles in maintaining the notochordal NP cell‐like phenotype in this cell line. Taken together, our data indicate that U‐CH1‐N may serve as a useful tool in studying the biology of intervertebral disc. © 2016 The Authors. Journal of Orthopaedic Research Published by Wiley Periodicals, Inc. on behalf of Orthopaedic Research Society. J Orthop Res 34:1341–1350, 2016.
Keywords: nucleus pulposus cell, notochord, chordoma, U‐CH1‐N
Abbreviations
- IVD
intervertebral disc
- NP
nucleus pulposus
- DAPI
diamidino‐2‐phenylindole
- TBRE
T‐box responsive element
- Luc
luciferase
- WT
wild‐type
- MT
mutant
- LV
lentivirus
- Sh
short‐hairpin
- GFP
green fluorescent protein
- MTT
3‐(4,5‐di‐methylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide, yellow tetrazole
- PCNA
proliferating cell nuclear antigen
- ECM
extracellular matrix.
Human intervertebral disc (IVD) is a heterogeneous soft tissue located between the vertebral bodies and provides flexibility and load support in the spine. Degeneration of IVD is causally related to chronic back pain, spinal deformities, and spinal canal stenosis.1, 2 These spinal disorders are the leading causes of disability in the workforce, resulting in large economic and social costs. Therefore, it is mandatory to learn more about the molecular mechanisms underlying IVD degeneration to establish prophylaxis and effective treatment modalities for spinal disorders.
IVD is composed of the inner and outer components; the nucleus pulposus (NP) and annulus fibrosus (AF), respectively. NP is an avascular cartilage‐like tissue that consists of extracellular matrix (ECM) proteins rich in proteoglycans. AF is a fibrous cartilage tissue composed of the inner and outer coaxial lamella.3 NP is derived from the notochord, a rod‐like structure of mesodermal origin. In humans, the number of notochordal NP cells decreases with age, and these cells are rarely present in the NP after adolescence.4 The decline in notochordal cell number in the NP is thought to be causally related to IVD degeneration.4, 5 Therefore, further understanding on the biology of notochordal NP cells may provide the basis for the mechanisms underlying IVD degeneration in humans. However, the study on notochordal NP cells has been proved formidable. Above all, it is highly challenging to obtain enough cells either from mice or human IVD for in vitro analyses. Furthermore, notochordal NP cells are highly sensitive to the changes in the micro‐environment and undergo dedifferentiation when they are cultured on plates, resulting in the loss of their phenotype.6 This problem can be circumvented, to some degree, by maintaining the cells on alginate beads or in agarose; however, gain‐ or loss‐of‐function experiments are not feasible under these experimental conditions.6, 7 For these reasons, a reliable cell line that retains the biological characteristics of notochordal NP cells has been long‐sought after by the researchers in the field of IVD biology.
Because many cell lines that are currently used were derived from tumor tissues, and both notochord and chordoma originate from the same progenitor cells, we sought to identify a chordoma‐derived cell line that retains the biological properties of notochordal NP cells. Most of human chordoma cell lines reported to date have a relatively long doubling time and are not suitable for in vitro analysis.8, 9, 10, 11, 12 In the course of screening, we found U‐CH1‐N, a cell line sub‐cloned from a chordoma‐derived cell line U‐CH1, as a potential candidate.13 U‐CH1‐N has a relatively short doubling‐time compared to other chordoma‐derived cell lines and maintains its phenotype, at least in terms of morphology, over passages under regular culture conditions.13 However, there has been no study to investigate whether U‐CH1‐N cells share biological characteristic with notochordal NP cells.
In the present study, we show that U‐CH1‐N exhibits important biological properties of the notochordal NP cells. U‐CH1‐N cells highly express the NP cell‐specific markers such as T brachyury transcription factor (hereafter referred to as T), keratin 19, and CD24, and produce chondrogenic ECM proteins, including type II collagen and aggrecan. Of note, we found that T, but not CD24, is essential for the maintenance of the NP‐like phenotype in U‐CH1‐N cells. Although further characterization of this cell line is mandatory, our data suggest that U‐CH1‐N will serve as a valuable tool to study the biology of notochordal NP cells in vitro.
MATERIALS AND METHODS
Human Samples and the Ethics Statement
Lumbar spine specimens including the disc tissues with AF and NP were collected from four fetuses (35–40 weeks of pregnancy) that were subjected to autopsy. Samples were immediately fixed in formalin (10%), embedded in paraffin, and cut into 4‐µm sections in sagittal plane. A written informed consent was obtained from all guardians of the participants. The study design and the consent procedure were approved by the Ethics Committee of Keio University School of Medicine (approval number, 20140333).
Cell Lines and Culture Conditions
U‐CH1‐N was generously provided by Dr. Takamitsu Kato, National Institute of Radiological Sciences, Japan.13 Cells were cultured in minimum essential medium (MEM)‐alpha (Sigma–Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS, JRH Biosciences, KS) and antibiotics, and maintained at 37°C in a humidified air atmosphere with 5% CO2. 293T cells were maintained in Dulbecco's Modified Eagles Medium (DMEM) and 10% FBS supplemented with antibiotics.
