Abstract
The aim of the study was to improve the oral absorption of the compound 25-OCH3-PPD with poor hydrophilicity and lipophilicity. 25-OCH3-PPD-phospholipid complex was prepared by solvent evaporation, then characterized by differential scanning calorimetry, scanning electron microscopy, and infrared absorption spectroscopy. The aqueous solubility and oil–water partition coefficient were compared with the free compound. A nanoemulsion loaded with 25-OCH3-PPD-phospholipid complex was developed by dissolving the complex in water in the presence of hydrophilic surfactant under sonication. After oral administration of the nanoemulsion and the suspension of 25-OCH3-PPD in rats, the concentrations of 25-OCH3-PPD in plasma were determined by high-performance liquid chromatography–tandem mass spectrometry method. The results showed that the solubility of the complex in water and n-octanol was enhanced. The oil–water partition coefficient improved 1.7 times. Peak plasma concentration and area under the curve(0–24 h) of the nanoemulsion of 25-OCH3-PPD-phospholipid complex were higher than that of free compound by 3.9- and 3.5-folds.
Keywords: 25-OCH3-PPD, phospholipid complex, solubility, bioavailability, LC–MS/MS
Introduction
Ginseng (Panax ginseng Meyer), a typical traditional medicinal herb listed in Shin-Nong-Bon-Cho-Kyung, has been used for thousands of years, and also applied as a functional food or therapeutic supplement in Western countries.1–5 A number of research investigations have been conducted to confirm its pharmacological and therapeutic effects in vitro or in vivo. Various beneficial activities of ginseng have been reported, including for cardiovascular system, immune function, and metabolism. Many investigations have focused on its advantage in cancer therapy in recent years.6–10
20(R)-25-methoxyl-dammarane-3β, 12β, 20-triol (25-OCH3-PPD) (Figure 1), a novel dammarane-type triterpene saponin, was isolated from Panax ginseng. The saponin exhibited 10- to 100-fold greater antitumor activities against several cancer cell lines than ginsenoside-Rg3, which has been listed as an adjuvant chemotherapy drug in the People’s Republic of China.11–13 The compound 25-OCH3-PPD has also shown inhibition of growth and proliferation, especially in breast cancer cell lines, inducing apoptosis and cell cycle arrest in the G1 phase.14 It displayed low toxic effects in an acute toxicity or a long toxicity test after oral administration. However, the oral bioavailability of 25-OCH3-PPD in rats was only 13% because of its poor water solubility.15,16
Figure 1.

Chemical structure of 20(R)-25-methoxyl-dammarane-3β, 12β, 20-triol.
Numerous approaches have been reported to enhance water solubility of hydrophobic drugs, such as salt formation, solid dispersion,17 encapsulation in cyclodextrins,18 nanoparticles,19 and lipid and surfactant-based dispersions.20 Phospholipids constitute a major part of the cell membrane and have many advantages as a carrier system, thus gaining increasing attention. Many studies have demonstrated that phospholipid complexes possess the advantages of both enhancing the solubility of a drug with low water solubility and improving the oral bioavailability of lipophilic drugs.21–29 The atom of hydroxyl oxygen upon phosphorus in the structure of phospholipid has a strong tendency to gain electrons while the nitrogen has a strong tendency to lose electrons. Under certain conditions, a complex may generate between the active compound with phospholipid. Therefore in this present study, for the purpose of improving the oral bioavailability of 25-OCH3-PPD, a phospholipid complex was prepared by the solvent evaporation method and its physicochemical properties were characterized by differential scanning calorimetry (DSC), infrared (IR), and scanning electron microscopy (SEM). The solubility in water and octanol/water partition coefficient (log P) of 25-OCH3-PPD-phospholipid was evaluated by comparing with pure 25-OCH3-PPD and physical mixture. Nanoemulsion of the phospholipid complex was prepared in order to investigate the pharmacokinetic behavior in rats for oral administration. The concentration of 25-OCH3-PPD in plasma was determined after oral administration of free 25-OCH3-PPD suspension and nanoemulsion phospholipid complex equivalent to 20 mg/kg of 25-OCH3-PPD.
Materials and methods
Materials and animals
25-OCH3-PPD (purity ≥98%) was prepared in our laboratory (Shenyang Pharmaceutical University, Shenyang, People’s Republic of China), soybean phospholipids (phosphatidyl choline, purity $96%) were provided by Tian-feng Biological Pharmaceutical Co., Ltd. (Shenyang, People’s Republic of China). Water, polyethylene glycol 400 (PEG 400) and methanol (high-performance liquid chromatography [HPLC] grade) were purchased from Kangkede Technology Co, Ltd (Tianjin, People’s Republic of China). n-Octanol was procured from Bodi Chemicals (Tianjin, People’s Republic of China). All other chemicals used were of analytical grade.
