Abstract
Background: Pneumonia and septicemia have the greatest impact on reduced life expectancy in persons with spinal cord injury (SCI). Fever is often the first presenting symptom of infection or inflammation. Thermoregulatory dysfunction in persons with SCI may preclude a typical febrile response to infection or inflammation and thus delay diagnostic workup. Objective: To determine the core temperature of persons with SCI in the setting of infection or inflammation and the frequency with which it meets criteria for the CDC definition of fever (>100.4°F). Methods: Retrospective review of hospitalized SCI patients over 5 years with a diagnosis of infection or inflammation (DI), defined by serum leukocytosis. In this study, 458 persons with paraplegia (PP) and 483 persons with tetraplegia (TP) had 4,191 DI episodes. Aural temperatures (Tau) on the day of DI, 7 days prior, and 14 days afterwards were abstracted from medical records. Main outcome measures were average Tau at DI, frequency of temperatures >100.4°F at DI, and average baseline temperatures before and after DI. Results: Average Tau at DI was 98.2°F (±1.5) and 98.2°F (±1.4) in the TP and PP groups, respectively, with only 11.6% to 14% of DI resulting in Tau >100.4°F. Baseline temperatures ranged from 97.9°F (±0.7) to 98.0°F (±0.8). Conclusion: SCI persons with leukocytosis infrequently mount a fever as defined by the CDC, and baseline temperatures were subnormal (<98.6°F). Thermoregulatory dysfunction likely accounts for these findings. Tau >100.4°F is not a sensitive predictor of infection or inflammation in persons with SCI. Clinicians should be vigilant for alternative symptoms of infection and inflammation in these patients, so diagnostic workup is not delayed.
Keywords: aural temperature, fever, infection, inflammation, spinal cord injury, thermoregulation
Introduction
According to the National Spinal Cord Injury Center statistical database, the most common causes of death with consequent reduced life expectancy for the SCI population are pneumonia and septicemia.1 More specifically, respiratory complications are the number one cause of death after the first year of SCI, while infections from secondary SCI-related sequalae are major cause of morbidity, length of hospital stay, and cost of care in the chronic stage.2–6 The most common secondary SCI-related conditions that predispose patients to infection are neurogenic bladder, insensate skin, neurogenic restrictive lung disease, unopposed parasympathetic input to the bronchi causing increased respiratory secretions, and spinal hardware placement and/or failure.7,8 A common, and some say reliable, presenting symptom of infection and/or inflammation that initiates a diagnostic workup is fever.9,10 Fever is currently defined by the Centers for Disease Control and Prevention (CDC) as a temperature greater than 100.4°F.11
The pathophysiology of fever requires an increase in vasomotor tone and shivering to elevate core temperature. Decentralization of the sympathetic nervous system after SCI reduces overall sympathetic activity below the level of injury, and thus vasomotor control is impaired.12 Furthermore, shivering responses are decreased and delayed in paralyzed muscles of SCI persons.13–16 Both the impaired vasomotor control and shivering responses have been shown to be proportional to the lesion level, with persons with tetraplegia being more impaired than persons with paraplegia.16 Persons with tetraplegia theoretically are more likely to have an impaired febrile response than those with paraplegia. On literature review, there are limited data available on this topic.
In the acute phase of SCI, incidence of fever ranges from 50% to 85%, with patients with complete injuries more likely to exhibit fever than patients who have incomplete injuries.5,17,18 During acute hospitalization post SCI, 73% to 81% of fevers have been reported to be of infectious etiology, with urinary tract and respiratory infections being most common.5,17,19,20 Furthermore, in a study of 88 SCI persons, temperatures greater than 101.5°F were more likely (but not always) to be of an infectious rather than noninfectious etiology.5 In summary, studies in persons with acute SCI show that fever results from noninfectious and infectious etiologies, however higher temperatures are more likely to result from infections. The caveats with these studies are the following: (1) the definition of “fever” varied from 99.5°F to 100.4°F (ie, there was a lack of a uniform fever definition), and (2) data were limited to persons with acute SCI.
