Abstract
The increased use of mobile phones has generated public concern about the impact of radiofrequency electromagnetic fields (RF‐EMF) on health. In the present study, we investigated whether RF‐EMFs induce molecular changes in amyloid precursor protein (APP) processing and amyloid beta (Aβ)‐related memory impairment in the 5xFAD mouse, which is a widely used amyloid animal model. The 5xFAD mice at the age of 1.5 months were assigned to two groups (RF‐EMF‐ and sham‐exposed groups, eight mice per group). The RF‐EMF group was placed in a reverberation chamber and exposed to 1950 MHz electromagnetic fields for 3 months (SAR 5 W/kg, 2 h/day, 5 days/week). The Y‐maze, Morris water maze, and novel object recognition memory test were used to evaluate spatial and non‐spatial memory following 3‐month RF‐EMF exposure. Furthermore, Aβ deposition and APP and carboxyl‐terminal fragment β (CTFβ) levels were evaluated in the hippocampus and cortex of 5xFAD mice, and plasma levels of Aβ peptides were also investigated. In behavioral tests, mice that were exposed to RF‐EMF for 3 months did not exhibit differences in spatial and non‐spatial memory compared to the sham‐exposed group, and no apparent change was evident in locomotor activity. Consistent with behavioral data, RF‐EMF did not alter APP and CTFβ levels or Aβ deposition in the brains of the 5xFAD mice. These findings indicate that 3‐month RF‐EMF exposure did not affect Aβ‐related memory impairment or Aβ accumulation in the 5xFAD Alzheimer's disease model. Bioelectromagnetics. 37:391–399, 2016. © 2016 The Authors Bioelectromagnetics published by Wiley Periodicals, Inc. on behalf of Bioelectromagnetics Society.
Keywords: Alzheimer's disease mice, β‐amyloid, memory impairment, RF‐EMF, hippocampus
INTRODUCTION
The brain can be exposed to radiofrequency electromagnetic fields (RF‐EMF) during use of mobile phones, which has raised public concern about possible adverse effects. A previous meta‐analysis suggested that long‐term exposure to mobile phones might be associated with the risk of brain tumors, including neuroma and glioma [Hardell et al., 2008]. In addition, the International Agency for Research on Cancer (IARC) has classified RF‐EMF as a possible human carcinogen (Group 2B) [Baan et al., 2011]. However, both the recent 13‐nation INTERPHONE study [Group, 2010] and numerous epidemiologic studies [Kan et al., 2008; Carlberg et al., 2013] have provided no evidence that RF‐EMF exposure could increase the risk of brain tumors.
Previous reports have suggested that RF‐EMF exposure could cause memory impairment in a mouse model [Ntzouni et al., 2011, 2013; Aldad et al., 2012], indicating a harmful effect of RF‐EMF on neurobehavioral function. In contrast, no effect of RF‐EMF exposure on spatial learning and working memory tasks was found [Sienkiewicz et al., 2000; Dubreuil et al., 2003; Maaroufi et al., 2014]. Moreover, other studies have reported a beneficial effect of RF‐EMF on cognitive function [Dragicevic et al., 2011; Arendash et al., 2012; Banaceur et al., 2013]. Thus, there is some controversy regarding the effects of RF‐EMF on cognitive functions and associated hippocampal functions, and further mechanistic investigations and time‐point analyses are needed. In addition, because most of the positive effects of RF‐EMF exposure were observed in Alzheimer's disease (AD) animal models [Arendash et al., 2010; Dragicevic et al., 2011; Arendash, 2012; Banaceur et al., 2013], suggesting that RF‐EMF may have a positive effect on AD pathology, biological effects of RF‐EMF on the function of the AD brain should be further investigated.