Male Sprague Dawley (SD) rats (15 weeks old) weighing 280–320 g were provided from the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, People’s Republic of China). All experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Shenyang Pharmaceutical University in Shenyang and the ethical approval was also approved by Shenyang Pharmaceutical University.
Nanoemulsion based on 25-OCH3-PPD-phospholipid complex
The complex was prepared with 25-OCH3-PPD and phospholipid at a suitable molar ratio (1:1, 1:1.5, and 1:2). The phospholipid was dissolved in ethanol by the ultrasonic method and then the required amount of 25-OCH3-PPD was added. The mixture was stirred at specified temperatures (40°C, 50°C, and 60°C) for some time. Then, the solution was evaporated using a rotary evaporator at 40°C to obtain a dry complex. The optimization of the preparation process was carried out based on the ratio of 25-OCH3-PPD to phospholipid and the temperature of complexation. DSC, IR, and SEM analyses were used to confirm the formation of phospholipid complex. Nanoemulsion of the phospholipid complex was prepared by dissolving the complex in 1% PEG400 water solution (2.5 mg/mL), and sonication was carried out for at 4°C at 400 W ultrasonic power 30 times to obtain a nanoemulsion with a small particle size. The average particle size of the nanoemulsion was measured by dynamic light scattering technique. The optimal formulation was selected according to the formation of the complex and droplet size.
HPLC analysis of 25-OCH3-PPD
All the samples were measured by HPLC (PUMP K-501, Unico Instruments Co., Ltd., Shanghai, People’s Republic of China) with an evaporative light scattering detector (Unimicro Technologies Inc, Shanghai, People’s Republic of China). The mobile phase consisted of methanol and water at a ratio of 87:13 (v/v). The flow rate of elution was set at 1.0 mL/min at 25°C and the parameter for the evaporative light scattering detector was set to a probe temperature of 50°C; the nebulizer for nitrogen gas was adjusted to 3 L/min. The calibration curve exhibited good linearity by fitting the peak area to the corresponding concentrations (A=7,266 C – 6,7354, R2=0.9990) in the concentration range 5–500 μg/mL for 25-OCH3-PPD.
DSC
DSC experiments were conducted using DSC-60. The samples were sealed in the aluminum crimp cell and heated from 30°C to 300°C at increments of 10°C/min under nitrogen atmosphere. The peak transition onset temperature of 25-OCH3-PPD-phospholipid complex, physical mixture (1:1), pure 25-OCH3-PPD, and phospholipid were determined and compared.
SEM
25-OCH3-PPD, 25-OCH3-PPD-phospholipid complex, physical mixture (1:1), and phospholipid were coated with platinum in a sputter coater and their surface morphology was viewed and photographed by field emission scanning electron microscopy (JEM-6700F, JEOL, Tokyo, Japan).
IR absorption spectroscopy
The IR spectra of 25-OCH3-PPD-phospholipid, physical mixture (1:1), 25-OCH3-PPD, and phospholipid were identified and compared by the IR spectrophotometric method with potassium bromide as supporter. The spectra were recorded in the range of 400–4,000 cm−1. Spectra were recorded on a Fourier transform IR spectrophotometer (Bruker IFS 55, Karlsruhe, Germany).
Solubility studies
The solubility analysis of 25-OCH3-PPD-phospholipid complex, physical mixture (1:1), and pure 25-OCH3-PPD were performed by adding excess of these samples to 5 mL of water or n-octanol in sealed glass vials and placing them in an air bath oscillator (THZ-82B, Medical Instrument Factory, People’s Republic of China) for 24 hours at 37°C. The samples were centrifuged at a speed of 15,000 g for 10 minutes at room temperature. The supernatant was filtered through a 0.22 μm membrane and diluted with methanol for quantification analysis of 25-OCH3-PPD by HPLC method. Each experiment was performed in triplicate.
Oil–water partition coefficient studies
25-OCH3-PPD, physical mixture (1:1), and 25-OCH3-PPD-phospholipid complex was put into 5 mL water in a sealed container and shaken at 37°C for 24 hours to reach equilibrium, separately. Then 5 mL of n-octanol was added to the mixture and mixed for 24 hours. The mixture was allowed to stand for completion of phase separation. After letting it stand for layering, the water and n-octanol were filtered using 0.22 μm membranes. The concentration of 25-OCH3-PPD in the filtered solutions were analyzed after dilution by mobile phase by HPLC—evaporative light scattering detector. The octanol–water partition coefficient of 25-OCH3-PPD was calculated based on their concentration in each phase. The experiment was carried out in triplicate.