Immunohistochemistry
The following antibodies were used in the present study; anti‐T (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100), anti‐keratin 19 (Novus, Littleton, CO; 1:100), anti‐CD24 (clone FL‐80, Santa Cruz Biotechnology; 1:100), anti‐CD73 (clone IE9, Santa Cruz Biotechnology; 1:100), anti‐CD90 (clone K‐16, Santa Cruz Biotechnology; 1:100), anti‐CD105 (clone H‐300, Santa Cruz Biotechnology; 1:100), and anti‐Laminin (DAKO, Agilent Technologies, Santa Clara, CA; 1:100). Alexa‐488‐conjugated antibodies against rabbit, mouse, and goat IgG were used as the secondary antibodies (Biosource, Camarillo, CA; 1:200). Antigen retrieval was achieved by pressure‐cooking in citrate buffer (pH 6.0) for 20 min. Diamidino‐2‐phenylindole (DAPI) (Wako, Osaka, Japan; 1:2000) was applied for nuclear staining.
Pellet Cultures in Atelocollagen Gel
U‐CH1‐N cells were suspended in 0.5% atelopeptide collagen solution (Kawaken Fine Chemicals, Japan), 5 mM NaOH, 26 mM NaHCO3, and 20 mM HEPES in α‐MEM (1 × 104 cells/µl). Aliquots of 20 µl in 15‐ml conical tubes (BD Bioscience, MA) were incubated for 30 min at 37°C to form a gel. Subsequently, 1 ml of DMEM with antibiotics was gently poured on the gel. The gel pellets were incubated at 37°C in a mixture of 95% air and 5% carbon dioxide for 21 days. Culture medium was changed every 7 days.14 After the incubation, the pellets were fixed with formalin (10%), embedded in paraffin, and cut into 4‐µm sections. For immunohistochemistry, HRP‐conjugated goat anti‐rabbit IgG (diluted 200‐fold; Sigma–Aldrich) was used as the secondary antibody. Staining was visualized using diamino benzidine (nacalai tesque, Kyoto, Japan). Nuclei were stained with hematoxylin.
Immunofluorescence Microscopy
Cells were plated on flat‐bottom 96‐well plates (5 × 103/well) and cultured overnight. After incubation, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X‐100 in phosphate buffered saline (PBS) for 10 min, blocked with PBS containing 5% FBS, and incubated with antibodies against aggrecan (Abcam, Cambridge, UK; 1:100) or type II collagen (Abcam; 1:100) at 4°C overnight. Following washing, cells were incubated with Alexa‐488‐conjugated anti‐mouse IgG, (Biosource, Camarillo, CA; 1:200) for 1 h at room temperature and with DAPI for nuclear staining. Images were captured using a fluorescence microscopy (Keyence, Osaka, Japan).
Alcian Blue Staining
To visualize the deposition of sulfated glycosaminoglycans, the formalin‐fixed specimens were incubated with 1% Alcian blue 8GX in 0.1 N HCl for 1 h and subsequently washed with 0.1 N HCl.
Real‐Time Reverse‐Transcriptase PCR Analysis
Total RNA was extracted from U‐CH1‐N cells using RNAeasy mini columns (Qiagen, Alameda, CA). cDNA synthesis was performed using oligo‐dT primer and reverse transcriptase (Wako). Quantitative PCR was performed using the SYBR Premix ExTaq II reagent and DICE Thermal cycler (Takara Bio Inc., Shiga, Japan), according to the manufacturer's instructions. The transcript level of β‐actin was used as an internal control. All primers were purchased from Takara Bio Inc. The primer sequences are presented in Table 1.
Table 1.
Gene‐Specific Forward and Reverse Primers
| Gene | Forward | Reverse |
|---|---|---|
| β‐actin | 5′‐TGGCACCCAGCACAATGAA‐3′ | 5′‐CTAAGTCATAGTCCGCCTAGAAGCA‐3′ |
| T | 5′‐GGTGACTGCTTATCAGAACGAGGA‐3′ | 5′‐GAGGATTTGCAGGTGGACACA‐3′ |
| COL2a1 | 5′‐CTCCAATGACGTGGAGA3′ | 5′‐GTCCATGGGTGCAATGTCAA‐3′ |
| AGC1 | 5′‐AGCAGTCACACCTGAGCAGCA‐3′ | 5′‐GTTCAGGCCGATCCSCTGGTA‐3′ |
| COL1a1 | 5′‐GCTTGGTCCACTTGCTTGAAGA‐3′ | 5′‐GAGCATTGCCTTTGATTGCTG‐3′ |
| SOX5 | 5′‐AGGAATAACGTTGGACCATTGTGAG‐3′ | 5′‐TTGAAGTGCAAAGTCAAGGTCAAGA‐3′ |
| SOX6 | 5′‐TGACATGCTGGCTGCTGTTTC‐3′ | 5′‐GGCCACACCCAGTGTTGCTA‐3′ |
| SOX9 | 5′‐CATCAAGACGGAGCAACTGA‐3′ | 5′‐TGTAGGTGAAGGTGGAGTAGAG‐3′ |
| CD24 | 5′‐GCCAGGGCAATGATGAATGAG‐3′ | 5′‐GCTCTTGTTGAATGGAAACAGGTG‐3′ |
Transfections and Dual Luciferase Assay
TBRE‐TK‐Luc‐WT (catalog #36246) and TBRE‐TK‐Luc‐MT (catalog #36245) were obtained from Addgene (Cambridge, MA). Luciferase assays were performed using the Dual‐Luciferase reporter assay system (Promega, Madison, WI). Cells were transfected with the designated reporter vector and the pRL‐TK vector (Promega), which bears the Renilla reniformis luciferase gene, using LipofectAMINE 2000 (Invitrogen) and incubated for 24 h. Luciferase activities were measured by a luminometer (TD‐20/20, Turner Designs, CA). Transcriptional activity was expressed as the ratio of firefly and Renilla luciferase activities.
