Abstract
T follicular helper (Tfh) cells are specialized subset of T helper (Th) cells necessary for germinal center reaction, affinity maturation and the differentiation of germinal center B cells to antibody-producing plasma B cells and memory B cells. The differentiation of Tfh cells is a multistage, multifactorial process involving a variety of cytokines, surface molecules and transcription factors. While Tfh cells are critical components of protective immune responses against pathogens, regulation of these cells is crucial to prevent autoimmunity and airway inflammation. Recently, it has been noted that Tfh cells could be potentially implicated either in cancer progression or prevention. Thus, the elucidation of the mechanisms that regulate Tfh cell differentiation, function and fate should highlight potential targets for novel therapeutic approaches. In this review, we summarize the latest advances in our understanding of the regulation of Tfh cell differentiation and their role in health and disease.
Keywords: Tfh cells, Th cell differentiation and plasticity, IL-4-expressing Tfh cells, Tfh cells in health and disease setting
1. Introduction
A major outcome of the immune response for clearance of pathogens is the production of high-affinity antibody-secreting plasma cells and memory B cells. This process requires direct contact between activated B and T cells in a specialized structure in secondary lymphoid organs known as the germinal centers (GCs) [1]. CD4+ T cells were found to be necessary for development of GCs where somatic hypermutation of Immunoglobulin (Ig) variable region genes and selection of high affinity B cells occur, followed by differentiation into memory B cells and long-lived plasma cells that secrete high-affinity antibodies [2]. For almost two decades the exact nature of CD4+ T cells that provide help to B cells remained mysterious. Early studies implicated Th2 cells as a major CD4+ T helper subset in helping B cell class switching towards IgE and IgG1 by secreting Interleukin (IL)-4 [3]. Th1 cells were also thought to contribute to antibody responses by inducing B cell class switching towards IgG2a [3]. However, GC formations and T cell dependent antibody responses were intact in the mice lacking key regulatory factors for Th1/Th2 development [4–6]. In early 2000s, T follicular helper (Tfh) cells have been identified and emerged as the key cells required for GC reactions [7, 8]. Similar to other Th subsets, Tfh cell differentiation involves a variety of cytokines, surface molecules and transcription factors. Understanding the development and function of Tfh cells is very important for generation of new therapeutic strategies against pathogens and vaccine development.
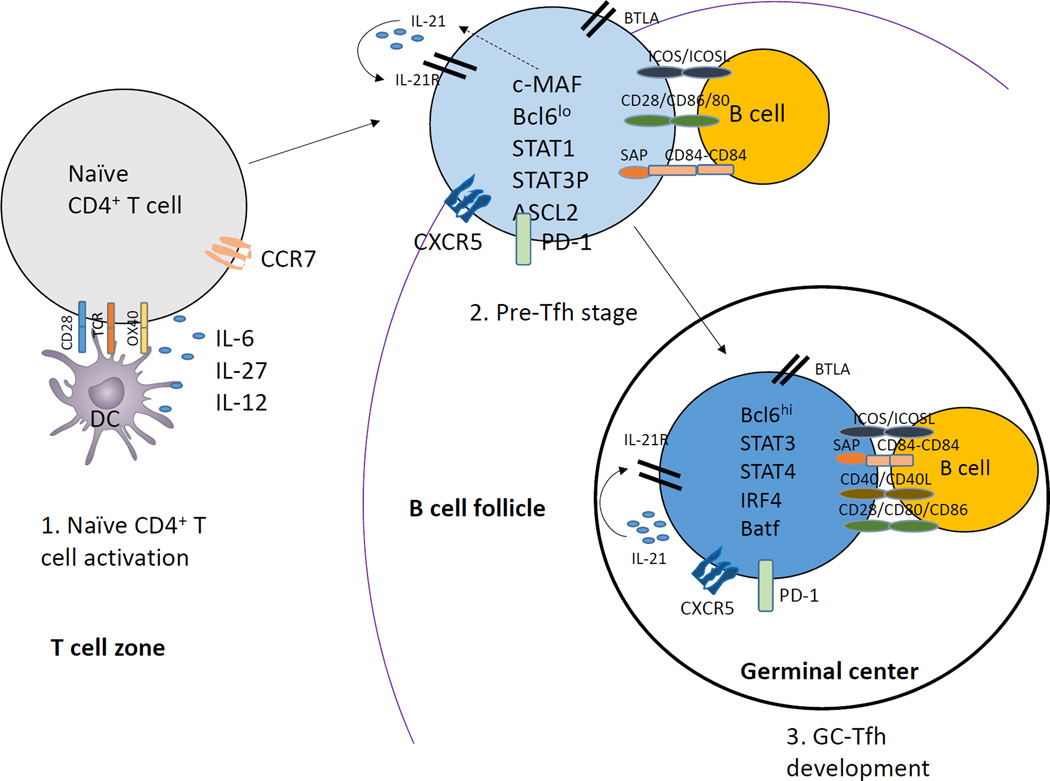
Tfh cell differentiation is a multistage, multifactorial process with significant heterogeneity [4, 9]. The Tfh differentiation process starts after naïve CD4+ T cells are primed with dendritic cells (DCs) in the T cell zone of the secondary lymphoid organ and become precursor Tfh (pre-Tfh) [5, 9]. Pre-Tfh cells that acquire C-X-C chemokine receptor type 5 (CXCR5) expression and down-regulate C-C chemokine receptor 7 (CCR7) migrate to T-B border where they interact with antigen-specific B cells [9, 10]. Further stimulation and antigen presentation by B cells helps the development of pre-Tfh cells to become fully programmed GC Tfh cells. GC Tfh cells provide help to B cells to differentiate into antibody-secreting plasma cells and memory