Chromatography and mass spectrometry conditions
The concentration of 25-OCH3-PPD in plasma samples were analyzed by liquid chromatography–tandem mass spectrometry. The HPLC was performed on an Agilent 1290 ultra performance liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA) with a Shiseido C18 column (150 mm ×4.6, 5 μm, Shiseido, Tokyo, Japan). The column was maintained at 30°C. The solvent consisted of a solution of acetonitrile and 5 mM aqueous ammonium acetate (73:27 v/v). The flow rate was 1.0 mL/min, and injection volume was 10 μL. The HPLC system was connected with an API 4,000 triple-quadrupole tandem mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA, USA) with an electrospray source, equipped with a turbo ion spray interface in the positive mode along with an ion-spray voltage of 4,500 V, a curtain gas pressure of 20 psi, a nebulizer gas pressure of 40 psi, a heater gas pressure of 40 psi, and a collision gas pressure of 4 psi. The source temperature was set at 500°C. All of the gases were nitrogen. The fragmentation transitions for the multiple reaction monitoring were m/z 493.5 → 425.4 amu for 25-OCH3-PPD and m/z 430.3 → 372.1 for mifepristone as internal standard substance.
Quantitative analysis of plasma samples
Frozen samples were allowed to thaw at room temperature. To 50 μL plasma sample in a glass tube was added 10 μL of the internal standard solution (6 μg/mL) and 100 μL acetonitrile. The mixture was vortexed for 30 seconds and centrifuged at 14,000 g for 5 minutes. Then, 10 μL of the supernatant was injected for liquid chromatography–tandem mass spectrometry canalysis. The whole analytical methodology was validated for accuracy and precision as previously described.16
Pharmacokinetics studies of free 25-OCH3-PPD and nanoemulsion 25-OCH3-PPD-phospholipid
For pharmacokinetic study, male SD rats were provided by the Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, People’s Republic of China). All animals were housed under controlled conditions (23°C±2°C, relative humidity 50%±5%) with a natural light–dark cycle for at least 1 week before starting the experiments. All the rats were given free access to water and standard rodent food for 1 week before administration of the phospholipid complex. Ten SD male rats were randomly assigned to two groups. All the rats were fasted but had free access to water for 12 hours. One group was treated with oral 25-OCH3-PPD suspension, which was prepared in 5% sodium carboxymethyl starch solution, at a dose of 20 mg/kg (1.25 mg/mL), while the other group was orally administered a nanoemulsion of 25-OCH3-PPD-phospholipid complex of equal dose.
The rats were anesthetized with ether and 300 μL of blood was collected into heparinized tubes from the eyeground veins at 0.25, 0.5, 1.0, 1.5, 2.5, 4.0, 6.0, 9.0, 12, 24 hours after oral administration. Thereafter, the blood was centrifuged at 3,500 g for 10 minutes, and was stored at −20°C before analysis.
Statistical analysis
Pharmacokinetic parameters were calculated using DAS 2.0 software (Shanghai Traditional Chinese Medicine University, Shanghai, People’s Republic of China). Analysis of variance of maximum concentration (Cmax) and area under the curve (AUC)(0–24 h) was performed, and the mean separation was done by least significant difference (P<0.05) using SPSS 16.0 program for windows (SPSS Inc., Chicago, IL, USA).
Results
Preparation of nanoemulsion based on 25-OCH3-PPD-phospholipid complex
The complex was prepared with 25-OCH3-PPD and phospholipid with molar ratio 1:1 at 40°C for 2 hours. The contents of 25-OCH3-PPD in the complex were 40.11%. The formation of phospholipid complex was confirmed by DSC, IR, and SEM analysis. Nanoemulsion of the phospholipid complex was prepared by dissolving 4 mg/mL of the complex in 1% of hydrophilic surfactant PEG 400 water solution, and the average particle size was 118.7 nm with polydispersity index of 0.124. Excessive temperatures were discouraged as they could promote the degradation of phospholipid.
DSC
DSC thermograms of pure 25-OCH3-PPD (A), phospholipid (B), physical mixture (C), and 25-OCH3-PPD-phospholipid (D) are demonstrated in Figure 2. The thermal curve of 25-OCH3-PPD showed a typical sharp peak at ~263.83°C, while phospholipid exhibited two different endothermal peaks. The first peak at 218.64°C was mild, suggesting phopsholipid polar parts. The second one at 254.82°C relatively sharp owing to the transition point from a gel-like state to liquid crystal state. The DSC curve for the 25-OCH3 PPD-phospholipid complex showed the endothermal peak at 241.77°C and the peak of 25-OCH3-PPD almost disappeared. Physical mixture of 25-OCH3-PPD and phospholipid exhibited a peak at 265.77°C, nearly the same as the of 25-OCH3-PPD.
