Abstract
We retrospectively analyzed the presenting features and survival of 194 newly diagnosed patients with multiple myeloma in the People’s Republic of China. Compared with older patients, younger patients had a higher percentage of IgD isotype, lower percentage of International Staging System Stage 3 disease, higher albumin level, and lower frequency of high β2-microglobulin and CD200 expression. There was no difference in sex, Durie–Salmon stage, bone lesion degree, creatinine, lactate dehydrogenase, fluorescence in situ hybridization, and expression of other antigens. Among all 940 newly diagnosed patients with multiple myeloma, those younger than 50 years had better overall survival and progression-free survival than older patients. Of these patients, 457 were treated with a bortezomib-containing regimen, and 450 received conventional therapy. Younger patients treated with bortezomib had better overall survival and progression-free survival than older patients. However, younger patients treated with conventional therapy had the same survival as older patients.
Keywords: multiple myeloma, age, survival, China
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder that rarely presents in children or adults younger 30 years. However, the incidence increases progressively with age thereafter: approximately 80% of patients are over 50 years of age, and the median age at diagnosis is approximately 70 years.1,2 Initially, MM in young patients was thought to have a more indolent course with atypical presentations.3–5 Myeloma in patients aged <50 years presents with more favorable features.6 However, a study challenged this view by reporting cases in which patients with MM were younger than 40 years and had similar presenting features and therapy response,7 and also did not have a superior prognosis8 to that of older patients with MM.
Cancer occurrence, type, and outcome are closely related to factors such as race and environment. In the People’s Republic of China, the median age of patients with MM at diagnosis is younger than that of Western countries.9 At present, large-scale, multicenter epidemiological studies for Chinese patients with MM are not available; in addition, the treatment paradigm for younger patients is different from that of Western countries, and high-dose treatment is not used as commonly as in Europe and the United States. Thus, it is necessary to understand age effects and the impact of novel agents in Chinese patients with MM.
The aim of this study was to describe presenting features and outcomes in the era of novel agents in patients with MM under the age of 50 years compared with older patients. This is a chart-review study including the three largest MM centers in mainland China. Over 70% of patients in the centers were referred from elsewhere in the country, all from hospitals affiliated with medical universities. Patients previously suspected to have MM in local hospitals were subsequently referred to these study hospitals.
Materials and methods
Study population and definition
This study continuously enrolled newly diagnosed inpatients in three centers, People’s Hospital of Peking University, Shanghai Changzheng Hospital, and Beijing Chaoyang Hospital, from January 2008 to December 2011. This study was designed to be retrospective and included complete follow-up data from the three hospitals. Thus, it did not intervene in the patients’ treatment, and all data were unmasked. Therefore, written informed consent was not required, as confirmed by the Clinical Research Ethics Approval Committee of People’s Hospital of Peking University. The study was approved by the Clinical Research Ethics Approval Committee of People’s Hospital of Peking University. To date, the largest study to compare younger patients with older patients was from the International Myeloma Working Group retrospective analysis,6 in which the age cutoff for younger patients was 50 years. We wished to know whether there are differences between Chinese patients and Western patients, and so 50 years was chosen as the cutoff age in our study as well.
MM was diagnosed by a pathologist using standard criteria. Patients with smoldering (asymptomatic) myeloma, primary amyloidosis, and monoclonal IgM-related disorders were excluded. In addition, the following information was available: sex, paraprotein level and type, hemoglobin level, platelet count, white blood cell (WBC) count, and serum levels of calcium, creatinine, uric acid, albumin, lactate dehydrogenase (LDH), and C-reactive protein (CRP). Tumor stage in patients was assessed according to the Durie–Salmon (DS) staging system and the International Staging System (ISS). Furthermore, the percentage of bone marrow plasma cell infiltration, the number of osteolytic lesions, and the combination with extramedullary plasmacytoma/disease (EMD) were recorded.
The results of conventional cytogenetics (G-banding) and interphase fluorescence in situ hybridization (FISH) included the value of RB1 deletion, the amplification of 1q21, p53 deletion, and D13S319 deletion. Cell sorting was performed using miniMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) per the manufacturer’s instructions.