AD is an important neurodegenerative disorder that is characterized by progressive cognitive impairment. Previous studies have reported that amyloid precursor protein (APP) is cleaved through the β‐secretase pathway to form amyloid‐beta protein (Aβ) [Shoji et al., 1992], which might be related to the pathology of AD. A major pathological hallmark of AD is abnormal accumulation of Aβ in the brain. The 5xFAD mouse, which overexpresses both APP, due to K670N/M671L (Swedish), 1716V (Florida), and V7171 (London) mutations, and PS1, due to M146L and L286 mutations, exhibits very aggressive Aβ deposition. Intraneuronal Aβ develops at 1.5 months, plaque appears at 2 months, memory deficits are evident at 4 months, and neuronal death occurs at 9 months of age [Oakley et al., 2006]. Because these mice exhibit Aβ pathology relatively early, they are very useful for studying AD‐like pathology, including memory and learning deficits. Previously, Aβ deposition and APP and carboxyl‐terminal fragment β (CTFβ) levels were found to be reduced in the hippocampus and cortex when 5xFAD transgenic mice were exposed to RF‐EMF for 8 or 8.5 months, indicating that long‐term RF‐EMF exposure might ameliorate AD‐like pathology [Arendash et al., 2010; Jeong et al., 2015]. In addition, it has been previously suggested that beneficial effects of RF‐EMF apparently occurred through anti‐aggregation effects on brain Aβ, mitochondrial enhancement, and enhanced neuronal activity in AD transgenic mice [Arendash, 2012]. However, little is known about the possible effects of intermediate exposure to RF‐EMF on APP processing, indicating that further investigations and specific time‐point analyses are needed. Furthermore, the precise mechanisms associated with effects of RF‐EMF on AD pathology remain unknown.
In the present study, we used behavioral tests to evaluate spatial and non‐spatial memory and APP processing‐related molecular pathology to determine the effect of 1950 MHz RF‐EMF (2 h per day, SAR 5 W/kg) on AD‐like pathology induced by Aβ production in 5xFAD mice.
MATERIALS AND METHODS
Animals
We used 5xFAD mice (a gift from Dr. Inhee Mook‐Jung, Seoul National University, Seoul, Korea), which express five mutant forms of human genes associated with AD: three APP genes (APPsw, APPfl, and, APPlon) and two presenilin1 (PS1, PSEN1) genes (PSEN1 M146L and PSEN1 L286V). The generation of 5xFAD mice has been described previously [Moon et al., 2014]. We used female 5xFAD mice and assigned the mice to two groups: 5xFAD sham exposure (n = 8) and 5xFAD RF exposure (n = 8). In addition, a non‐transgenic wild‐type littermate (WT; n = 1) served as a negative control group for Western blotting. RF‐EMF exposure was started at the age of 1.5 months, when the animals are still adolescents with immature brains, because intraneuronal Aβ aggregation develops in 5xFAD mice beginning at this point. All mouse procedures were approved by the Institutional Animal Care and Use Committee of the Korea Institute of Radiological and Medical Sciences (IACUC permit number: KIRAMS2013‐67).
RF Exposure System
Whole‐body exposure to 1950 MHz RF‐EMF was performed in a reverberation chamber (ERE‐MRC‐1.5, ERETEC, Gyeonggi‐do, Korea) designed for in vivo experiments. Detailed descriptions of the system, uniformity of the field dose, and specific absorption rate (SAR) have been provided previously [Lee et al., 2012]. Briefly, the 1950 MHz RF‐EMF signal was generated using a microprocessor unit chip on which WCDMA‐formatted code controlled a central processing unit. Subsequently, the signal was amplified using an additional high‐power amplifier (PCS60WHPA_CW, Kortcom, Anyang, Korea) after it was passed through a separate digital attenuator. An 11‐bit digital PIN diode attenuator (Model 349, General Microwave, Farmingdale, NY) was used to control the output power level (maximum: 60 W). Transmitting antennae were purchased commercially (patch type, KCAN1900PA, Korea Telecommunication Components, Gyeonggi‐do, Korea), and a computer was used to control exposure level and time. External dimensions of the reverberation chamber were 2295 × 2293 × 1470 mm3; walls were made of stainless steel with a thickness of 2.3 mm. To measure field uniformity, five cages were placed in the test area, and field strength was measured for 1 min at each of 24 points. Distribution of the electric field inside the chamber was determined using a three‐axis isotropic probe (HI‐6005, ETS‐Lindgren, Cedar Park, TX). Any difference in field distribution was much less than 3 dB. SAR distribution for each caged mouse was calculated using a mouse phantom (Chungnam National University, Daejon, Korea); the simulation featured 40 tissues and a voxel size of 1 mm. The power output was controlled at 52 W to achieve an average whole‐body SAR of 5 W/kg. The reverberation chamber was placed in the animal facility, and ventilation, temperature, and humidity were controlled. Animals were exposed to 1950 MHz RF‐EMF according to the following schedule: SAR 5 W/kg, 2 h/day, 5 days/week for 3 months. Sham‐irradiated mice were placed inside the chambers for the same period of time without RF‐EMF signals. During radiofrequency (RF) exposure, air temperature in the test area inside was maintained at 20 ± 3 °C. Rectal temperatures were measured before RF‐EMF exposure and immediately thereafter. Body temperature did not increase by over 0.5 °C (changes ranged from −1.9 to +0.5 °C), and body temperature was thus maintained within the normal range.