Flow Cytometry
Cells were stained with anti‐CD24 (BD PharMingen, San Diego, CA; clone HIS50, 1:100) followed by Alexa488‐conjugated anti‐mouse IgG (1:200) and anti‐CD90‐APC, CD73‐CF5, CD105‐perCP, and a negative marker cocktail (CD45, CD34, CD11b, CD79A, and HLA‐DR) (R&D Systems, Minneapolis, MN, 1:200). Flow cytometry was performed using FACS Vantage (Becton–Dickinson Immunocytometry Systems, San Jose, CA).
Protein Extraction and Western Blotting
Culture plates were placed on ice and washed with ice‐cold Hank's balanced salt solution. Total cell protein was extracted using a mammalian protein extraction reagent (MPER; Pierce, Rockford, IL), containing a protease inhibitor cocktail (Roche, Indianapolis, IN), NaF (5 mM), and Na3VO4 (200 μM). Proteins were resolved on 8–12% sodium dodecyl sulfate‐polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Bio‐Rad, Hercules, CA). The membranes were blocked with Tris‐buffered saline and Tween 20 (TBST) (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% Tween 20) containing 5% non‐fat dry milk and incubated overnight at 4°C in TBST containing 3% non‐fat dry milk and the primary antibody. Membranes were washed several times and incubated with the secondary antibody. The bound antibodies were detected using the ECL reagent (Amersham Biosciences, Piscataway, NJ). The following antibodies were used for Western blotting; anti‐CD24 (Santa Cruz Biotechnology; clone FL‐80, 1:1000), anti‐T (Santa Cruz Biotechnology, 1:1000), anti‐PCNA (BioNovus Life Sciences, Cherrybrook, Australia; clone 16D10, 1:1000), anti‐β‐actin (Cell Signaling Technology, 1:1000), and HRP‐conjugated goat anti‐rabbit and rat IgG (Sigma–Aldrich, 1:2000).
Lentiviral Transduction
For stable gene‐silencing of T and CD24 in U‐CH1‐N cells, lentivirus‐mediated short‐hairpin RNA system was utilized. Lentiviral particles (#SHC003, T: #SHCLNV‐NM_003181, and CD24: #SHCLNV‐NM_013230) were purchased from Sigma–Aldrich (Tokyo, Japan). U‐CH1‐N cells culutred on 10 cm dishes (50–60% confluency) were incubated with 10 ml of conditioned media containing 20 µl/l viral particles and 6 µg/ml polybrene for 24 h. After the incubation, the conditioned media was replaced with fresh medium and the cells were further incubated for 4 day before analyses.
Evaluation of Cell Viability
Cell viability were evaluated by an MTT‐based assay (Cell Counting Kit‐8, Dojindo Molecular Technologies, Kumamoto, Japan) and using a microplate reader (Analytical Instruments, MN).
Statistical Analysis
All measurements were performed in triplicate and data are presented as the mean ± standard error of the mean (SEM). Differences between the two groups were evaluated by the Student t‐test. p < 0.05 was considered statistically significant.
RESULTS
U‐CH1‐N Cells Express the Notochordal NP Cell‐Specific Markers
We first performed a comparative microarray analyses using U‐CH1‐N, a chondrosarcoma cell line OUMS27, and an osteosarcoma cell line MG63 to obtain an idea about the gene expression profiles of U‐CH1‐N cells. In this preliminary analysis, we found that the transcripts of the NP markers, such as T (encodes T brachyury transcription factor), KRT19 (encodes keratin 19), and CD24, were highly expressed in U‐CH1‐N cells compared to those in OUMS27 or MG63 (data not shown). High level of expression (>95% of cells) of T, keratin 19, and CD24 in both U‐CH1‐N cells and human fetal IVD was confirmed by immunostaining (Fig. 1A and B). Consistently, flow cytometric analysis showed a high level of cell‐surface expression of CD24, a cell‐surface marker for notochordal NP cells and chordoma,9 in U‐CH1‐N cells (Fig. 1C). To test if T expressed in U‐CH1‐N cell is functional as a transcription factor, we introduced a luciferase reporting vector bearing a wild‐type (WT) or mutant (MT) T‐box responsive element (TBRE) sequence into U‐CH1‐N cells and 293T cells (Fig. 1D). 293T cells do not express T at a detectable level (data not shown) and were used as a negative control. Introduction of the WT reporter vector into U‐CH1‐N cells showed a significantly higher luciferase activity compared to those transfected with the MT reporter vector, indicating that T expressed in U‐CH1‐N cells is functional (Fig. 1E). Very low levels of luciferase activity were observed in 293T cells transfected with either the WT or MT reporter vector.