Immunophenotyping results were also collected using four-color fluorescently labeled anti-human monoclonal antibodies, including cluster of differentiation (CD38, CD138, CD117, CXCR4, CD19, CD45, CD56, CD9, CD20, CD22, CD33, CD28, CD200, CD13, ckappa, and clambda [BD Biosciences, San Jose, CA, USA]). Data of 10,000 events were collected and analyzed using identical parameters throughout the collection process using a FACSort instrument (BD Biosciences). CellQuest software (BD Biosciences) was used. To optimally detect antigen expression, we identified plasma cells based on expression of CD138, CD38, and CD45. The initial analysis gate was devised using CD38 vs CD138 expression, whereas the second one used CD38 brightly positive vs CD45-positive and -negative cells. Cases were considered positive if the percentage of plasma cells showing positive expression for a given antigen was higher than 20%. EMD was considered positive when one of the following criteria was met: pleural effusion with clonal plasma cell in the pleural fluid, clonal plasma cells in the ascitic fluid, clonal plasma cells in the cerebrospinal fluid, biopsy from organ or tissue demonstrating plasmacytoma, or computed tomography or positron emission tomography–computed tomography identifying a plasmacytoma (plasmacytoma extending beyond a bone was not included).
Overall survival (OS) and progression-free survival (PFS) were calculated. PFS was defined as the duration from the start of the treatment to disease progression or death, whichever came first. OS referred to the time interval measured from the date of diagnosis to the date of death or last follow-up. Death from all causes was included. All data were reviewed based on Good Clinical Practice criteria by an independent academic contract research organization.
Statistical analysis
Chi-squared test, t-test, and Fisher’s exact test were used to compare demographic characteristics. Curves for OS and PFS were plotted according to the Kaplan–Meier method and were compared to those of patients older than 50 years using the log-rank test. We constructed a Cox proportional hazards model to evaluate the association between patient characteristics and OS. P-value <0.05 was considered statistically significant. Hazard ratios and 95% confidence intervals (CIs) were generated, with hazard ratio <1.0 indicating a survival benefit (or reduced mortality). Calculations were performed using SPSS 19.0 (IBM Corporation, Armonk, NY).
Results
Baseline characteristics comparing patients under and over 50 years
Data from a total of 1,153 cases were collected; after excluding cases with incomplete records, 940 cases remained. The median age was 59 years (23–88 years). There were 570 men (60.6%) and 370 (39.4%) women; 194 (20.6%) patients were younger than 50 years. Among the patients under the age of 50, most (145, 75%) were between 40 and 50 years. Only 42 patients (22%) were between 30 and 40 years, and seven patients were younger than 30 years. The median age of the 746 patients in the >50 years age group was 62 years (range, 50–88 years), and the median age of the 194 patients under the age of 50 was 46 years (range, 20–49 years).
Compared with the patients over the age of 50, those under the age of 50 were more likely to have ISS Stage 1 (29.8% vs 17.4%, P<0.001) but less likely to have ISS Stage 3 (39.4% vs 50.7%, P=0.007), whereas the frequency of ISS Stage 2 did not differ (30.9% vs 31.9%, P=0.774). In addition, younger patients were less likely to have the adverse prognostic factor and low serum albumin levels (<35 g/L) and were more likely to have high β2-microglobulin (≥3.5 mg/dL) serum levels (37.1% vs 57.9% and 45.8% vs 62.0%, P<0.001 and P<0.001, respectively); meanwhile, the median serum albumin levels were 37.3 g/L vs 33.5 g/L, respectively (P<0.001). Moreover, other adverse prognostic factors, such as low hemoglobin (<10 g/dL, 56.1% vs 61.3%, P=0.265), creatinine (≥2 mg/dL, 21.4% vs 25.1%, P=0.300), and CRP (≥10 mg/L, 19.0% vs 19.4%, P=0.891), did not differ significantly between the groups in either frequency or median value. Patients below 50 years of age more frequently had IgD isotype (10.3% vs 5.5%, P=0.015); however, the other isotypes did not differ.
There were no differences in sex, platelet count, WBC counts, and serum levels of calcium, uric acid, and LDH, nor in DS stage, percentage of bone marrow plasma cell infiltration, numbers of osteolytic lesions, and the combination of EMD. Patient characteristics and laboratory parameters for patients under and over 50 years are listed in Table 1.
Table 1.