Open Field Test
The open field test was used to assay general activity of the mice. Mice were placed in the central area, and video tracking was started. Mice were allowed to move in the open field apparatus without restrictions for 10 min. The footpath of all animals was recorded by the automated video‐tracking system (Viewer3, BIOSERVE, St. Augustin, Germany). The tracking system was used to quantify the mean velocity, the total distance traveled, and the time spent in the central region of the apparatus.
Novel Object Recognition Memory Test
The novel object recognition memory test was used to examine non‐spatial working memory. Immobile plastic objects that were 3.5 cm in height but different shapes were used in the recognition test. The chamber and all objects were cleaned with 70% (v/v) ethanol between trials to prevent buildup of olfactory cues. During training, two randomly selected, differently shaped objects were presented to each mouse for 10 min. At 24 h after training, another set of objects (one previously presented object and one novel object) was presented to the trained mice. The mouse was considered to be exploring the object when it sniffed, touched, or moved toward the object in such a way that distance between the tip of the nose and object was less than 1 cm. Total time spent exploring each object was recorded by a trained observer blind to exposure condition using a video‐tracking system (Viewer3). If the mouse retained memory of a previously encountered object, the animal would prefer the novel object. Exploration of the novel object during testing was expressed as a percentage of total exploration time: time spent exploring the novel object divided by total time spent interacting with either object.
Y‐Maze Test
The Y‐maze test was used to measure working memory and reference memory, which were assessed by recording spontaneous alternation behavior. Activity was recorded for 8 min and analyzed by a computer program (Viewer3). Alternation was defined as successive entries into three different arms on overlapping triplet sets. Percentage alternation was calculated as the ratio of actual to possible alternation (defined as total number of arm entries −2) ×100, as follows: % Alternation = (No. of alternations)/(Total arm entries −2) ×100.
Morris Water Maze Test
The Morris water maze test was used to assess hippocampus‐dependent spatial memory [Morris, 1984]. Mice were individually trained in a circular pool (100 cm diameter, 30 cm height) filled with water that was maintained at 25 °C and made opaque using a non‐toxic washable white paint. The maze was located in a lit room that contained extramaze cues. The escape platform (10 cm diameter) was placed in the center of a designated quadrant of the pool with its top positioned 1 cm below the water surface. Mice were not allowed to swim in the pool before training. During visible platform training, the platform was marked by a flag (5 cm tall). Mice were subjected to three trials daily. Three trials per day were conducted at 10‐min intervals. Each trial lasted for 60 s unless the mouse reached the platform. Time that elapsed until the mouse reached and climbed onto the platform was scored as the escape latency. If the mouse failed to find the platform within 60 s, it was gently navigated to the platform by hand. Regardless of whether the mouse found or failed to find the platform within 60 s, it was allowed to stay on the platform for 20 s. After the visible platform training, mice were further subjected to hidden platform training, during which the platform was placed 1 cm below the surface of the opaque water. Position of the platform was fixed, and starting positions were pseudorandomly varied between trials. Mice were subjected to three trials at 1‐h intervals daily for 4 consecutive days. The tracking system (Viewer3) was used to quantify time to platform and swim speed.
Tissue Preparation
The mice were sacrificed after completion of behavioral tests at the age of 4.5 months, and brain tissue was harvested. For histological analysis, the brain of left hemisphere (six mice per group) was fixed in a 4% paraformaldehyde solution. The hippocampus and cortex of right hemisphere were dissected (six mice per group) and stored at −80 °C for Western blotting analysis.