Figure 2.
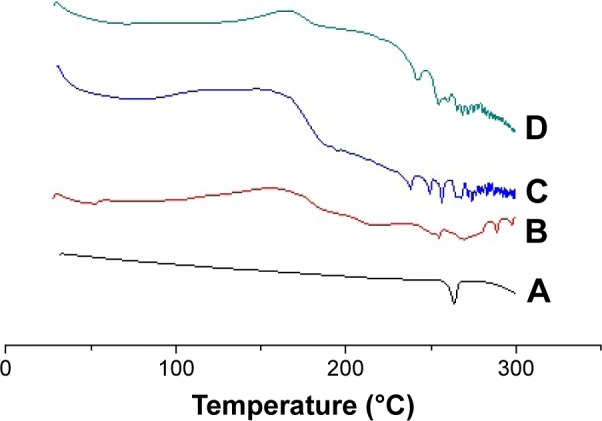
Differential scanning calorimetry thermograms of 25-OCH3-PPD (A), phospholipid (B), physical mixture (C), and complex (D).
Scanning electron microscopy
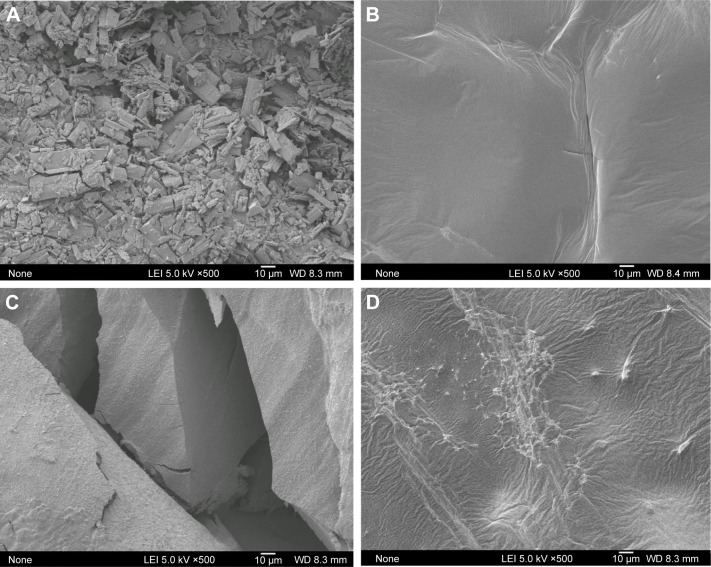
The surface morphology of 25-OCH3-PPD-phospholipid complex, physical mixture, and phospholipid are shown in Figure 3. With ×500 magnification, pure 25-OCH3-PPD appeared as a column crystal structure (Figure 3A). Whereas it was evident that 25-OCH3-PPD was uniformly dispersed in the 25-OCH3-PPD-phospholipid complex in the amorphous state (Figure 3B), the shape of 25-OCH3-PPD and phospholipid (Figure 3D) appeared in the physical mixture (Figure 3C).
Figure 3.
Scanning electron microscopic pictures of 25-OCH3-PPD (A), 25-OCH3-PPD-phospholipid complex (B), physical mixture (C), and phospholipid (D) at ×500 magnification.
Infrared absorption spectroscopy
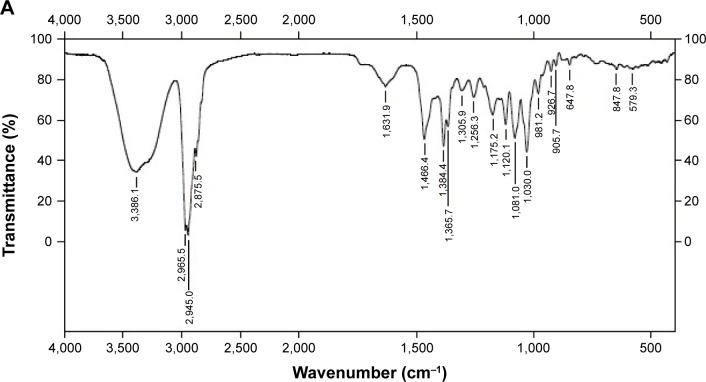
The complex confirmed by Fourier transform IR spectroscopy and the spectra of 25-OCH3-PPD (A), phospholipid (B), physical mixture (C), and 25-OCH3-PPD-phospholipid (D) are shown in Figure 4. The remarkable feature of 25-OCH3-PPD was the hydroxyl stretching vibration at 3,386.1 cm−1 (Figure 4A), which was 3,286.1 cm−1 (Figure 4D) in the phospholipid complex and 3,400.7 cm−1 (Figure 4C) in the physical mixture. In addition, the P-O bond stretching vibration in phospholipid molecules was sharp at 1,242.9 cm−1 (Figure 4B), which was 1,246.2 cm−1 (Figure 4C) in the physical mixture but weak at 1,246.9 cm−1 (Figure 4D) in the phospholipid complex. The results suggested that the hydroxyl in 25-OCH3-PPD interacted with P-O group in phospholipid.