Although it is clear from the aforementioned studies that persons with acute SCI are able to raise their core temperature in response to infection, incidence and conditions of febrile response in persons with chronic SCI are not reported and are under-investigated. Literature review found 3 case reports that describe infection in the absence of a febrile response in persons with chronic SCI.21 Ohry et al proposed that persons with chronic SCI may respond to infection in a manner similar to the elderly who can exhibit “silent sepsis” where infection occurs without typical presenting symptoms, such as fever.21 Furthermore, reports of subnormal resting baseline temperatures have been reported by a few.22–25 The largest cohort studied consisted of 50 persons with chronic tetraplegia, of whom 66% had resting core temperatures lower than 97.7°F in the absence of infection.22 This relatively lower resting core temperature in the SCI versus non-SCI population has been previously attributed to failure of the mechanisms of heat conservation (ie, vasoconstriction) and production (ie, shivering) that require an intact spinal cord and its hypothalamic feedback.14,15,23–25 This subnormal resting core temperature, combined with impaired vasomotor and shivering responses in persons with SCI, has the potential to preclude core temperature elevation above 100.4°F to reach the CDC definition; however the literature is silent on this topic.
Little is known on the ability of persons with chronic SCI to mount a febrile response to infection and inflammation. If thermoregulatory dysfunction precludes a febrile response to infection and/or inflammatory processes, workup and treatment may be delayed and thus lead to increased morbidity, mortality, health care costs, and lengths of stay.4,5,18 We hypothesize that the absence of fever as a presenting symptom may play a role in contributing to the great impact of pneumonia and septicemia on reducing life expectancy in persons with SCI.
This study aims to examine core temperatures of persons with chronic SCI in noninfectious/inflammatory versus infectious/inflammatory conditions and the effect of level of injury in each condition using retrospective analysis of medical records. To our knowledge, this study is the first to examine the ability of persons with chronic SCI to mount a fever as defined by the CDC (>100.4°F) in the setting of infectious or inflammatory processes.
Methods
Retrospective chart review was conducted of hospitalized patients with chronic SCI (>1 year post SCI) from 5 Veterans Affairs (VA) centers who had a documented diagnosis of infection or inflammation (DI) during the 5-year period between 2008 and 2013. There were 458 persons with paraplegia (PP) and 483 persons with tetraplegia (TP) who had 4,191 DI episodes. DI was defined by elevated serum white blood cell count (>10×109/L) and then stratified into 3 groups: a definitively high white blood cell (WBC) count group (>15×109/L), a borderline group (10–15×109/L), and normal group (<10×109/L). Aural temperature (Tau) was documented every 8 hours in the inpatient units from which data were gathered. Average on the day of DI was Tau measured.
To compare core temperature in noninfectious/inflammatory conditions, Tau 7 days before DI and 14 days after DI were abstracted from the data (Figure 1). Fourteen days after DI was chosen to allow for 7 days of treatment for the infectious/inflammatory process in addition to 7 days of returning to baseline resting noninfectious/inflammatory conditions. We did abstract, but did not report, data on days 2 to 6 post DI, as this is the time frame during which antibiotic treatment is given and pyrogens potentially still present; temperature on these days would not reflect a true baseline/noninfectious state. After treatment for 7 days following DI, circulating pyrogenic cytokines should be minimal to none and antibiotics and/or anti-inflammatory treatments completed or in full therapeutic effect, and thus fever (if present) should be fully resolved.26–28 To determine reliability of baseline temperatures, we abstracted baseline temperatures (BT) 7 days before (BT1) and 8 to 14 days after DI (BT2) so they could be compared. DI was labeled as day 0 (D0) (Figure 1).
Figure 1.
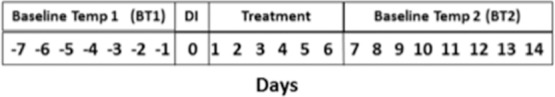
Days of aural temperature (Tau) data collection. DI = diagnosis of infection or inflammation.
D0 temperatures were dichotomized as high being 100.4°F and above and normal being less than 100.4°F. Average Tau on both the day before DI (day-1) and day after DI (day+1) were compared to D0 to evaluate trends of temperatures immediately before and after DI. Daily Tau averaged over 7 days before DI was labeled baseline temperature 1 (BT1) and 7 days (days 7–14 after D0) after DI was labeled baseline temperature 2 (BT2).