Immunohistochemistry
Brain samples were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. Sections were immunostained with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) following the manufacturer's protocol. For antigen retrieval, sections were placed in citrate buffer (pH 6.0) and heated in boiling water for 30 min. For immunoperoxidase labeling, endogenous peroxidase activity was blocked by incubating the sections in 0.3% H2O2 in absolute methanol for 15 min at room temperature (RT), followed by blocking with normal horse serum (Vector Laboratories). Next, sections were incubated overnight at 4 °C with a mouse anti‐6E10 antibody (1:1000, SIG‐39320, Covance, Emeryville, CA). After sections were washed, they were incubated with a biotinylated horse anti‐mouse IgG (Vector ABC Elite Kit, Vector Laboratories) for 30 min at RT and then washed and incubated for 30 min at RT with an avidin–biotin peroxidase complex (Vector ABC Elite Kit) prepared according to manufacturer's instructions. After sections were washed, peroxidase reaction proceeded using a diaminobenzidine substrate (contained in the DAB kit; Vector Laboratories) prepared according to manufacturer's instructions.
Images of immunohistochemically stained sections were captured using a BX‐53 microscope (CCD DP73, Olympus, Tokyo, Japan). For Aβ burden analysis, images were converted to grayscale, and the threshold was adjusted for every image for background subtraction. The number of amyloid plaques in the hippocampus or cortex was identified with ImageJ software (NIH, Bethesda, MD). All measurements were performed by the same individual who was blind to experimental conditions. Mean plaque burden of the sham exposure group was assigned the value of 100, and changes were expressed relative to this value. Relative value per group was averaged and expressed as mean ± standard error (SE; n = 6 mice per group).
Western Blotting
Hippocampal and cortical tissues were minced and placed in tissue lysate buffer (Pro‐prep, Intron, Sungnam, Korea). The protein concentration was determined by protein assay (Bio‐Rad, Hercules, CA), and Western blot analysis was performed using a mouse anti‐6E10 antibody (1:2,000, Covance) for APP and CTFβ as well as a mouse anti‐β‐actin antibody (1:5,000, Sigma, St. Louis, MO).
Enzyme‐Linked Immunospecificassay (ELISA)
Blood was collected from 5xFAD mice into ethylenediaminetetraacetic acid‐coated microtainer tubes (BD Biosciences, Franklin Lakes, NJ) and was separated into blood cells and plasma. Plasma samples were snap‐frozen on dry ice and stored at −80 °C for ELISA analysis. ELISA was performed to measure the Aβ level in the plasma, and Aβ 40 and 42 proteins were quantified using commercial ELISA kits (Invitrogen, Carlsbad, CA; KHB3481 and KHB3441, respectively) following manufacturer's protocol. Briefly, samples were diluted in sample diluent to detect Aβ40 and Aβ42 levels within optimal working range of each standard at known concentrations (0–500 pg/ml). After samples were incubated with antibody solutions, concentrations of proteins in the sample were determined by colorimetric assay via absorbance at 620 nm (A620). The A620 values were converted to pg Aβ40 or pg Aβ42/mg by correcting for dilution factors, and were assigned the value of Aβ42/Aβ40 ratio. The Aβ42/Aβ40 ratio per group was averaged and expressed as the mean ± standard error (SE; n = 7–8 mice per group).
Statistical Analysis
Behavioral and imaging data were analyzed by two‐tailed Student's t‐tests using GraphPad Prism 6.0 (GraphPad software, San Diego, CA). All data are presented as mean ± standard error of the mean (SEM). Significance was defined as a P‐value less than 0.05.
RESULTS
Locomotor Activity
We first explored whether RF‐EMF exposure affected basic locomotor activity in 5xFAD mice. 5xFAD mice aged 4.5 months exhibited similar exploratory locomotor activity (measured by average velocity and total distance traveled) following sham‐ or RF‐EMF exposure (Fig. 1A and B). In addition, no difference in amount of time spent in the center was found between sham‐ and RF‐EMF‐exposed 5xFAD mice, indicating that RF‐EMF does not alter anxiety‐related behavior in 5xFAD mice (Fig. 1C).
Figure 1.

RF‐EMF exposure does not affect basal locomotor activity in 5xFAD mice. Open field tests were conducted for general activity/exploratory activity. Mean velocity (A), total distance traveled (B), and amount of time mice stayed in center zone (C) were measured. Values are presented as the mean ± SEM (n = 8).
Spatial and Non‐Spatial Memory Deficits of 5xFAD Mice
We evaluated the effect of RF‐EMF on spatial memory using the Y‐maze test. The 5xFAD mice that had been exposed to RF‐EMF for 3 months showed no differences in spontaneous alternation in the Y‐maze test (Fig. 2A).
Figure 2.