Characteristics and laboratory parameters in patients <50 vs ≥50 years
| Characteristics/parameters | Age categories
|
P-values | |
|---|---|---|---|
| <50 years | ≥50 years | ||
| N (%) | 194 (21) | 746 (79) | – |
| Male/female (%) | 113/81 (58.3) | 457/289 (61.2) | 0.459 |
| IgA (%) | 28/194 (14.4) | 141/746 (18.9) | 0.149 |
| IgG (%) | 75/194 (38.7) | 341/746 (45.7) | 0.078 |
| IgD (%) | 20/194 (10.3) | 41/746 (5.5) | 0.015 |
| Nonsecretory (%) | 6/194 (3.1) | 21/746 (2.8) | 0.837 |
| Light-chain only (%) | 64/194 (33.0) | 195/746 (26.1) | 0.057 |
| DS stage 2/3 (%) | 33/159 (17.2) | 99/639 (13.4) | 0.201 |
| ISS stage 1–2/3 (%) | 114/74 (60.6) | 355/365 (49.3) | 0.007 |
| EMD (%) | 16/162 (9.2) | 47/644 (6.8) | 0.316 |
| >3 lytic lesions | 82/109 | 334/403 | 0.569 |
| Creatinine ≥2 mg/dL | 41/151 (21.4) | 185/553 (25.1) | 0.300 |
| β2 (mg/dL) | 5.96±6.94 | 7.12±8.22 | 0.090 |
| β2 ≥3.5 mg/dL (%) | 45.8 | 62 | <0.001 |
| ALB (g/L) | 37.3±7.5 | 33.5±7.5 | <0.001 |
| ALB <35 g/L (%) | 37.1 | 57.9 | <0.001 |
| LDH (U/L) | 213.06±192.17 | 193.29±134 | 0.201 |
| Uric acid (U/L) | 377.57±174.42 | 398.64±172.83 | 0.210 |
| Creatinine (μmol/L) | 149.29±201.67 | 141.91±145.60 | 0.648 |
| CRP (mg/L) | 10.69±25.83 | 9.96±21.80 | 0.718 |
| CRP ≥10 mg/L (%) | 19.0 | 19.4 | 0.891 |
| Bone marrow plasma cell (%) | 31.62±27.39 | 31.79±25.61 | 0.936 |
| WBC | 5.49±2.74 | 5.65±2.32 | 0.550 |
| Hb | 97.73±27.26 | 93.56±26.12 | 0.094 |
| Plt | 172.75±94.2 | 168.47±81.57 | 0.574 |
Abbreviations: DS, Durie–Salmon; ISS, International Staging System; EMD, extramedullary plasmacytoma/disease; ALB, albumin; LDH, lactate dehydrogenase; CRP, C-reactive protein; WBC, white blood cell; Hb, hemoglobin; Plt, platelet.
The patients under the age of 50 were further divided into two subgroups: patients under 40 years and patients between 40 and 50 years. Table 2 lists a comparison of different factors in the under 40 years age group. There was a trend toward male predominance that did not reach statistical significance (67.9% vs 55.3%, P=0.18). Patients under 40 years had a less elevated CRP and lower erythrocyte sedimentation rate than those patients 40–50 years of age. The other baseline characteristics did not differ between patients under 40 years and patients 40–50 years, including the isotype of myeloma, ISS stage, DS stage, WBC counts, hemoglobin levels, platelet counts, LDH, uric acid, creatinine, albumin, EMD, and the number of plasma cells in the bone marrow.
Table 2.
Characteristics and laboratory parameters in patients <40 vs 40–50 years
| Characteristics/parameters | Age categories
|
P-values | |
|---|---|---|---|
| <40 years | 40–50 years | ||
| N (%) | 49 (25) | 145 (75) | – |
| Male/female (%) | 68 | 55.3 | 0.180 |
| Age, years | 38 (23–40) | 47 (41–50) | 0.000 |
| CRP (mg/L) | 5.58±9.23 | 11.46±27.71 | 0.040 |
| ESR (mm/hr) | 53.70±50.57 | 76.24±47.78 | 0.050 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Cytogenetics and phenotypic findings comparing those over or under 50 years
The frequencies of P53 deletion, RB1 deletion, and 1q21 amplification by FISH did not differ significantly between the groups (18.7% vs 17.4%, P=0.771; 35.1% vs 41.1%, P=0.507; and 33.3% vs 44.4%, P=0.064, respectively; Table 3). Conventional cytogenetic analysis was available in 153 patients and also showed no difference in the frequency of clonal cytogenetic abnormalities and the percentage of del 13 by cytogenetics. Among the seven younger patients with clonal cytogenetic abnormalities, three were hypodiploid, three were hyperdiploid, and one was pseudodiploid; among the 32 cases with clonal cytogenetic abnormality in older patients, 17 were hypodiploid, eight were hyperdiploid, and seven were pseudodiploid.