Statistical analyses of the dichotomized temperature (high vs normal) and the level of injury (TP vs PP groups) were conducted using Pearson's chi-square and logistic regression model; comparison of average temperature at DI among 3 stratified levels of WBC (normal, borderline, and high) and between the level of injury groups were examined using generalized linear model. Comparisons of Tau between TP and PP groups on day-1, D0, and day+1 and BT1, D0, and BT2 were examined using 2-way analysis of variance (ANOVA) with repeated measures. The p value <.05 was considered to be significant with 2-sided analysis. All analyses were performed by SAS V9.4 (SAS Institute, Inc., Cary, NC).
Results
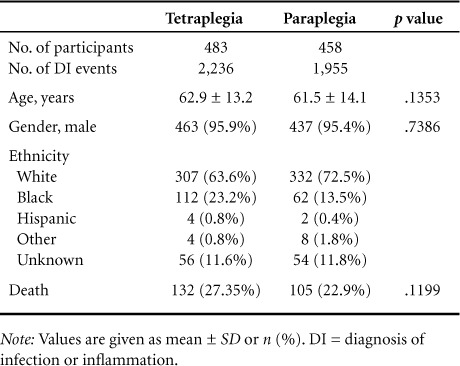
Nine hundred forty-one subjects (TP = 483, PP = 458) had 4,191 documented DI events over 5 years. There were 1,955 events in the PP group and 2,236 in the TP group. The demographics of the groups are outlined in Table 1. There were no significant differences in age, number of males versus females, or number of deaths between TP and PP groups.
Table 1.
Demographics
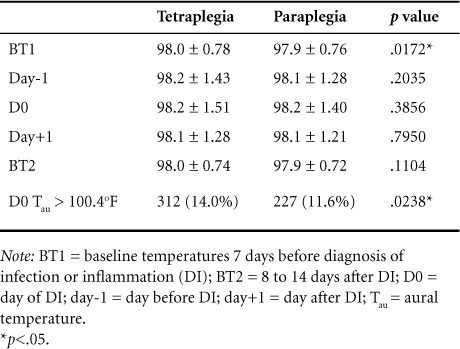
The mean Tau at D0 in the TP group was 98.2°F (±1.5), and in the PP group it was 98.2°F (±1.4), with no significant difference between the 2 groups (p = .3856). In 312 (14%) of the DI events in TP group and 227 (11.6%) in the PP group, Tau was greater than 100.4°F, with a significant difference between groups (Table 2).
Table 2.
Aural temperature (°F) by days in patients with tetraplegia and paraplegia
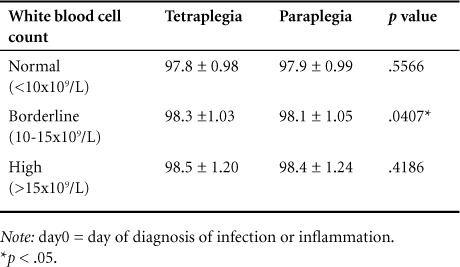
Results of D0 Tau after participants were subdivided into 3 groups based on degree of leukocytosis (ie, high, borderline, and normal) are seen in Table 3. There was a positive correlation between higher WBCs and higher average temperature (p < .0001), however average DI temperature of the high WBC group remained significantly below the CDC definition of fever (>100.4°F), with a p value less than .0001. Interestingly, TP temperatures were higher than PP temperatures in all groups, with a significant difference in DI temperatures in the borderline group (p = .0407).
Table 3.
Aural temperature (°F) on day0 by degree of leukocytosis in patients with tetraplegia and paraplegia
The maximum Tau at DI for TP and PP groups was 104.8°F and 104.1°F, respectively. Logistic regression model indicated that persons with tetraplegia were 1.23 times more likely to have Tau greater than 100.4°F compared with persons with paraplegia.
Average daily Tau 7 days before infection to 14 days after infection is depicted in Figure 2. Two-way ANOVA with repeated measures analyzed average baseline temperature of day-7 to day-1 (BT1), average DI temperature (D0), and average baseline temperature of day7 to day14 (BT2) between 2 groups and within each group (Table 2). There was a significant difference in BT1 between groups (p = .017), with BT1 higher in the TP group. Within each group, there were significant differences in Tau from BT1 to D0 (TP, p = .0009; PP, p = .0216) and D0 to BT2 (TP, p ≤ 0001; PP: p ≤.0001), showing that the DI/D0 temperatures were significantly higher than baseline temperatures before and after DI in both groups. There were significant differences in BT1 and BT2 within both groups (TP, p = .0003; PP, p = .0018), with BT1 being higher on average than BT2.