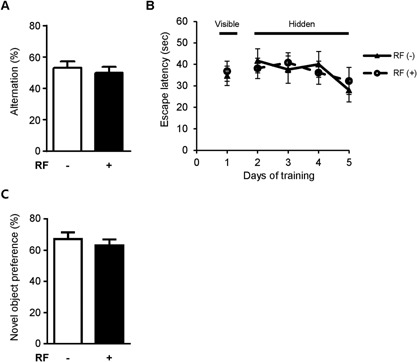
Effect of RF‐EMF exposure on Y‐maze and Morris water maze performance in 5xFAD mice. (A) Y‐maze test was used to evaluate spatial memory in mice. Percentage alternation indicates frequency of non‐overlapping entries compared to total number of entries into three arms. (B) Morris water maze data derived after 3 months of RF‐EMF exposure. During visible platform training on day 1, mice in different groups showed similar latencies to find visible platform. During hidden platform training from days 2 to 5, no differences in escape latency were observed between groups. (C) Object recognition memory test data derived after 3 months of RF‐EMF exposure. No differences in preference for novel object were observed during testing. Values are presented as mean ± SEM (n = 8).
We further examined whether RF‐EMF exposure affected hippocampus‐dependent spatial memory in AD mice using the Morris water maze test (Fig. 2B). During the visible platform training, mice learned to find the escape platform, which had an attached visual cue. There was no significant difference in escape latency between the groups during visible platform training. In addition, 5xFAD animals that were exposed to RF‐EMF for 3 months exhibited escape latencies that were similar to those of the sham‐exposed mice, indicating that spatial memory, as examined by the Morris water maze test, was not affected by 3 months of RF‐EMF exposure.
To evaluate hippocampus‐related non‐spatial memory in 5xFAD mice, the object recognition memory test was performed after the 3 months of RF‐EMF exposure. Twenty‐four hours after the training sessions, mice were tested in terms of novel object recognition. No differences in the preference for the novel object was observed between the sham‐exposed control mice and the RF‐EMF‐exposed mice (Fig. 2C), indicating that 3 months of RF‐EMF exposure did not affect this type of learning and memory.
Aβ Deposition in 5xFAD Brain
To examine, whether the exposure to RF‐EMF affected the levels of Aβ deposition in the brains of the 5xFAD mice, brain sections were stained with the Aβ‐specific 6E10 antibody. The animals were sacrificed at 3 months after RF‐EMF exposure (4.5 months old). Immunohistochemical analysis for Aβ in the brain sections from the RF‐exposed 5xFAD mice focused on the hippocampus and cortex, which are known to be primary sites of AD lesions and pathology. This analysis revealed the absence of differences in amyloid plaque deposition in both the hippocampus and cortex between the sham‐ and RF‐EMF‐exposed groups (Fig. 3A and B). These data suggest that 3 months of 1950 MHz RF‐EMF exposure did not change the accumulation of Aβ and its burden in the 5xFAD mouse model of AD.
Figure 3.
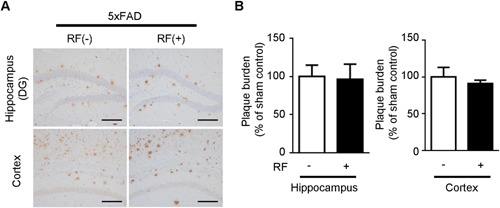
Effects of RF‐EMF exposure on protein levels and Aβ deposition in 5xFAD mice. (A) Immunohistochemistry analysis of 6E10‐positive Aβ deposition in 5xFAD brain sections following chronic RF‐EMF exposure. Scale bar: 100 μm (B) Quantification of Aβ deposition in 5xFAD mice following RF‐EMF exposure. Values are presented as mean ± SEM (n = 6).
APP Processing in 5xFAD Mice
To further elucidate the effect of RF‐EMF on APP processing, Western blotting was performed in the hippocampus and cortex of 5xFAD mice following RF‐EMF exposure. Although high levels of APP were observed in the hippocampus and cortex of 5xFAD mice compared to those in an age‐matched wild‐type mouse, RF‐EMF exposure did not cause any significant differences in APP protein levels (Fig. 4A and B). In addition, CTFβ levels in the hippocampus and cortex of 5xFAD mouse brains also were not changed by RF‐EMF exposure (Fig. 4A and B). We also analyzed Aβ 40 and 42 levels in plasma to further evaluate effects of RF‐EMF on APP processing. RF did not increase plasma Aβ levels in 5xFAD mice. These results indicate that 3 months of RF‐EMF exposure did not affect APP processing in the brains of 5xFAD mice.