Figure 4.
Infrared radiation of 25-OCH3-PPD (A), phospholipid (B), physical mixture (C), and complex (D).
Solubility and oil–water partition coefficient of 25-OCH3-PPD-phospholipid complex
Table 1 displayed the solubility of 25-OCH3-PPD, physical mixture, 25-OCH3-PPD-phospholipid in water and in n-octanol at 37°C. The results showed that the solubility of 25-OCH3-PPD-phospholipid in water and in n-octanol exhibited enhancement of 4.9 and 1.4 times, respectively. Lipophilicity is usually measured as partition coefficient as log P. The apparent partition coefficients of pure 25-OCH3-PPD, physical mixture, and complex in n-octanol/water system were 1.28, 1.53, and 2.19. The results illustrated that log P of phospholipid complex was 1.7 times higher compared with free compound.
Table 1.
Apparent solubility of 25-OCH3-PPD, physical mixture and complex in water and in n-octanol at room temperature
| Samples | Solubility (μg/mL)
|
|
|---|---|---|
| Water | n-octanol | |
| 25-OCH3-PPD | 9.44±0.11 | 518.87 |
| Physical mixture | 22.59±1.13 | 454.11 |
| 25-OCH3-PPD-phospholipid complex | 46.87±1.17 | 715.95 |
Note: n=3, mean ± SD.
Abbreviation: SD, standard deviation.
Pharmacokinetics experiments
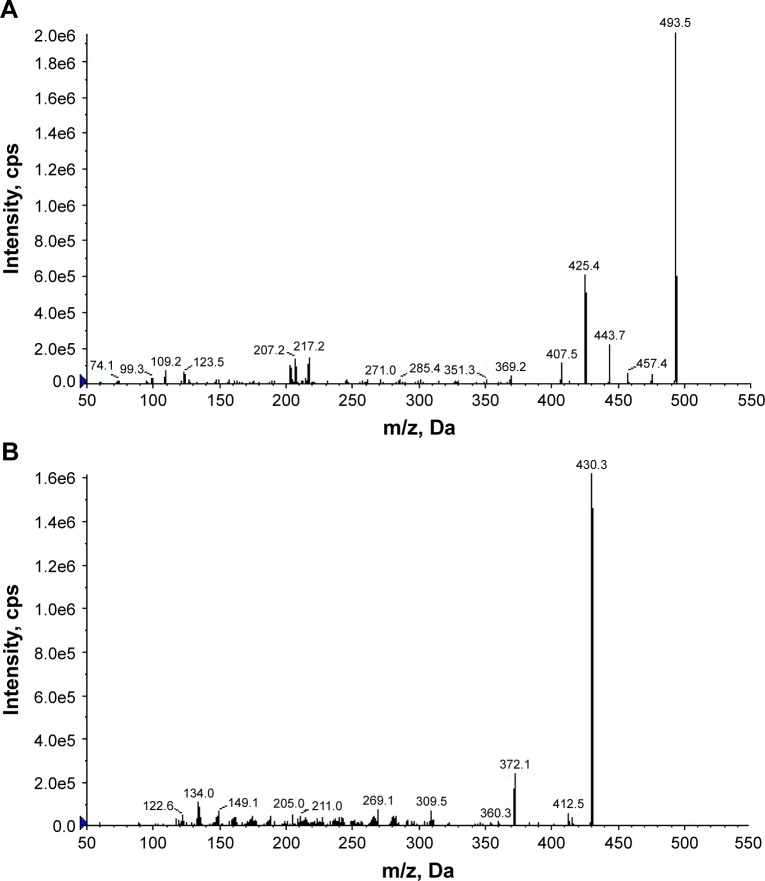
The responses of 25-OCH3-PPD and mifepristone to electron spray ionization were evaluated by recording the full-scan mass spectra in both positive and negative ionization modes. The response in the negative mode was better than the positive mode. These tandem mass spectrometry fragmentations are shown in Figure 5. The fragmentation transitions for the multiple reaction monitoring were m/z 479.4 → m/z 461.5 amu for 25-OCH3-PPD, and m/z 430.3 → m/z 372.1 amu for mifepristone. Peak area ratios of the analyte to internal standard were calculated and calibration curves were established by fitting the ratio (y) to the corresponding concentrations (x) by least squares regression with 1/X2 as weighing factor. Quantification range for 25-OCH3-PPD was 2.5–1,000 ng/mL and the calibration curve was: y=0.00193x+0.000853 (r=0.9967).