Figure 2.
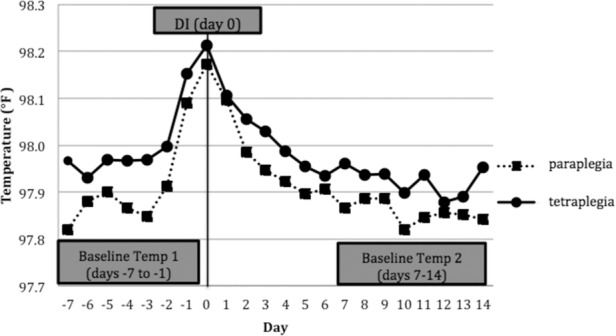
Average temperatures from −7 day (before infection) to 14 days (after infection) for tetraplegia and paraplegia groups. DI = diagnosis of infection or inflammation.
To examine the trend in Tau within the 24 hours within D0, Tau the day before (day-1), and day after (day+1), DI was compared and reported in Table 2. In both TP and PP groups, there was no significant difference in Tau between day-1 to D0 (TP, p = .1627; PP: p = .5425), however there were significant differences were seen between Tau on D0 and day+1 within both groups (TP, p ≤ .0001; PP, p = .0043).
Discussion
Fever is one of the most common presenting symptoms of an infection or inflammatory process. Given autonomic dysfunction (impairing vasoconstriction) and paralysis (impairing shivering) in SCI patients, there may not be an adequate neuro-humoral response to mount a fever greater than 100.4°F per the CDC definition.13,26
Pathophysiology of fever
The molecular pathophysiology of fever from infection or inflammation begins with activation of blood monocytes and macrophages to produce pyrogenic cytokines (eg, interleukin-1 and 6, tumor necrosis factor, and interferon).13 These endogenous pyrogens travel across the blood-brain barrier to indirectly affect the hypothalamus to increase its thermostatic set point. Once the thermostatic set point is raised, the core temperature is deemed cold so the hypothalamic efferents stimulate heat-producing processes. Activation of sympathetic centers of the hypothalamus results in peripheral vasoconstriction and increased metabolism. Motor activation of skeletal muscles results in shivering that converts ATP to ADP and produces heat. Core temperature rises as a result of these processes and continues until reaching the higher thermostatic set point. This rise in temperature to the higher set point is known as “fever.” When inflammatory cells cease releasing pyrogenic cytokines, the thermostatic set point drops back to baseline.26 To decrease core temperature, the hypothalamus inhibits sympathetic activity on the blood vessels for convective cooling via passive vasodilation and activates sympathetic cholinergic activity on sweat glands for evaporative cooling.
The interruption of the connection between hypothalamus and efferent nerves (sympathetic and motor) responsible for raising core temperature may attenuate or completely preclude the febrile response in SCI persons. Sympathetic nerves exit the spinal cord at the thoracolumbar levels, whereas motor nerves exit via the anterior horn at all levels.29 Thus persons with tetraplegia theoretically have no ability to vasoconstrict or increase metabolism, while persons with paraplegia have some ability to raise their core temperature dependent on their lesion level. In all persons with SCI, shivering would only occur above the motor level of injury, with persons with tetraplegia shivering in fewer muscle groups than persons with paraplegia.13 As a result of neurological injury in the spinal cord, persons with SCI may have an impaired ability to elevate core temperature above 100.4°F in the setting of infection or inflammation. As a result, diagnostic workup and treatment may often be delayed; this may play a role in explaining why pneumonia and sepsis have the greatest impact on life expectancy in the SCI population.1
While the literature previously mentioned is limited, it seems that some persons with acute SCI do have potential to elevate temperature above the CDC fever cut-off of 100.4°F and this is more likely when infection is present; however in chronic SCI, the incidence and conditions (infections vs noninfectious) of fever as defined by the CDC are unknown. This study examined the incidence of fever as defined by the CDC in response to infection in persons with tetraplegia and paraplegia through retrospective chart review of SCI inpatients with chronic SCI over 5 years.
DI temperatures
In 941 SCI persons with 4,191 DI events, only 11% to 14% of the DI events met CDC definition of fever greater than 100.4°F, with the TP group more likely to meet criteria than the PP group.