Figure 4.
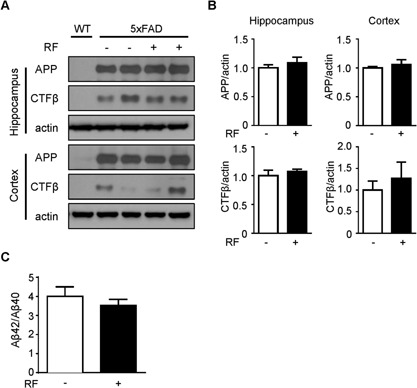
Effects of RF‐EMF exposure on protein levels of APP and CTFβ in Tg‐5xFAD mice. (A) Western blotting for APP and CTFβ using hippocampal and cortical extracts from 5xFAD mice following RF‐EMF exposure. A WT mouse was included for comparison. (B) Graph shows quantification of APP and CTFβ protein levels in 5xFAD mice (n = 6) based on band intensity. (C) Ratio of Aβ42/Aβ40 in plasma of 5xFAD mice was measured by colorimetric ELISA assay, and graph displays ratio of Aβ42/Aβ40 (n = 7–8). Values are presented as mean ± SEM.
DISCUSSION
Since the exponential increase of mobile phone use in daily life, public concern has been raised about possible side effects of RF‐EMF on human health, especially brain function. Alongside previous in vivo and in vitro studies that have reported that RF‐EMF induced oxidative stress [Ilhan et al., 2004; Xu et al., 2010], a possible association between RF‐EMF and neurodegeneration, such as AD, has been suggested. A previous in vivo study demonstrated that exposing C57BL/6 mice to GSM 1.8 GHz cell phone signals for 8 weeks induced transient impairments in spatial and non‐spatial memory [Ntzouni et al., 2013]. Furthermore, exposing rats to 2450 MHz caused spatial reference memory deficits, as measured by water maze performance [Wang and Lai, 2000]. In addition, impaired neurodevelopment and behavior were found in mice that were exposed to 800–1900 MHz‐rated cell phone signals in utero [Aldad et al., 2012]. However, there have also been reports that exposure to 900–2450 MHz did not induce spatial or non‐spatial memory deficits in rats [Dubreuil et al., 2003] or mice [Yamaguchi et al., 2003] when memory was evaluated behaviorally. Therefore, whether RF‐EMF exposure induces memory impairment remains controversial.
AD is an important neurodegenerative disease that is characterized by progressive cognitive impairment. Although the exact etiology of AD is poorly understood, senile plaques composed of Aβ protein deposits, particularly in the cortex and hippocampus, is one of the possible mechanisms of AD [McKhann et al., 1984; Findeis, 2000]. Intriguingly, a positive effect of RF‐EMF was reported in an AD mouse model. Previous studies have demonstrated that long‐term RF‐EMF exposure may have beneficial effects on cognitive deficits of AD transgenic mice [Arendash et al., 2010; Jeong et al., 2015], and this effect might be related to reduced Aβ deposition. Therefore, we used the 5xFAD transgenic mouse model, which shows AD‐like pathology, such as early Aβ plaque formation in brain parenchyma and behavioral deficits [Oakley et al., 2006]. We exposed 5xFAD mice to RF‐EMF for 3 months to evaluate the effect of RF‐EMF on memory impairment and Aβ pathology. To examine effects of RF‐EMF on behavioral function, we performed behavioral tests, including Y‐maze, Morris water maze, and object recognition test. We observed no effects of RF‐EMF on behavioral function and APP processing, including Aβ deposition, in 5xFAD mice. However, long‐term RF‐EMF exposure (918 MHz for 8 months) protected against cognitive impairment, which was demonstrated by radial arm water maze and cognitive interference task reported in transgenic APPsw mice [Arendash et al., 2010]. In addition, 8 months exposure to 1950 MHz RF‐EMF has been reported to significantly improve Y‐maze and passive avoidance performance in 5xFAD mice [Jeong et al., 2015]. These previous studies suggested that long‐term RF‐EMF exposure might be required to improve cognitive performance in AD mice, although some previous studies also showed a beneficial memory effect following exposure for periods shorter than 3 months. For example, in old (21–27 months old) APPsw AD model mice, 2 months of RF‐EMF exposure seemed to produce a cognitive benefit, as demonstrated by Y‐maze and radial arm water maze performance [Arendash et al., 2012]. In the triple transgenic mouse model of AD (3xTg‐AD), exposure to 2.4 GHz EMF signals for 28 days resulted in memory enhancement when mice were assessed via cognitive interference and Barnes maze test [Banaceur et al., 2013]. These differences may stem from the use of different genetically engineered animal models or time of onset of RF‐EMF exposure. We may not have observed a beneficial effect of 3‐month RF‐EMF exposure on spatial and non‐spatial memory performance because we used the 5xFAD mouse model, which has a relatively swiftly progressing AD phenotype. Therefore, more comprehensive studies that investigate effects of multiple durations of RF‐EMF exposure on neurocognitive functions in various AD animal models are necessary.