Figure 1.
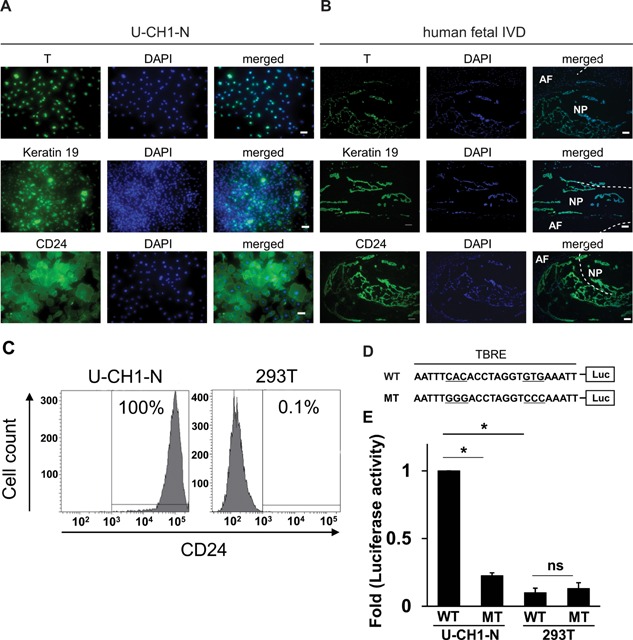
Expression of the NP markers in U‐CH1‐N cells and human fetal NP cells (NP). (A and B) U‐CH1‐N cells (A) and human fetal IVD (B) were immunostained for T, keratin 19, and CD24, and counterstained with DAPI. Scale bar, 50 µm. (C) Flow cytometric analysis of CD24 expression in U‐CH1‐N cells and 293T cells. (D) A schematic of WT and MT T‐box responsive element (TBRE) constructs used for reporter assay. (E) Luciferase reporter assay using WT and MT reporter constructs. Data are represented as mean ± SEM of three independent experiments performed in triplicate (n = 3). *p < 0.05; ns, not significant.
Expression of Cell Surface Markers of Mesenchymal Stem Cell in U‐CH1‐N Cells and Human Fetal IVD
To further characterize the biological properties of U‐CH1‐N cells, we evaluated the frequency of positive cells for several cell surface markers. Flow cytometry analysis showed that approximately 40% of the cells are positive for a mesenchymal stem cell marker CD90. On the other hand, there was no expression of CD105, CD73, CD45, CD34, CD11b, CD79A, or HLA‐DR in U‐CH1‐N cells (Fig. 2A). Lack of expression of CD73, CD90, and CD105 in human fetal NP cells was confirmed by immunohistochemistry (Fig. 2B).
Figure 2.
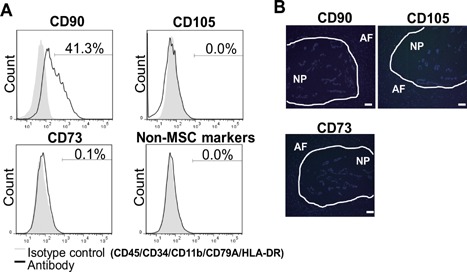
Analysis of cell surface markers in U‐CH1‐N cells and human fetal NP cells. (A) Flow cytometry analysis of cell surface markers in U‐CH1‐N cells. (B) Human fetal IVD sections immunostained for CD73, CD90, and CD105 and counterstained with DAPI. Scale bar, 50 µm.
U‐CH1‐N Cells Exhibit Chondrogenic Capacity
We next assessed whether U‐CH1‐N cells have chondrogenic capacity, one of the characteristics of primary notochordal NP cells. Immunocytochemistry showed that both aggrecan and type II collagen were highly expressed in U‐CH1‐N cells (Fig. 3A and B). Accordingly, U‐CH1‐N cells cultured for 10 days were stained positive for Alcian blue (Fig. 3C), as observed in the notochordal NP cells derived from human fetal IVD (Fig. 3D). Furthermore, we evaluated the chondrogenic capacity of U‐CH1‐N cells in atelocollagen pellet culture. Of interest, cells incubated in the pellets showed vacuoles that were not apparent when these cells were cultured on plates. The cells expressed high levels of CD24, keratin19, T, and Laminin, and the matrix was stained positive for Alcian blue (Fig. 4). Taken together, these observations show that U‐CH1‐N cells express high levels of the NP cell‐specific markers and retain the chondrogenic capacity as observed in the primary notochordal NP cells.
Figure 3.
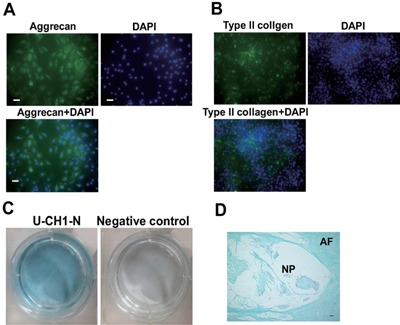
U‐CH1‐N cells exhibit chondrogenic capacity (A) U‐CH1‐N cells immunostained for aggrecan (A) and type II collagen (B), and counterstained with DAPI. Scale bar, 50 µm. (C) U‐CH‐1 N cells cultured for 10 days and stained with Alcian blue. (D) A human fetal IVD section stained with Alcian blue. Scale bar, 50 µm.