Core temperatures at DI in both TP (98.2°F) and PP (98.2°F) groups were significantly below the CDC definition of fever (<100.4°F; p < .0001 for both groups). They are also well below fever thresholds of 99.5°F and 99.9°F as previously reported in SCI literature.5,18 Most notably, they were also below 98.6°F, which is the “normal” core temperature of a healthy resting adult human. While this could have been a result of averaging 3 temperatures of the day of DI or due to administration of antipyretic medication as standard of care, it was a statistically significant rise from baseline temperature before DI (BT1) in both TP and PP groups (p = .0009 and .0216, respectively), so it was a true increase from baseline Tau. This low DI temperature could be attenuated due to an impaired ability to vasoconstrict and shiver to raise core temperature in response to pyrogenic cytokines or subnormal resting body temperatures from thermal dyregulation and poikilothermia that increase the difference between resting values and 100.4°F.13,22,23,30
When D0 data were subdivided into groups based on degree of leukocytosis, there was a direct correlation between higher WBCs and higher average D0 temperature. Despite this, D0 temperatures in all groups (normal, high, and borderline WBCs) remained significantly below 100.4°F, even in the high WBC group. This further demonstrates the impaired ability of SCI persons to elevate core temperature regardless of the degree of inflammatory responses and/or numbers of circulating pyrogenic cytokines.
Fever in tetraplegia vs paraplegia
The average maximum DI temperature for the TP group was higher than the PP group, and TP persons were also more likely to have elevated core temperature above 100.4°F at DI. Although these findings were unexpected given that persons with paraplegia should theoretically have greater ability to mount a typical febrile response, one other study has reported no difference in fever incidence between TP and PP groups in the acute stage of SCI.20 Our data demonstrate that, similar to the acute injury stage, persons with chronic tetraplegia can indeed mount a febrile response despite having greater thermoregulatory dysfunction than persons with paraplegia. According to Schmidt and Chan, the ability to increase core temperature to the hypothalamic set point in persons with tetraplegia may be due to the following untested theories: (a) Pyrogenic cytokines may affect anatomic sites other than the hypothalamus (eg, directly on blood vessels to vasoconstrict or cells to increase metabolism); (b) hypothalamus may regulate core temperature by mechanisms other than efferent neurological pathways (eg, where humoral factors are released and result in peripheral vasoconstriction or increased cellular metabolism); or (c) autonomic spinal cord tracts are more resistant to injury and remain intact despite complete motor and sensory interruption.13 Meanwhile, tested theories show that impaired convective and evaporative cooling mechanisms as a result of vasomotor and sudomotor dysfunction, respectively, preclude lowering of core temperature in hyperthermic states.31 These processes are more impaired in persons with tetraplegia than paraplegia and thus could potentially prolong the fever duration and the time for core temperature to return to baseline.
Baseline temperatures
Baseline temperatures (BT1 and BT2 data) within this cohort ranged from a minimum of 97.9°F (±0.7) to a maximum of 98.0°F (±0.8). Baseline temperatures were below the “normal” resting core temperature of 98.6°F in both groups. As mentioned previously, this phenomena of subnormal resting core body temperature in up to 66% of persons with SCI has been previously reported and is considered to be a result of thermoregulatorydysfunction.16,22,32 Similar studies reporting subnormal resting core temperatures in the elderly and immunocompromised populations have caused clinicians to advocate for a lower fever threshold that is more sensitive in detecting infection in these persons.33–35 Based on SCI data presented here, one could argue that a lower fever threshold may be needed in the SCI population to detect infectious/inflammatory processes sooner than later.
Regarding differences in BT1 and BT2, we found a significant difference between BT1 and BT2 within the TP and PP groups, with BT2 being lower than BT1 in both groups. This is likely due to the temperature beginning to rise 1 to 2 days before DI; these days are included in the BT1 data (Figure 2). In addition, BT1 was significantly higher in the TP group when compared to the PP group. One possible explanation is that the rise in temperature during the febrile response is faster in persons with tetraplegia. This was demonstrated by evaluating temperatures immediately before (day-1) and at D0. For the TP group, there was a higher temperature elevation of 0.048°F compared to 0.021°F in the PP group from day-1 to D0. Therefore, BT2 maybe a more accurate representation of respective baseline temperatures for each SCI group. There were no significant differences in BT2 between TP and PP groups, indicating that there were no differences in resting core body temperatures in chronic SCI patients no matter the injury level.
Alternative fever definition in SCI?