Table 3.
Cytogenetics in patients <50 vs ≥50 years
| Variables | Age categories, number with factor/number with valid data for factor (%)
|
P-values | |
|---|---|---|---|
| <50 years | ≥50 years | ||
| Any clonal CA | 7/33 (21) | 32/120 (27) | 0.524 |
| Del 13a | 3/33 (9) | 9/120 (8) | 0.767 |
| Del 13b | 13/37 (35) | 58/141 (41) | 0.507 |
| 1q21 amplificationb | 49/87 (33) | 139/313 (44) | 0.064 |
| Del P53b | 17/91 (19) | 61/351 (17) | 0.771 |
Notes:
Cytogenetics;
FISH.
Abbreviations: CA, cytogenetic abnormality by G-banding; FISH, fluorescence in situ hybridization; Del, deletion.
The phenotype of the plasma cells, including expression of CD38, CD138, CD56, CXCR4, CD20, CD117, CD9, and CD200, showed no difference between the groups except for CD200 (45% vs 61%, P<0.05). The presenting FACS features of the patients younger than 50 years vs those over 50 years are listed in Table 4.
Table 4.
Immunophenotype in patients <50 vs ≥50 years
| Variables | Age categories, number with factor/number with valid data for factor (%)
|
P-values | |
|---|---|---|---|
| <50 years | ≥50 years | ||
| CD38 | 62/62 (100) | 261/262 (100) | 1.0 |
| CD138 | 62/62 (100) | 264/267 (99) | 1.0 |
| CD56 | 32/56 (57) | 163/246 (66) | 0.198 |
| CXCR4 | 18/41 (44) | 60/184 (33) | 0.169 |
| CD20 | 8/51 (16) | 53/217 (25) | 0.181 |
| CD117 | 22/52 (42) | 89/219 (41) | 0.826 |
| CD9 | 30/46 (65) | 120/194 (62) | 0.672 |
| CD28 | 8/31 (26) | 27/141 (19) | 0.404 |
| CD200 | 36/80 (45) | 112/184 (61) | 0.017 |
Abbreviation: CXCR, chemokine receptor.
Treatment and outcome comparing those over or under 50 years
Among the 940 newly diagnosed patients with MM, patients under the age of 50 had better OS and PFS than did the patients who were over the age of 50 (OS: 53 months (95% CI: 49.2–56.3) vs 44 months (95% CI: 42.1–46.4); P<0.001; and PFS: 35 months (95% CI: 30.0–39.3) vs 28.2 months (95% CI: 26.0–30.3); P=0.009; Figures 1 and 2).
Figure 1.
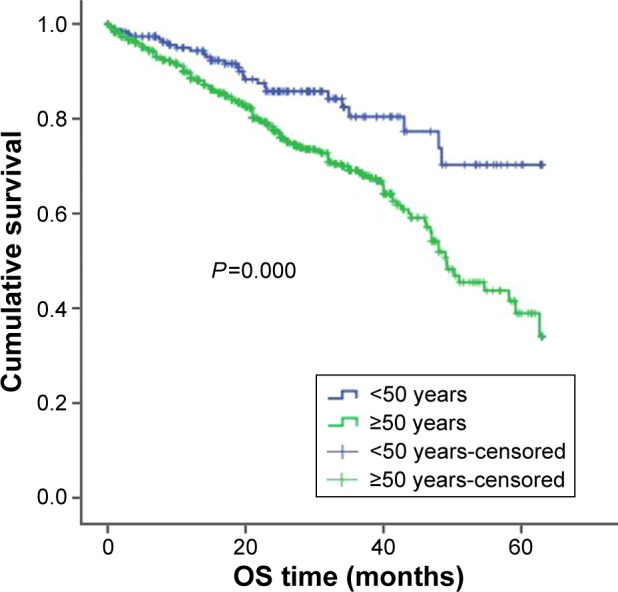
OS in younger and older patients.
Abbreviation: OS, overall survival.