Figure 5.
Product ion mass spectra of [M+H]+ ions. Product ion mass spectra of 25-OCH3-PPD (m/z 493.5 → 425.4) (A) and mifepristone (m/z 430.3 → 372.1) (B).
The mean plasma concentration versus time profiles of 25-OCH3-PPD after oral administration of nanoemulsion and suspension at a dose of 20 mg/kg are depicted in Figure 6. The pharmacokinetic parameters of 25-OCH3-PPD obtained from the study are shown in Table 2. The results indicate that Cmax of nanoemulsion was 3.9-fold higher compared to Cmax of pure compound. Meanwhile, t1/2 of the nanoemulsion and pure compound was 3.58 and 2.51 hours, respectively. The AUC(0–24 h) of 25-OCH3-PPD-phospholipid complex (97.24 ng/mL⋅h) was 3.65-fold higher than that of pure compound (26.65 ng/mL·h). This increase of AUC(0–24 h) in the nanoemulsion resulted in a relative bioavailability of 365% compared to pure compound.
Figure 6.

The plasma concentration–time curve of 25-OCH3-PPD in rats after the oral administration of 25-OCH3-PPD in Carboxyl methyl Cellulose (CMC) and the complex (20 mg/kg, 25-OCH3-PPD).
Note: Data are presented as mean ± SD, (n=5).
Abbreviation: SD, standard deviation.
Table 2.
Pharmacokinetic parameters of 25-OCH3-PPD and complex in rats after oral administration
| Parameters | 25-OCH3-PPD | 25-OCH3-PPD-phospholipid |
|---|---|---|
| t1/2 (hours) | 2.51±0.74 | 3.58±0.34 |
| Tmax (hours) | 2.25±2.47 | 2.10±0.55 |
| Cmax (ng/mL) | 7.18±3.39 | 28.07±19.52* |
| AUC(0–24 h) (ng/mL hours) | 26.65±7.11 | 97.24±40.17* |
| AUC(0–∞) (ng/mL hours) | 44.52±16.40 | 114.02±44.84 |
Notes: Data are presented as mean ± SD (n=5),
P<0.05.
Abbreviations: AUC, area under the curve; SD, standard deviation.
Discussion
Phospholipids, the major building blocks of cell membranes, are miscible in both water and lipid environments and thus can accompany the compound through biological membranes and improve absorption and bioavailability. Drug–phospholipid complexes are drug carrier systems that were introduced by the Italian researcher Bombardelli during his studies on liposomes.30 The phospholipid complexes of active compounds from traditional Chinese medicine, including oxymatrine, quercetin, and mangiferin improved gastrointestinal absorption and increased bioavailability.31–33
25-OCH3-PPD has low water and lipid solubility, and exhibits low oral absorption. This group previously developed a self-microemulsifying drug delivery system to enhance the oral bioavailability of 25-OCH3-PPD due to enhancing permeation across the intestinal membrane.34 However, self-microemulsifying drug delivery system formulation included more surfactants, which would be harmful to human health. Complex with phospholipids may considerably improve the physicochemical properties of active components derived from herbal medicines, meanwhile enhance bioavailability. The nanoemulsion of 25-OCH3-PPD was prepared based on phospholipid complex in order to administrate to rats easily. In the present study, the solubilities of this compound in water and n-octanol were enhanced after formation with phospholipid. Phospholipid that acts as amphiphilic surfactant increases the solubility because of its wetting function, thus lowering the interfacial tension. The increased oral absorption of 25-OCH3-PPD in rats can be attributed to the increased hydrophilic and lipophilic property of the compound, possibly enhancing the rate and the extent of entry into intestinal fluids and penetration of the intestinal mucosa.
25-OCH3-PPD-phospholipid complex was characterized by DSC, SEM, and IR analyses, which are reliable and rapid methods to screen drug and excipient compatibility, and provide maximum information about the possible interactions.23 The peak of 25-OCH3-PPD almost disappeared and a new peak occurred in the DSC curve of 25-OCH3-PPD-phospholipid complex. The surface morphology of 25-OCH3-PPD-phospholipid complex was different from the free compound. The spectrum of IR demonstrated the force of hydrogen bond between phospholipid and 25-OCH3-PPD, while there existed little differences between the IR spectra of 25-OCH3-PPD-phospholipid complex and physical mixture. 25-OCH3-PPD was uniformly dispersed in the amphiphilic structure of phospholipid, and the quantity of phospholipid was more than that of 25-OCH3-PPD, so signals of phospholipid overshadowed many weaker signals from smaller quantities of 25-OCH3-PPD. These findings suggest that some weak physical interactions occurred during the complex formation. There was no crystal habit present on surface morphology studies, so it could be concluded the complex was formed.