The low incidence of fever (11%–14%) in the setting of infectious or inflammatory processes challenges the sensitivity of this symptom in persons with SCI. It may be that the definition of fever in SCI as an absolute cut-off of 100.4°F is of inadequate sensitivity; change in temperature from baseline may be a more appropriate definition. The subnormal baseline temperatures [ranging from 97.9°F (±0.7) to 98.0°F (± 0.8)] could partly be responsible for a febrile response below 100.4°F. While, for example, a 2°F increase from subnormal baseline values would not raise temperature above 100.4°F, such a change in Tau might be a more sensitive clinical indicator of active infection or inflammation. Within our large cohort studied here, 0.1°F was a statistically significant change from BT1 to DI in both TP and PP groups. Unfortunately, 0.1°F is a small change that would be difficult to detect in a clinical setting, even if the baseline temperatures of patients were known. Furthermore, similar to the recommendations in immunocompromised and elderly patients, a lower core body temperature may be a more sensitive predictor of infection/inflammation in SCI persons. Future SCI studies should address whether fever should be defined differently in persons with SCI, via a lower fever threshold or a change in Tau. Because the presentation of infection/inflammation is often atypical in SCI patients, particularly in terms of thermal responses, clinical evaluations and judgment may be the key to safer care for such patients.
Implications for primary care providers
Primary care providers of persons with SCI need to be educated on such sequelae of thermoregulatory dysfunction and not rely on fever as the sole initial presenting symptom of infectious or inflammatory pathology. Approximately 90% of people with SCI identify family physicians as their regular doctors and only 63% see SCI specialists as needed.36 Family practice literature reports small numbers of persons with SCI in a typical family medicine case load36–38; as a result, there is “uncertainty of how to provide persons with SCI optimal standard of care.”36(p1207) Furthermore, outcome data on quality of care and patient satisfaction reveal that “family medicine does not serve patients with SCI as well as non-SCI patients.”36(p1207) In light of this, we suspect that family physicians/general practitioners may be less aware of the extent of attenuated febrile responses in SCI persons and education may help prevent morbidity and mortality from delayed diagnosis.
Fever in various infection types
Previous literature identified respiratory and urinary tract infections to be the most common infectious etiologies of fever in SCI patients.5,18 In pilot data collection for this study, we also identified urinary tract infections as the most common cause, followed by pneumonia; however, due to inconsistencies in coding across various VA hospitals for infection, we were unable to establish parameters for clearly identifying specific types of infection in the larger cohort examined here.39 When examining temperatures in SCI persons with pneumonia versus urinary tract infection in our pilot data, there was a higher maximum aural temperature in patients with pneumonia than urinary tract infection. The inflammatory response of some infectious processes such as pneumonia and sepsis may be more robust and thus result in a higher hypothalamic set point/febrile response. This larger cohort data support the concept that greater degree of leukocytosis correlates with a higher core temperature rise. In the future, the effect of infection type on febrile responses should be examined in larger SCI populations.
Limitations
There are 3 main limitations that should be considered. First, DI was defined as serum leukocytosis. Serum leukocytosis can also present in conditions that are noninfectious or noninflammatory such as cancer (eg, myelodysplasia and lymphoma) and during corticosteroid administration.5 These conditions were not excluded, so temperature data gathered in these conditions (which is likely very few, if any) would attenuate the average core temperature response to DI and this bias toward the null hypothesis. Second, persons with complete SCI likely have more thermoregulatory dysfunction than those with incomplete SCI, however the data set we utilized was unable to separate persons by completeness of injury. Some have reported that persons with complete injuries were more likely to have a fever than persons with incomplete injuries; we were unable to verify the effect of completeness on fever responses.5,18 Finally, not all persons were hospitalized over the entire 21 days data were collected, so the BT1 and BT2 data sets represent only those patients who were hospitalized during those days, which equated to 83 SCI persons (TP = 50; PP = 33) with 1,139 DI events (TP = 639; PP = 500).