Figure 4.
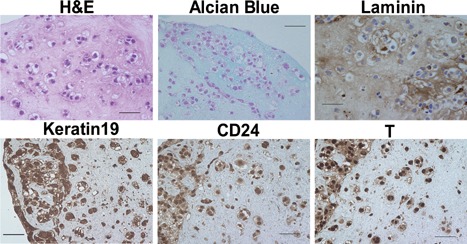
Atelocollagen pellet culture of U‐CH1‐N cells. U‐CH1‐N cells incubated in the pellets have vacuoles and express high levels of CD24, keratin 19, T, and Laminin. The pellet was stained positive for Alcian blue (Fig. 4). Scale bar, 50 µm.
The T Transcription Factor Is Essential for the Cell Growth and Chondrogenic Capacity in U‐CH1‐N Cells
Using U‐CH1‐N cells, we next explored the potential role of T in notochordal NP cells. Knockdown of T expression in U‐CH1‐N cells was achieved by lentivirus‐mediated transduction of a short‐hairpin RNA vector against T transcripts (LV‐Sh‐T). Virus vector bearing a green fluorescent protein gene (LV‐GFP) was used to monitor the transfection efficiency and as a negative control. A transduction efficiency of approximately 80% was achieved using LV‐GFP vector (Fig. 5A). Quantitative RT‐PCR and Western blot analysis showed a significant decrease in the transcription levels of T in LV‐Sh‐T‐transfected cells compared to those with LV‐GFP (Fig. 5B and C). U‐CH1‐N cells transfected with LV‐Sh‐T appeared more spindle‐shaped and grew slower compared to those with LV‐GFP (Fig. 5D). MTT assay revealed that T‐knockdown markedly suppressed cell viability (Fig. 5E). Consistently, the expression level of PCNA was significantly decreased in LV‐Sh‐T‐transfected U‐CH1‐N cells compared to those with LV‐GFP (Fig. 5F). Furthermore, we found that knockdown of T transcripts compromises the chondrogenic capacity of U‐CH1‐N cells. The expression of the transcripts for type II collagen and aggrecan, but not that of type I collagen, was markedly suppressed in LV‐Sh‐T‐transfected U‐CH1‐N cells compared to those with LV‐GFP, suggesting that T is involved in the production of chondrogenic ECM proteins in notochordal NP cells (Fig. 6A–C). To gain further insight into the molecular mechanisms by which T regulates the expression of chondrogenic ECM proteins, we quantified the mRNA expression of the SOX trio genes (SOX5, SOX6, and SOX9) in LV‐Sh‐T‐transfected U‐CH1‐N cells. Interestingly, we found that the suppression of T expression resulted in a decrease in SOX5 and SOX6, but not SOX9, transcripts levels in U‐CH1‐N cells, suggesting that T regulates the expression of chondrogenic ECM proteins through promoting the expression of SOX5 and SOX6 (Fig. 6D–F).
Figure 5.
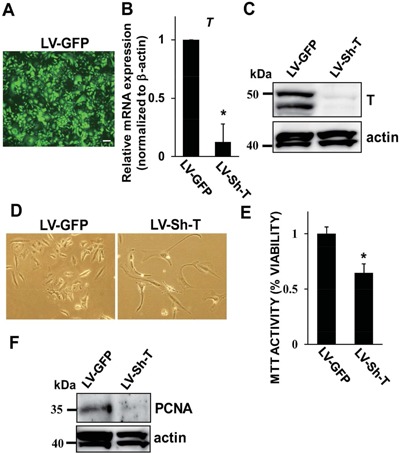
Gene silencing of T suppresses the cell growth in U‐CH1‐N cells. (A) A photomicrograph of U‐CH1‐N cells transduced with LV‐GFP. Scale bar, 25 µm. (B) Assessment of gene silencing efficacy of T in U‐CH1‐N cells transduced with LV‐GFP or LV‐Sh‐T by quantitative RT‐PCR (B) and Western blot (C). Relative expression of the T transcripts in U‐CH1‐N cells transduced with LV‐GFP was normalized to that of β‐actin and set to one. (D) Photomicrographs of U‐CH1‐N cells transduced with LV‐GFP or LV‐Sh‐T. Magnification ×20. (E) Cell survival rate of U‐CH1‐N cells transduced with LV‐GFP or LV‐Sh‐T was evaluated using an MTT‐based assay. MTT activity of U‐CH1‐N cells transduced with LV‐GFP was set to one. (F) Western blot analysis of cell lysate collected from U‐CH1‐N cells transduced with LV‐GFP or LV‐Sh‐T. Data are represented as mean ± SEM of three independent experiments performed in triplicate (n = 3). *p < 0.05; ns, not significant.
Figure 6.