Figure 2.
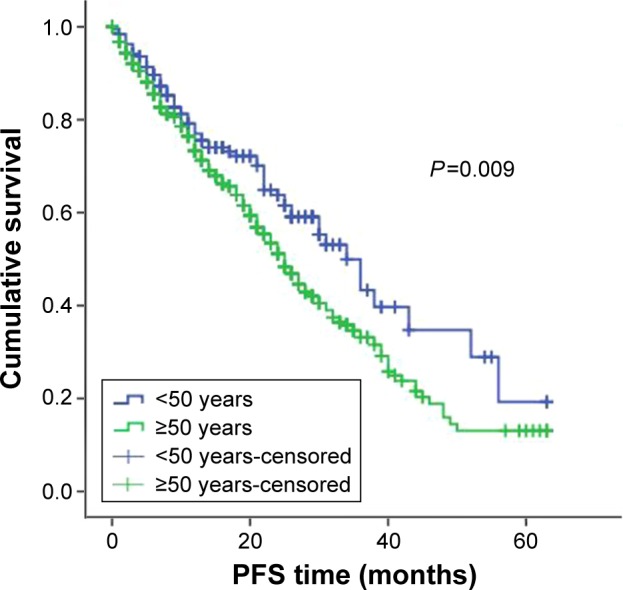
PFS in younger and older patients.
Abbreviation: PFS, progression-free survival.
For treatment, 457 patients received bortezomib-containing regimens and 450 patients received conventional therapy (melphalan-prednisolone [MP] and vincristine-adriamycin-dexamethasone [VAD]). When subgroup analysis was completed according to the treatment plan, among patients receiving bortezomib therapy, younger patients had better OS and PFS compared with older patients. The PFS was 33 months in younger patients treated with bortezomib and 25 months in older patients treated with bortezomib, P=0.043; the OSs were 52 months and 42 months, respectively, P=0.003 (Figures 3 and 4). For patients who got MP/VAD treatment, PFS was 33 months in younger patients and 31 months in older patients, P=0.225; OSs were 51 months and 46 months, respectively, P=0.121 (Figures 5 and 6). Otherwise, among the patients who got conventional therapy, younger patients had the same survival as older patients. Only 14.4% had received an autologous transplantation. There were 67 younger patients (34.5%) and 68 older patients (9.1%) who underwent autologous transplantation (P<0.01). For patients who underwent transplantation but not receive bortezomib, the younger patients had better OS than did the older patients, 51 months and 40 months, respectively (P=0.01), but the PFS did not differ significantly, 31 months and 25 months, respectively (P=0.09).
Figure 3.
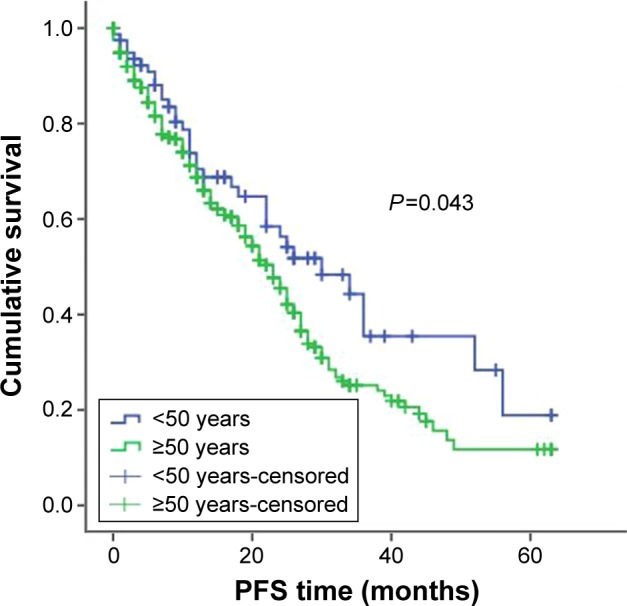
PFS in younger and older patients receiving bortezomib and MP/VAD.
Abbreviations: PFS, progression-free survival; MP, melphalan-prednisolone; VAD, vincristine-adriamycin-dexamethasone.
Figure 4.