The water solubility of 25-OCH3-PPD in phospholipid complex was 4.9-fold compared with free compound. The partition coefficients (log P) of 25-OCH3-PPD in the form of phospholipid complex was 1.7 times compared with free compound. The increased log P was due to both the lipophilicity of phospholipid and the intermolecular forces between the compound and phospholipid.25 After oral administration of free compound suspension and emulsion of phospholipid complex in rats, the AUC(0–24 h) and Cmax of 25-OCH3-PPD in the phospholipid complex were about 3.5 and 3.9-fold of free compound. Furthermore, Tmax with nanoemulsion (2.1 hours) was little shorter than free compound (2.25 hours) and may be attributed to the quick absorption due to the small size of vesicles, modified lipophilicity, and similarity between the emulsion and biomembranes.
Conclusion
The present investigation reports the development of nanoemulsion of 25-OCH3-PPD based on a phospholipid complex. Phospholipid complex of 25-OCH3-PPD was prepared and evaluated in vitro and in vivo. Solubility studies showed there was a higher solubility in water (4.9 times) and n-octanol (1.4 times) for the phospholipid complex. The in vivo pharmacokinetic results are promising in demonstrating the improved oral bioavailability of 25-OCH3-PPD using nanoemulsion prepared by phospholipid complex.
Acknowledgments
This work was financially supported by Technology Platform of Industrialization Chromatographic Preparation for Standard Extract of Traditional Chinese Medicine (2010ZX09401-304-105B) and the National Science Foundation of China (grant number 81273389).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen S, Wang Z, Huang Y, et al. Ginseng and anticancer drug combination to improve cancer chemotherapy: a critical review. Evid Based Complement Alternat Med. 2014;2014:168940. doi: 10.1155/2014/168940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H, Høiby N, Yang L, Givskov M, Song Z. Effects of Radix Ginseng on microbial infections: a narrative review. J Tradit Chin Med. 2014;34(2):227–233. doi: 10.1016/s0254-6272(14)60083-2. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi MH, Siddiqi MZ, Ahn S, et al. Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res. 2013;37(3):8–29. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MS, Lim HJ, Yang HJ, Lee MS, Shin BC, Ernst E. Ginseng for managing menopause symptoms: a systematic review of randomized clinical trials. J Ginseng Res. 2013;37(1):30–36. doi: 10.5142/jgr.2013.37.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Kim TH, Choi TY, Lee MS. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. Plos One. 2013;8(4):e59978. doi: 10.1371/journal.pone.0059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Chen D, Li W, Xiao D. 20(S)-Protopanaxadiol saponins inhibit SKOV3 cell migration. Oncol Lett. 2016;11(3):1693–1698. doi: 10.3892/ol.2016.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CZ, Cai Y, Anderson S, Yuan CS. Ginseng metabolites on cancer chemoprevention: an angiogenesis link? Diseases. 2015;3(3):193–204. doi: 10.3390/diseases3030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytother Res. 2016;30(5):741–753. doi: 10.1002/ptr.5586. [DOI] [PubMed] [Google Scholar]
- 9.Xiao J, Chen D, Lin XX, et al. Screening of drug metabolizing enzymes for the ginsenoside compound K in vitro: an efficient anti-cancer substance originating from Panax ginseng. PLoS One. 2016;11(2):e0147183. doi: 10.1371/journal.pone.0147183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Liu J, Chen W, et al. 20(S)-Protopanoxadiol derivative overcomes multi-drug resistance by antagonizing ATP-binding cassette subfamily B member 1 transporter function. Oncotarget. 2016;7(8):9388–9403. doi: 10.18632/oncotarget.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang LH, Jia YL, Lin XX, et al. AD-1, a novel ginsenoside derivative, shows anti-lung cancer activity via activation of p38 MAPK pathway and generation of reactive oxygen species. BBA-Biomembranes. 2013;1830(8):4148–4159. doi: 10.1016/j.bbagen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine – ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16(22):2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao JM, Li N, Zhang H, Wu CF, Piao HR, Zhao YQ. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bloorg Med Chem Lett. 2011;21(3):1027–1031. doi: 10.1016/j.bmcl.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Liu YF, Yuan HN, Bi XL, et al. 25-Methoxyl-protopanaxadiol derivatives and their anti-proliferative activities. Steroids. 2013;78(14):1305–1311. doi: 10.1016/j.steroids.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Xu J, Zhang D, Gu J, Zhao Y. The study of pharmacokinetics of 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol and its active metabolite after oral and intravenous administration in rat. Xenobiotica. 2009;39(6):457–464. doi: 10.1080/00498250902810951. [DOI] [PubMed] [Google Scholar]