Conclusion
Persons with SCI rarely elevate core temperatures consistent with the CDC definition of fever (>100.4°F) in the setting of DI. This challenges the sensitivity of the febrile response as a sign of developing infectious/inflammatory processes in persons with SCI. Furthermore, baseline temperatures in noninfectious/inflammatory states were subnormal in the TP and PP groups. Impaired vasoconstriction from decentralization of the sympathetic nervous system and impaired shivering responses from paralyzed muscles likely contribute to these findings. Clinicians providing medical care of persons with SCI need to be vigilant for symptoms of infection or inflammation in the absence of fever defined by the CDC (>100.4°F), so diagnosis is not delayed and morbidity and mortality increased. Alternative signs and symptoms, lower fever thresholds, or change in temperature may be more sensitive predictors of infection/inflammation in persons with SCI and should be investigated further.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Audie L Murphy VA Medical Center, San Antonio, Texas.
REFERENCES
- 1. National Spinal Cord Injury Statistical Center. . Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2015. [Google Scholar]
- 2. DeVivo MJ, Black KJ, Stover SL.. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993; 74( 3): 248– 254. [PubMed] [Google Scholar]
- 3. Cardenas DD, Hoffman JM, Kirshblum S, McKinley W.. Etiology and incidence of rehospitalization after traumatic spinal cord injury: A multicenter analysis. Arch Phys Med Rehabil. 2004; 85( 11): 1757– 1763. [DOI] [PubMed] [Google Scholar]
- 4. Winslow C, Bode RK, Felton D, Chen D, Meyer PR Jr.. Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest. 2002; 121( 5): 1548– 1554. [DOI] [PubMed] [Google Scholar]
- 5. McKinley W, McNamee S, Meade M, Kandra K, Abdul N.. Incidence, etiology, and risk factors for fever following acute spinal cord injury. J Spinal Cord Med. 2006; 29( 5): 501– 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeVivo MJ, Krause JS, Lammertse DP.. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999; 80( 11): 1411– 1419. [DOI] [PubMed] [Google Scholar]
- 7. Waites KB, Canupp KC, Chen Y, DeVivo MJ, Moser SA.. Bacteremia after spinal cord injury in initial versus subsequent hospitalizations. J Spinal Cord Med. 2001; 24( 2): 96– 100. [DOI] [PubMed] [Google Scholar]
- 8. McKinley WO, Gittler MS, Kirshblum SC, Stiens SA, Groah SL.. Spinal cord injury medicine. 2. Medical complications after spinal cord injury: Identification and management. Arch Phys Med Rehabil. 2002; 83( 3 suppl 1): S58– 64, S90– 58. [DOI] [PubMed] [Google Scholar]
- 9. Atkins E, Bodel P.. Fever. N Engl J Med. 1972; 286( 1): 27– 34. [DOI] [PubMed] [Google Scholar]
- 10. Dinarello CA, Bunn PA Jr.. Fever. Semin Oncol. 1997; 24( 3): 288– 298. [PubMed] [Google Scholar]
- 11. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM.. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16( 3): 128– 140. [DOI] [PubMed] [Google Scholar]
- 12. Popa C, Popa F, Grigorean VT, . et al. Vascular dysfunctions following spinal cord injury. J Med Life. 2010; 3( 3): 275– 285. [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt KD, Chan CW.. Thermoregulation and fever in normal persons and in those with spinal cord injuries. Mayo Clin Proc. 1992; 67( 5): 469– 475. [DOI] [PubMed] [Google Scholar]
- 14. Downey JA, Chiodi HP, Darling RC.. Central temperature regulation in the spinal man. J Appl Physiol. 1967; 22( 1): 91– 94. [DOI] [PubMed] [Google Scholar]
- 15. Downey JA, Darling RC, Chiodi HP.. The response of tetraplegia patients to cold. Arch Phys Med Rehabil. 1967; 48( 12): 645– 649. [PubMed] [Google Scholar]
- 16. Downey JA, Myers SJ, Gonzalez EG.. Physiological Basis of Rehabilitation Medicine. 2nd ed. Stoneham, MA: Butterworth-Heinemann; 1994. [Google Scholar]