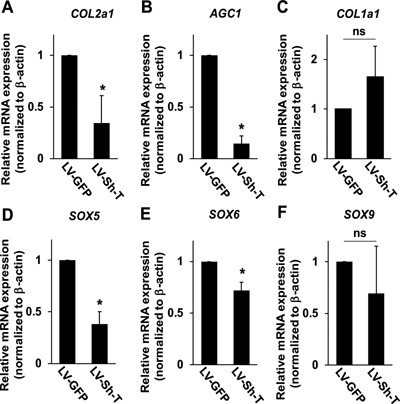
T regulates the expression of the transcripts for chondrogenic ECM proteins in U‐CH1‐N cells. Quantitative RT‐PCR analysis using the transcripts collected from U‐CH1‐N cells transduced with LV‐GFP or LV‐Sh‐T. The expression level of each gene is normalized to that of β‐actin. Relative expression level of each gene in U‐CH1‐N cells transduced with LV‐GFP is set to one. Data are represented as mean ± SEM of three independent experiments performed in triplicate (n = 3); *p < 0.05; ns, not significant.
CD24 Is Dispensable for the Notochordal NP Cell‐Like Phenotype in U‐CH1‐N Cells
Because CD24 is highly expressed in notochordal NP cells,15 we asked whether gene‐silencing of CD24 also leads to phenotypical changes in U‐CH1‐N cells. However, on contrary to our expectation, knockdown of CD24 in U‐CH1‐N cells showed no change in the morphology or viability (data not shown). In accordance, gene‐silencing of CD24 did not affect the expression of the transcripts for type II collagen, aggrecan, or the SOX trios genes; on the other hand, there was a slight, yet statistically significant, increase in the transcription level for type I collagen (Fig. 7). Taken together, the loss‐of‐function experiments suggest that T is indispensable for the homeostasis and chondrogenic capacity of notochordal NP cells and that the lack of CD24 can largely be compensated, at least under the current experimental conditions.
Figure 7.
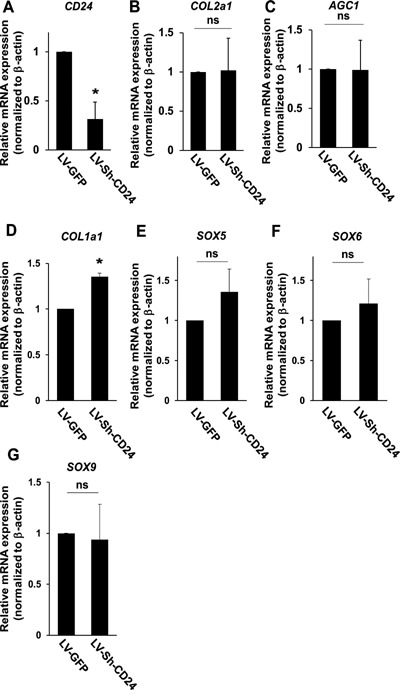
Silencing of CD24 does not affect the transcription of chondrogenic ECM proteins in U‐CH1‐N cells. Quantitative RT‐PCR analysis using the transcripts collected from U‐CH1‐N cells transduced with LV‐GFP and LV‐Sh‐CD24. The expression level of each gene is normalized to that of β‐actin. The relative expression level of each gene in U‐CH1‐N cells transduced with LV‐GFP is set to one. Data are represented as mean ± SEM of three independent experiments performed in triplicate (n = 3); *p < 0.05; ns, not significant.
DISCUSSION
The present study demonstrates that a chordoma‐derived cell line, U‐CH1‐N, expresses high levels of the NP markers, including T, keratin 19, and CD24 and exhibits chondrogenic capacity. These results show that U‐CH1‐N cells share some of the important biological properties with human notochordal NP cells. Furthermore, using this cell line, we found that T is an essential transcriptional factor for maintaining the notochordal NP cell‐like phenotypes and cell proliferation in U‐CH1‐N cells. Although there are several limitations as discussed below, we would like to purpose U‐CH1‐N as a notochordal NP cell‐like cell line that can be used to investigate the biology of IVD.
The number of notochordal NP cells decreases with age and they are rarely present after adolescence in humans.4 Therefore, it is nearly impracticable to isolate enough number of human notochordal NP cells for biological research. In the field of musculoskeletal biology, cell lines such as MC3T3‐E1, ATDC5, C2C12, and RAW264.7 cells, have been widely used to investigate the functions and development of osteoblasts, chondrocyte, skeletal muscle, and osteoclasts, respectively.16, 17, 18, 19 To the best of our knowledge, there have been only a few attempts to establish immortalized notochordal NP cell lines in the past20, 21, 22; however, none of these cell lines can be reliably used or widely recognized as notochordal NP cell‐like cell line. Inevitably, lack of such a cell line has hampered the progression of IVD biology.
Chordoma is a malignant tumor that usually arise from the skull and vertebra. Of note, chordoma is caused by a malignant transformation of the cellular remnants of the embryonic notochord.23, 24 While there are several human chordoma cell lines reported in the literature,8, 9, 10, 11, 12 most of these cell lines have a relatively long doubling time and are not suitable for practical use. For example, the first validated chordoma cell line, U‐CH1 has a doubling time of approximately 7 day.8 In this respect, U‐CH1‐N, a subpopulation derived from U‐CH1 chordoma cells,13 has an exceptionally short doubling time and is feasible for research applications.