- 16.Shi CH, Zhang X, Suo H, et al. Simultaneous determination by LC-MS/MS of 25-methoxydammarane-3β, 12β, 20-triol epimers and active metabolites in rat plasma after intravenous administration. Xenobiotica. 2013;43(10):868–874. doi: 10.3109/00498254.2013.789149. [DOI] [PubMed] [Google Scholar]
- 17.Kubo Y, Terashima Y, Yagi N, Nochi H, Tamoto K, Sekikawa H. Enhanced bioavailability of probucol following the administration of solid dispersion systems of probucol-polyvinylpyrrolidone in rabbits. Biol Pharm Bull. 2009;32(11):1880–1884. doi: 10.1248/bpb.32.1880. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Xiao H, Li J, Zhong Y. Drug carrier systems based on water-soluble cationic β-cyclodextrin polymers. Int J Pharm. 2004;278(2):329–342. doi: 10.1016/j.ijpharm.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Io T, Fukami T, Yamamoto K, et al. Homogeneous nanoparticles to enhance the efficiency of a hydrophobic drug, antihyperlipidemic probucol, characterized by solid-state NMR. Mol Pharm. 2010;7(1):299–305. doi: 10.1021/mp900254y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen FS, Petersen KB, Mullertz A. Bioavailability of probucol from lipid and surfactant based formulations in minipigs: influence of droplet size and dietary state. Eur J Pharm Biopharm. 2008;69(2):553–562. doi: 10.1016/j.ejpb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Singh C, Bhatt TD, Gill MS, Suresh S. Novel rifampicin-phospholipid complex for tubercular therapy: synthesis, physicochemical characterization and in-vivo evaluation. Int J Pharm. 2014;460(1–2):220–227. doi: 10.1016/j.ijpharm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Y, Song Y, Chen Z, Ping Q. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 2006;307(1):77–82. doi: 10.1016/j.ijpharm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Zhang M, Liu Z, et al. Development of quercetin-phospholipid complex to improve the bioavailability and protection effects against carbon tetrachloride-induced hepatotoxicity in SD rats. Fitoterapia. 2016;113:102–109. doi: 10.1016/j.fitote.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Prasanna H, Smita M, Ramesh K, Sagar J, Rashmi V, Venkatrao K. Preparation and evaluation of Bacopa–phospholipid complex for anti-amnesic activity in rodents. Drug Invent Today. 2013;5(1):13–21. [Google Scholar]
- 25.Maiti K, Mukherjee K, Gangtait A, Ahamed HN, Saha BP, Mukherjee PK. Enhanced therapeutic benefit of quercetin–phospholipid complex in carbon tetrachloride induced acute liver injury in rats: a comparative study. Int J Phamr Tech. 2005;4:84–90. [Google Scholar]
- 26.Maiti K, Mukherjee K, Gangtait A, Saha BP, Mukherjee PK. Enhanced therapeutic potential of naringenin-phospholipid complex in rats. J Pharm Pharmacol. 2006;58(9):1227–1233. doi: 10.1211/jpp.58.9.0009. [DOI] [PubMed] [Google Scholar]
- 27.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330(1–2):155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114(2):242–250. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Semalty A, Semalty M, Rawat BS, Singh D, Rawat MS. Development and evaluation of pharmacosomes of aceclofenac. Indian J Pharm Sci. 2010;72(5):576–581. doi: 10.4103/0250-474X.78523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bombardelli E. Phytosome: new cosmetic delivery system. Boll Chim Farm. 1991;130(11):431–438. [PubMed] [Google Scholar]
- 31.Yue PF, Yuan HL, Li XY, Yang M, Zhu WF. Process optimization, characterization and evaluation in vivo of oxymatrine-phospholipid complex. Int J Pharm. 2010;387(1–2):139–146. doi: 10.1016/j.ijpharm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Maiti K, Mukherjee K, Gantait A, Ahamed HN, Saha BP, Mukherjee PK. Enhanced therapeutic benefit of quercetin-phospholipid complex in carbon tetrachloride-induced acute liver injury in rats: a comparative study. Iran J Pharmacol Ther. 2005;4(2):84–90. [Google Scholar]
- 33.Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. 2014;93:54–61. doi: 10.1016/j.fitote.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Cai S, Shi C, Zhang X, et al. Self-microemulsifying drug-delivery system for improved oral bioavailability of 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol: preparation and evaluation. Int J Nanomed. 2014;9:913–920. doi: 10.2147/IJN.S56894. [DOI] [PMC free article] [PubMed] [Google Scholar]