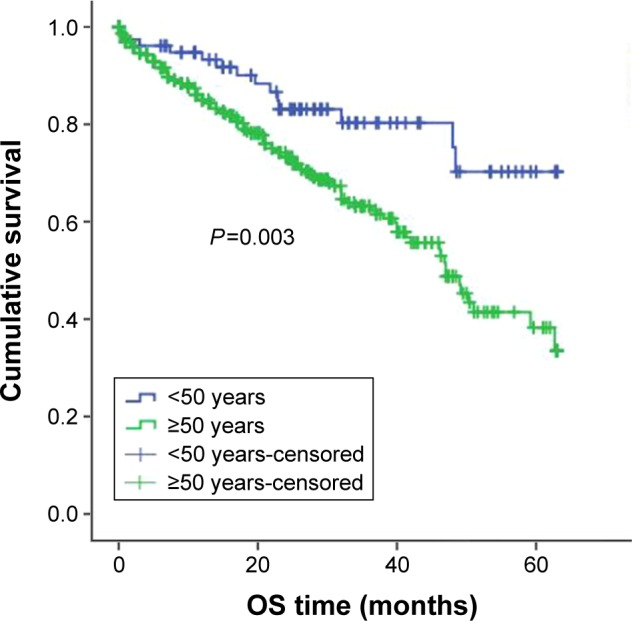
OS in younger and older patients receiving bortezomib and MP/VAD.
Abbreviations: OS, overall survival; MP, melphalan-prednisolone; VAD, vincristine-adriamycin-dexamethasone.
Figure 5.
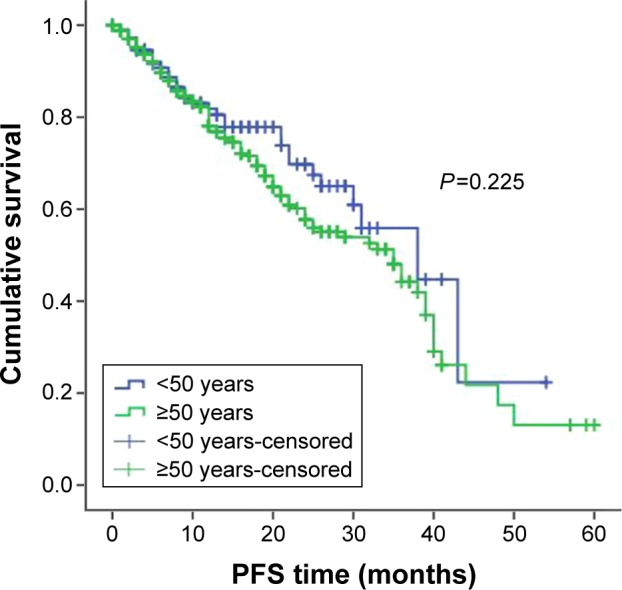
PFS in younger and older patients receiving MP/VAD treatment.
Abbreviations: PFS, progression-free survival; MP, melphalan-prednisolone; VAD, vincristine-adriamycin-dexamethasone.
Figure 6.
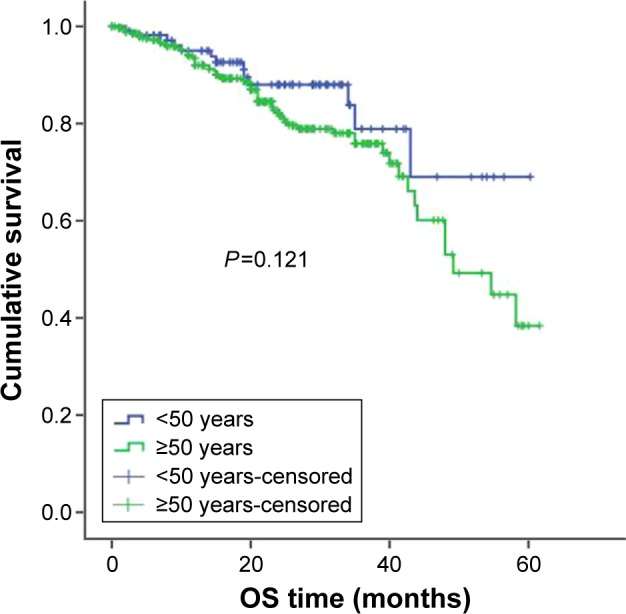
OS in younger and older patients receiving MP/VAD treatment.
Abbreviations: OS, overall survival; MP, melphalan-prednisolone; VAD, vincristine-adriamycin-dexamethasone.