Although T is well characterized as an NP cell marker, its potential role in notochordal NP cells or mature NP cells remains poorly understood. T is required for the differentiation of the axial midline mesoderm into the notochord25 and misexpression of T gives rise to a fully differentiated ectopic notochord.26 In the present study, we found that T promotes the transcription of SOX5 and SOX6, which encode essential transcriptional factors for the synthesis of chondrogenic ECM proteins, indicating that T is involved in the maintenance of the extracellular milieu of NP. Interestingly, our results also indicate that that the expression of one of the SOX trio genes, SOX9, is not associated with the expression of T. This discrepancy with other two SOX genes remains to be elucidated.
CD24 is a GPI‐anchored cell surface protein that is highly expressed in notochordal NP cells and the expression of its transcripts remains almost unchanged with aging.15, 27 Studies using Cd24‐deficient mice showed that CD24 plays a role in modulating proliferation and apoptosis of B‐cell precursors and thymocytes.28, 29, 30 In humans, the expression level of CD24 is associated with the progression of multiple sclerosis and systemic lupus erythematosus.31, 32, 33 Although the physiological functions of CD24 in humans are widely unknown, the expression pattern of CD24 strongly indicates that it has crucial roles in notochordal NP cells. However, our data revealed that knockdown of CD24 does not have any significant impact on the proliferation or chondrogenic capacity in U‐CH1‐N cells, at least under the present experimental conditions. Phenotypical analyses of Cd24‐deficent mice are in progress to address these issues.
As is the case for other well‐known cell lines, there are limitations in using U‐CH1‐N as a notochordal NP cell‐like cell line. Because U‐CH1‐N was derived from a tumor specimen and is immortal, it is likely that some of the fundamental cell surviving mechanisms are different from those in notochordal NP cells. Most importantly, the T transcription factor, which is indispensable for the development of notochord, is also critically involved in the pathogenesis of chordoma. Therefore, the functions of T are likely to be context‐dependent and not identical between chordoma and notochordal NP cells. In this regard, functional analysis of the T transcription factor using U‐CH1‐N must be undertaken with caution. Furthermore, largely due to a paucity of data on IVD biology, we do not fully understand what criteria define “notochordal NP cell‐like cell line.” Although further studies are mandatory to address these issues, based on the observations of the past34, 35 and present studies, we would like to propose the expression of notochordal NP cell‐specific markers, especially T and CD24, and the chondrogenic capacity as potentially the necessary conditions for “notochordal NP cell‐like cell line.” Nevertheless, bearing the limitations in mind, U‐CH1‐N is probably one of the best cell lines available to date to study the biology of notochordal NP cells.
CONCLUSIONS
Our data suggest that a chordoma‐derived cell line U‐CH1‐N shares some of the biological properties with notochordal NP cells and has a potential to be used as “notochordal NP cell‐like cell line” for IVD research. Our data using this cell line also suggest that the notochordal‐specific transcription factor T is indispensable to maintain the phenotype of notochordal NP cells. Although further studies are required to fully characterize and understand the biological properties of U‐CH1‐N, we believe that this cell line will serve as a useful tool in the field of IVD research.
AUTHORS’ CONTRIBUTIONS
NF conceived the study, secured funding, participated in its design and coordination, performed the statistical analysis and drafted the manuscript. SS and RW carried out the experiments and performed the statistical analysis. KW and KI participated in the interpretation of the data and helped drafting manuscript. MS prepared the human sections and participated in the interpretation of the data. KT performed flow cytometry analysis and participated in the interpretation of the data. YT secured funding and participated in the interpretation of the data. KH participated in the design and coordination of the study and helped to draft the manuscript. TM secured funding and participated in the interpretation of the data. MN secured funding and helped to interpret the data. MM secured funding, participated in the design and coordination of the study. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank Dr. Takamitsu Kato for providing U‐CH1‐N and Yui Sato for technical support.
Conflict of interest: None.