5xFAD mice showed massive Aβ accumulation, considerably impaired behavioral functions, and early AD‐like pathology. APP cleavage can cause accumulation of amyloid deposits, and accumulation of amyloid plaques in the brain parenchyma might be a possible mechanism for cognitive impairment in AD. A previous immunostaining study demonstrated that Aβ deposits in both the hippocampus and entorhinal cortex were significantly lower when Tg mice were exposed to RF‐EMF for 8.5 months [Arendash et al., 2010]. In addition, after 8 months of RF‐EMF exposure, Aβ deposition in the hippocampus and cortex was significantly down‐regulated in exposed Tg mice compared with control Tg mice, consistent with the observation of a beneficial effect on memory function [Jeong et al., 2015]. In the present study, positive Aβ immunostaining was detected in the brains of 4.5‐month‐old 5xFAD mice, consistent with results of a previous study that demonstrated that 5xFAD mice exhibit Aβ plaques at 2 months of age [Oakley et al., 2006]. However, in this study, RF‐EMF exposure did not seem to have an effect on Aβ deposition in either the cortex or hippocampus in AD mice. In addition, no differences were found in protein levels of APP and CTFβ, which are produced by APP cleavage by β‐secretase. Thus, these data suggest that 3 months of RF‐EMF exposure did not affect APP processing and Aβ accumulation in the brains of 5xFAD mice. Although we did not observe a beneficial effect of RF‐EMF on AD, the present study may provide a basis for further studies to determine beneficial or detrimental effects of RF‐EMF in a variety of neurodegenerative animal models (e.g., for AD, Parkinson's disease, or amyotrophic lateral sclerosis).
The present study aimed to investigate the effect of RF‐EMF on AD based on Aβ pathology. We found no significant effects of exposure to 1950 MHz RF‐EMF for 3 months on spatial and non‐spatial memory. In addition, no protective or detrimental effect of RF‐EMF on Aβ deposition was detected in 5xFAD brains, consistent with unaltered APP processing in 5xFAD mice. Thus, these data suggest that 3 months of 1950 MHz RF‐EMF exposure did not aggravate AD‐like pathology in 5xFAD mice.
Conflict of interest: None.
REFERENCES
- Aldad TS, Gan G, Gao XB, Taylor HS. 2012. Fetal radiofrequency radiation exposure from 800 to 1900 MHz‐rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep 2:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Sanchez‐Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M, Wang L, Zhang G, Sava V, Tan J, Cao C. 2010. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer's disease mice. J Alzheimers Dis 19:191–210. [DOI] [PubMed] [Google Scholar]
- Arendash GW. 2012. Transcranial electromagnetic treatment against Alzheimer's disease: Why it has the potential to trump Alzheimer's disease drug development. J Alzheimers Dis 32:243–266. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Dorsey M, Gonzalez R, Tajiri N, Borlongan C. 2012. Electromagnetic treatment to old Alzheimer's mice reverses beta‐amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE 7:e35751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R, Grosse Y, Lauby‐Secretan B, El Ghissassi F, Bouvard V, Benbrahim‐Tallaa L, Guha N, Islami F, Galichet L, Straif K, Group WHOIAfRoCMW. 2011. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol 12:624–626. [DOI] [PubMed] [Google Scholar]
- Banaceur S, Banasr S, Sakly M, Abdelmelek H. 2013. Whole body exposure to 2.4 GHz WIFI signals: Effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer's disease (3xTg‐AD). Behav Brain Res 240:197–201. [DOI] [PubMed] [Google Scholar]
- Carlberg M, Soderqvist F, Hansson Mild K, Hardell L. 2013. Meningioma patients diagnosed 2007–2009 and the association with use of mobile and cordless phones: A case‐control study. Environ Health 12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Bradshaw PC, Mamcarz M, Lin X, Wang L, Cao C, Arendash GW. 2011. Long‐term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer's transgenic mice and normal mice: A mechanism for electromagnetic field‐induced cognitive benefit? Neuroscience 185:135–149. [DOI] [PubMed] [Google Scholar]