In multivariate analysis, male sex (P=0.006), age above 50 years (P=0.011), and higher ISS (P<0.001) were identified as independent risk factors for shorter survival; other parameters, including DS stage (3 vs 1 and 2), albumin level (<35 g/L), hemoglobin level (<10 g/dL), β2-microglobulin level (≥3.5 mg/dL), and treatment regimen were correlated with survival in univariate analysis but not in multivariate analysis.
Discussion
Younger age has been reported to be correlated with improved survival and has been recognized as an important prognostic factor.6,10,11 However, when undergoing upfront autologous stem cell transplant, age below 40 years does not confer a superior prognosis in patients with MM.12 MM survival has improved among younger but not among older patients since the mid-1990s. The improved survival of younger patients coincided with increasing trial participation and the increasing use of high-dose chemotherapy as well as autologous stem cell transplantation.8 Therefore, we aimed at investigating the features of patients under the age of 50 vs those over the age of 50 and to find differences between the groups.
In a retrospective analysis of 940 Chinese patients newly diagnosed with MM, we found that 20.6% of patients were younger than 50 years, and 75% of them were 40–50 years old. These values were similar to those reported by Ludwig et al,6 who found that 16% of patients with MM were younger than 50 years, with 82% of these being 40–50 years old. Compared with older patients, younger patients had a higher percentage of IgD isotype, more frequent ISS Stage 1, higher albumin levels, and a lower frequency of high β2-microglobulin and CD200 expression. Ludwig et al6 also reported that younger patients had more ISS Stage 1 and higher albumin levels. Unlike Ludwig et al,6 however, we did not find a higher frequency of IgA and light chain isotype in younger patients, but we found a higher frequency of IgD; this difference may be related to the difference in ethnicity between the study populations.
In our study, we found that patients younger than 50 years more often had IgD isotype (10.3% vs 5.5%, P=0.015), which is regarded as a poor prognosis factor. IgD isotype is seen in 2% of all MM cases. IgD MM has been reported previously to have a poorer prognosis, at least after conventional therapy. Chong et al13 compared the survival following high-dose melphalan and autologous stem cell transplantation (ASCT) between patients with IgD MM and those with other subtypes and found that IgD MM had the lowest survival among MM subtypes (P<0.01). Reece et al14 conducted a retrospective analysis of patients with MM who underwent transplantation in 1995–2005 to describe the characteristics and results of ASCT in IgD MM (n=36) and IgM MM (n=11) among 3,578 patients with MM who underwent ASCT during this period. They found that relapse and progression were higher, while PFS was lower in patients with IgD MM, but no striking differences were apparent in the post-ASCT outcomes of patients with IgD and IgM MM.
In addition, compared with older patients, CD200 expression was lower among younger patients (45% vs 61%; P=0.017). CD200 is a membrane glycoprotein that imparts an immunoregulatory signal through CD200R, leading to the suppression of T-cell (R) C-mediated immune responses. Patients lacking CD200 expression on their MM cells have an increased event-free survival compared with patients with CD200-positive MM cells after high-dose therapy and stem cell transplantation.15 CD200 plays an immunoregulatory function by switching cytokine production from a Th1 to Th2 pattern, thus reducing cytotoxic responses while indirectly enabling tumor escape and growth. Therefore, CD200 is a target for a novel humanized monoclonal antibody. Anti-CD200 inhibits CD200 binding to its receptor, thus modifying cytokine production and improving T-cell-mediated cytotoxic responses, and could represent a new therapeutic target in MM and other B-cell malignant diseases.16 Our results showed that CD200 expression was less frequently observed in younger patients, suggesting that the patients under the age of 50 had not only poor but also favorable prognostic factors. In addition, younger patients also had some other good prognostic factors, such as fewer patients with ISS Stage 3 and higher levels of albumin. Therefore, bad and good prognostic factors seem to coexist in young patients.
There was no difference in sex, DS stage, bone lesion degree, creatinine, lactate dehydrogenase, FISH, and expression of other antigens. Among all 940 newly diagnosed MM patients, patients under the age of 50 had better OS and PFS than did older patients. For treatment, 457 patients received bortezomib-containing regimens and 450 patients received conventional therapy; younger patients treated with bortezomib had better OS and PFS than older patients. Otherwise, younger patients treated with conventional therapy had the same survival as older patients. The younger patients had better survival in part due to high-dose therapy, since the proportion of young patients treated with high-dose therapy was almost four times as high (34.5%) as that of older patients (9.1%; P<0.001), and those younger patients who got high-dose therapy but not bortezomib had better OS than older patients.