REFERENCES
- 1. Livshits G, Popham M, Malkin I, et al. 2011. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 70:1740–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts S, Evans H, Trivedi J, et al. 2006. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 88:10–14. [DOI] [PubMed] [Google Scholar]
- 3. Chelberg MK, Banks GM, Geiger DF, et al. 1995. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat 186:43–53. [PMC free article] [PubMed] [Google Scholar]
- 4. Hunter CJ, Matyas JR, Duncan NA. 2004. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunter CJ, Matyas JR, Duncan NA. 2003. The three‐dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat 202:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan M, Leong KW, Chan BP. 2011. Three‐dimensional culture of rabbit nucleus pulposus cells in collagen microspheres. Spine J 11:947–960. [DOI] [PubMed] [Google Scholar]
- 7. Chiba K, Andersson GB, Masuda K, et al. 1998. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine 23:1821–1827. [DOI] [PubMed] [Google Scholar]
- 8. Scheil S, Brüderlein S, Liehr T, et al. 2001. Genome‐wide analysis of sixteen chordomas by comparative genomic hybridization and cytogenetics of the first human chordoma cell line, U‐CH1. Genes Chromosomes Cancer 32:203–211. [DOI] [PubMed] [Google Scholar]
- 9. Ricci‐Vitiani L, Pierconti F, Falchetti ML, et al. 2006. Establishing tumor cell lines from aggressive telomerase‐positive chordomas of the skull base. Technical note. J Neurosurg 105:482–484. [DOI] [PubMed] [Google Scholar]
- 10. Ostroumov E, Hunter CJ. 2007. The role of extracellular factors in human metastatic chordoma cell growth in vitro. Spine 32:2957–2964. [DOI] [PubMed] [Google Scholar]
- 11. Ostroumov E, Hunter CJ. 2008. Identifying mechanisms for therapeutic intervention in chordoma: c‐Met oncoprotein. Spine 33:2774–2780. [DOI] [PubMed] [Google Scholar]
- 12. Karikari IO, Gilchrist CL, Jing L, et al. 2014. Molecular characterization of chordoma xenografts generated from a novel primary chordoma cell source and two chordoma cell lines. J Neurosurg Spine 21:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato TA, Tsuda A, Uesaka M, et al. 2011. In vitro characterization of cells derived from chordoma cell line U‐CH1 following treatment with X‐rays, heavy ions and chemotherapeutic drugs. Radiat Oncol 6:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ota N, Takaishi H, Kosaki N, et al. 2009. Accelerated cartilage resorption by chondroclasts during bone fracture healing in osteoprotegerin‐deficient mice. Endocrinology 150:4823–4834. [DOI] [PubMed] [Google Scholar]
- 15. Fujita N, Miyamoto T, Imai J, et al. 2005. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun 338:1890–1896. [DOI] [PubMed] [Google Scholar]
- 16. Tsunoi M, Hakeda Y, Kurihara N, et al. 1984. Effect of transferrin on alkaline phosphatase activity and collagen synthesis in osteoblastic cells derived from newborn mouse calvaria. Exp Cell Res 153:240–244. [DOI] [PubMed] [Google Scholar]
- 17. Shukunami C, Shigeno C, Atsumi T, et al. 1996. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation‐dependent gene expression of parathyroid hormone (PTH)/PTH‐related peptide receptor. J Cell Biol 133:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bains W, Ponte P, Blau H, et al. 1984. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol 4:1449–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka Y, Amano F, Kishi H, et al. 1989. Degradation of arachidonyl phospholipids catalyzed by two phospholipases A2 and phospholipase C in a lipopolysaccharide‐treated macrophage cell line RAW264.7. Arch Biochem Biophys 272:210–218. [DOI] [PubMed] [Google Scholar]
- 20. Sakai D, Mochida J, Yamamoto Y, et al. 2004. Immortalization of human nucleus pulposus cells by a recombinant SV40 adenovirus vector: establishment of a novel cell line for the study of human nucleus pulposus cells. Spine 29:1515–1523. [DOI] [PubMed] [Google Scholar]
- 21. Liu MC, Chen WH, Wu LC, et al. 2014. Establishment of a promising human nucleus pulposus cell line for intervertebral disc tissue engineering. Tissue Eng Part C Methods 20:1–10. [DOI] [PubMed] [Google Scholar]
- 22. van den Akker GG, Surtel DA, Cremers A, et al. 2014. Novel immortal human cell lines reveal subpopulations in the nucleus pulposus. Arthritis Res Ther 16:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salisbury JR, Isaacson PG. 1985. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol 9:791–797. [DOI] [PubMed] [Google Scholar]
- 24. Risbud MV, Schaer TP, Shapiro IM. 2010. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn 239:2141–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schulte‐Merker S, Ho RK, Herrmann BG, et al. 1992. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116:1021–1032. [DOI] [PubMed] [Google Scholar]
- 26. Takahashi H, Hotta K, Erives A, et al. 1999. Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev 13:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujita N, Imai J, Suzuki T, et al. 2008. Vascular endothelial growth factor‐A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun 372:362–372. [DOI] [PubMed] [Google Scholar]
- 28. Chappel MS, Hough MR, Mittel A, et al. 1996. Cross‐linking the murine heat‐stable antigen induces apoptosis in B cell precursors and sup‐presses the anti‐CD40‐induced proliferation of mature resting B lymphocytes. J Exp Med 184:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hough MR, Chappel MS, Sauvageau G, et al. 1996. Reduction of early B lymphocyte precursors in transgenic mice overexpressing the murine heat‐stable antigen. J Immunol 156:479–488. [PubMed] [Google Scholar]
- 30. Hough MR, Takei F, Humphries RK, et al. 1994. Defective development of thymo‐cytes overexpressing the costimulatory molecule, heat‐stable antigen. J Exp Med 179:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Lin S, Rammohan KW, et al. 2007. A dinucleotide deletion in CD24 confers protection against autoimmune diseases. PLoS Genet 3:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Q, Rammohan K, Lin S, et al. 2003. CD24 is a genetic modifier for risk and progression of multiple sclerosis. Proc Natl Acad Sci USA 100:15041–15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Liu R, Li D, et al. 2012. A hypermorphic SP1‐binding CD24 variant associates with risk and progression of multiple sclerosis. Am J Trans Res 4:347–356. [PMC free article] [PubMed] [Google Scholar]
- 34. Risbud MV, Schoepflin ZR, Mwale F, et al. 2015. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res 33:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodrigues‐Pinto R, Berry A, Piper‐Hanley K, et al. 2016. Spatiotemporal analysis of putative notochordal cell markers reveals CD24 and keratins 8, 18 and 19 as notochord‐specific markers during early human intervertebral disc development. J Orthop Res [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