- Dubreuil D, Jay T, Edeline JM. 2003. Head‐only exposure to GSM 900‐MHz electromagnetic fields does not alter rat's memory in spatial and non‐spatial tasks. Behav Brain Res 145:51–61. [DOI] [PubMed] [Google Scholar]
- Findeis MA. 2000. Approaches to discovery and characterization of inhibitors of amyloid beta‐peptide polymerization. Biochimica et Biophysica Acta 1502:76–84. [DOI] [PubMed] [Google Scholar]
- Group IS. 2010. Brain tumour risk in relation to mobile telephone use: Results of the INTERPHONE international case‐control study. Int J Epidemiol 39:675–694. [DOI] [PubMed] [Google Scholar]
- Hardell L, Carlberg M, Soderqvist F, Hansson Mild K. 2008. Meta‐analysis of long‐term mobile phone use and the association with brain tumours. Int J Oncol 32:1097–1103. [PubMed] [Google Scholar]
- Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M, Akyol O, Ozen S. 2004. Ginkgo biloba prevents mobile phone‐induced oxidative stress in rat brain. Clin Chim Acta 340:153–162. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Kang GY, Kwon JH, Choi HD, Pack JK, Kim N, Lee YS, Lee HJ. 2015. 1950 MHz electromagnetic fields ameliorate abeta pathology in Alzheimer's disease mice. Curr Alzheimer Res 12:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan P, Simonsen SE, Lyon JL, Kestle JR. 2008. Cellular phone use and brain tumor: A meta‐analysis. J Neurooncol 86:71–78. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Jin YB, Kim TH, Pack JK, Kim N, Choi HD, Lee JS, Lee YS. 2012. The effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic fields on rat testicular function. Bioelectromagnetics 33:356–364. [DOI] [PubMed] [Google Scholar]
- Maaroufi K, Had‐Aissouni L, Melon C, Sakly M, Abdelmelek H, Poucet B, Save E. 2014. Spatial learning, monoamines and oxidative stress in rats exposed to 900 MHz electromagnetic field in combination with iron overload. Behav Brain Res 258:80–89. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Moon M, Cha MY, Mook‐Jung I. 2014. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J Alzheimers Dis 41:233–241. [DOI] [PubMed] [Google Scholar]
- Morris R. 1984. Developments of a water‐maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. [DOI] [PubMed] [Google Scholar]
- Ntzouni MP, Stamatakis A, Stylianopoulou F, Margaritis LH. 2011. Short‐term memory in mice is affected by mobile phone radiation. Pathophysiology 18:193–199. [DOI] [PubMed] [Google Scholar]
- Ntzouni MP, Skouroliakou A, Kostomitsopoulos N, Margaritis LH. 2013. Transient and cumulative memory impairments induced by GSM 1.8 GHz cell phone signal in a mouse model. Electromagn Biol Med 32:95–120. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet‐Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. 2006. Intraneuronal beta‐amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. J Neurosci 26:10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG. 1992. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258:126–129. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz ZJ, Blackwell RP, Haylock RG, Saunders RD, Cobb BL. 2000. Low‐level exposure to pulsed 900MHz microwave radiation does not cause deficits in the performance of a spatial learning task in mice. Bioelectromagnetics 21:151–158. [DOI] [PubMed] [Google Scholar]
- Wang B, Lai H. 2000. Acute exposure to pulsed 2450‐MHz microwaves affects water‐maze performance of rats. Bioelectromagnetics 21:52–56. [PubMed] [Google Scholar]
- Xu S, Zhou Z, Zhang L, Yu Z, Zhang W, Wang Y, Wang X, Li M, Chen Y, Chen C, He M, Zhang G, Zhong M. 2010. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res 1311:189–196. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Tsurita G, Ueno S, Watanabe S, Wake K, Taki M, Nagawa H. 2003. 1439 MHz pulsed TDMA fields affect performance of rats in a T‐maze task only when body temperature is elevated. Bioelectromagnetics 24:223–230. [DOI] [PubMed] [Google Scholar]