The survival outcome reported in our study was similar to those of previous reports6,10,11 but was different from the results reported by Cheema et al,12 who found a trend toward prolonged OS and PFS in patients under the age of 50 only in patients treated with bortezomib.
Conclusion
In patients under the age of 50, poor and favorable prognostic factors coexist, and these patients had better survival compared with patients over 50 years of age. This result indicated that patients under the age of 50 do not have inferior survival, and unique features, such as MM subtype, staging, surface antigen expression, etc, are important to a particular patient. Furthermore, MM subtype and surface antigen expression provide a meaningful index.
Acknowledgments
This study was sponsored by Celgene Pharmaceutical (ShangHai) Co. Ltd. The study was partly supported by a National Natural Science Fund of China grant to Doctor Jin Lu (grant number 81372535). Additionally, we thank the Chinese Medical Doctor Association Hematology Branch for their help.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wisloff F, Andersen P, Andersson TR, et al. Has the incidence of multiple myeloma in old age been underestimated? The myeloma project of health region I in Norway. I. Eur J Haematol. 1991;47(5):333–337. doi: 10.1111/j.1600-0609.1991.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergsagel D. The incidence and epidemiology of plasma cell neoplasms. Stem Cells. 1995;13(Suppl 2):1–9. [PubMed] [Google Scholar]
- 3.Hewell GM, Alexanian R. Multiple myeloma in young persons. Ann Intern Med. 1976;84(4):441–443. doi: 10.7326/0003-4819-84-4-441. [DOI] [PubMed] [Google Scholar]
- 4.Lazarus HM, Kellermeyer RW, Aikawa M, Herzig RH. Multiple myeloma in young men. Clinical course and electron microscopic studies of bone marrow plasma cells. Cancer. 1980;46(6):1397–1400. doi: 10.1002/1097-0142(19800915)46:6<1397::aid-cncr2820460618>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 5.Geetha N, Jayaprakash M, Rekhanair A, Ramachandran K, Rajan B. Plasma cell neoplasms in the young. Br J Radiol. 1999;72(862):1012–1015. doi: 10.1259/bjr.72.862.10673955. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–4047. doi: 10.1182/blood-2007-03-081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blade J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93(2):345–351. doi: 10.1046/j.1365-2141.1996.5191061.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaapveld M, Visser O, Siesling S, Schaar CG, Zweegman S, Vellenga E. Improved survival among younger but not among older patients with multiple myeloma in the Netherlands, a population-based study since 1989. Eur J Cancer. 2010;46(1):160–169. doi: 10.1016/j.ejca.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Hou J, Du X, Jin J, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013;6:41. doi: 10.1186/1756-8722-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagaster V, Kaufmann H, Odelga V, et al. Chromosomal abnormalities of young multiple myeloma patients (<45 yr) are not different from those of other age groups and are independent of stage according to the International Staging System. Eur J Haematol. 2007;78(3):227–234. doi: 10.1111/j.1600-0609.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheema PK, Zadeh S, Kukreti V, et al. Age 40 years and under does not confer superior prognosis in patients with multiple myeloma undergoing upfront autologous stem cell transmplant. Biol Blood Marrow Transplant. 2009;15(6):686–693. doi: 10.1016/j.bbmt.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Chong YP, Kim S, Ko OB, et al. Poor outcomes for IgD multiple myeloma patients following high-dose melphalan and autologous stem cell transplantation: a single center experience. J Korean Med Sci. 2008;23(5):819–824. doi: 10.3346/jkms.2008.23.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reece DE, Vesole DH, Shrestha S, et al. Outcome of patients with IgD and IgM multiple myeloma undergoing autologous hematopoietic stem cell transplantation: a retrospective CIBMTR study. Clin Lymphoma Myeloma Leuk. 2010;10(6):458–463. doi: 10.3816/CLML.2010.n.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreaux J, Hose D, Reme T, et al. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108(13):4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 16.Kretz-Rommel A, Bowdish KS. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert Opin Biol Ther. 2008;8(1):5–15. doi: 10.1517/14712598.8.1.5. [DOI] [PubMed] [Google Scholar]