- 17. Colachis SC 3rd, Otis SM.. Occurrence of fever associated with thermoregulatory dysfunction after acute traumatic spinal cord injury. Am J Phys Med Rehabil. 1995; 74( 2): 114– 119. [PubMed] [Google Scholar]
- 18. Unsal-Delialioglu S, Kaya K, Sahin-Onat S, Kulakli F, Culha C, Ozel S.. Fever during rehabilitation in patients with traumatic spinal cord injury: Analysis of 392 cases from a national rehabilitation hospital in Turkey. J Spinal Cord Med. 2010; 33( 3): 243– 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugarman B, Brown D, Musher D.. Fever and infection in spinal cord injury patients. JAMA. 1982; 248( 1): 66– 70. [PubMed] [Google Scholar]
- 20. Beraldo PS, Neves EG, Alves CM, Khan P, Cirilo AC, Alencar MR.. Pyrexia in hospitalised spinal cord injury patients. Paraplegia. 1993; 31( 3): 186– 191. [DOI] [PubMed] [Google Scholar]
- 21. Ohry A, Heim M, Rozin R.. Peculiar septic responses in traumatic tetraplegic patients. Paraplegia. 1983; 21( 5): 318– 321. [DOI] [PubMed] [Google Scholar]
- 22. Khan S, Plummer M, Martinez-Arizala A, Banovac K.. Hypothermia in patients with chronic spinal cord injury. J Spinal Cord Med. 2007; 30( 1): 27– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Attia M, Engel P.. Thermoregulatory set point in patients with spinal cord injuries (spinal man). Paraplegia. 1983; 21( 4): 233– 248. [DOI] [PubMed] [Google Scholar]
- 24. Altus P, Hickman JW, Nord HJ.. Accidental hypothermia in a healthy quadriplegic patient. Neurology. 1985; 35( 3): 427– 428. [DOI] [PubMed] [Google Scholar]
- 25. Menard MR, Hahn G.. Acute and chronic hypothermia in a man with spinal cord injury: Environmental and pharmacologic causes. Arch Phys Med Rehabil. 1991; 72( 6): 421– 424. [PubMed] [Google Scholar]
- 26. Biddle C. The neurobiology of the human febrile response. AANA J. 2006; 74( 2): 145– 150. [PubMed] [Google Scholar]
- 27. Dinarello CA. Thermoregulation and the pathogenesis of fever. Infect Dis Clin North Am. 1996; 10( 2): 433– 449. [DOI] [PubMed] [Google Scholar]
- 28. Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J Endotoxin Res. 2004; 10( 4): 201– 222. [DOI] [PubMed] [Google Scholar]
- 29. Lin VW. Spinal Cord Medicine: Principles and Practice. New York: Springer Publishing Company; 2003. [Google Scholar]
- 30. Essiet A, Onuba O.. Hyperpyrexia in spinal injury patients. Paraplegia. 1992; 30( 5): 339– 342. [DOI] [PubMed] [Google Scholar]
- 31. Griggs KE, Price MJ, Goosey-Tolfrey VL.. Cooling athletes with a spinal cord injury. Sports Med. 2015; 45( 1): 9– 21. [DOI] [PubMed] [Google Scholar]
- 32. Weaver LC, Fleming JC, Mathias CJ, Krassioukov AV.. Disordered cardiovascular control after spinal cord injury. Handb Clin Neurol. 2012; 109: 213– 233. [DOI] [PubMed] [Google Scholar]
- 33. Falsey AR, Baran A, Walsh EE.. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses. 2015; 9 ( suppl 1): 23– 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brusaferro S, Regattin L, Viale P.. Should we change the definition of fever in nosocomial infection surveillance? J Infect. 2008; 57( 5): 420– 422. [DOI] [PubMed] [Google Scholar]
- 35. Norman DC, Grahn D, Yoshikawa TT.. Fever and aging. J Am Geriatr Soc. 1985; 33( 12): 859– 863. [DOI] [PubMed] [Google Scholar]
- 36. McColl MA, Aiken A, McColl A, Sakakibara B, Smith K.. Primary care of people with spinal cord injury: Scoping review. Can Fam Physician. 2012; 58( 11): 1207– 1216, e1626– 1235. [PMC free article] [PubMed] [Google Scholar]
- 37. Middleton JW, Leong G, Mann L.. Management of spinal cord injury in general practice - part 2. Aust Fam Physician. 2008; 37( 5): 331– 332, 335– 338. [PubMed] [Google Scholar]
- 38. Middleton JW, Leong G, Mann L.. Management of spinal cord injury in general practice - part 1. Aust Fam Physician. 2008; 37( 4): 229– 233. [PubMed] [Google Scholar]
- 39. Li C. YS, Trbovich M.. Does the CDC definition of fever (100.4°F) accurately define infection in persons with spinal cord injury? Paper presented at: American Spinal Injury Professionals; September 1–4, 2013; Las Vegas. [Google Scholar]