B cells within GCs [5, 9, 10]. The generation and function of Tfh cells is regulated at multiple checkpoints along the process of early priming in T zones and throughout to the effector stage of differentiation in GCs (Fig. 1). IL-6 and IL-21 signaling, possibly via STAT (signal transduction and activator of transcription) 3/STAT1 and B cell lymphoma 6 (Bcl6) which is a key transcription factor are required for Tfh lineage commitment [4, 11] In addition , other markers are critical for Tfh development and function including surface molecules OX40, Inducible costimulatory (ICOS), IL-21R, IL-6R, Signaling Lymphocytic Activation Molecule (SLAM)-Associated Protein (SAP), PD (Programmed Death)-1, B and T-lymphocyte attenuator (BTLA) along with transcription factors such as STAT3, Basic Leucine Zipper Transcription Factor (Batf), Interferon regulatory factor (IRF4) (Fig. 1). On the other hand, STAT5, B lymphocyte-induced maturation protein (Blimp)-1 and IL-2 are known to negatively regulate Tfh cell development.
Figure 1. Developmental stages of Tfh cells.
1) Naïve CD4+ T cells get primed by MHC/Antigen interaction on DCs leading to expression of CXCR5 and ICOS. 2) Interactions of CXCR5+ CD4+T cells with B cells promotes further differentiation of Tfh cells with help from ICOS leading to c-MAF upregulation that subsequently leads to IL-21 production by Tfh cells. Other transcription factors also begin to get expressed such as Bcl6, STAT1, STAT3, Ascl2. 3) Finally, the IL-21 produced by Tfh cells functions in an autocrine manner and leads to high expression of Bcl6 which determines the final differentiation state of Tfh cells. Other transcription factors also get up-regulated at this stage such as STAT3, STAT4, IRF4 and Batf.
In this review, we discuss the recent advances in the understanding of the requirements for the generation and acquisition of effector function of Tfh cells including signaling pathways activated downstream of costimulatory molecules and cytokines, and the consequent activation of subset-specific transcriptional factors. We also elaborate on Tfh cells as an alternative source of IL-4 production and discuss the transcriptional regulation driving IL-4 production by Tfh cells. Further, we review some of the recent advances on the role of Tfh cells in different disease settings.
2. Discovery and identification of T follicular helper cells
A fundamental function of Th cells is to provide help to B cells and to regulate their proliferation and immunoglobulin class switching, especially in the GCs [12]. Discovery of CXCR5 receptor on B cells in 1993 helped in the identification of a specific B-cell helper subset, Tfh cells [13, 14]. In the early 2000s, studies on CD4+ T cells in the human tonsils showed that cells expressing high level of CXCR5 and low level CCR7 have a capacity to induce Ig production in B cells [7, 8, 13, 15]. Similar to B cells, CXCR5 expression of T cells is indispensable for T cell migration to the B-cell follicles [16, 17]. Interestingly, while other Th cells transiently express CXCR5 only at the T cell priming stage, Tfh cells maintain CXCR5 expression and differentiate into GC Tfh cells [18]. By upregulation and sustained CXCR5 expression and downregulation of CCR7, Tfh cells can move from T zones towards CXCL13-rich B-cell follicles, interact with B cells for further differentiation and provide help to B cells [9, 16, 19]. Similarly specialized B helper and tissue inflammatory CD4+ T cell subsets expressing CXCR5 were found in mice [20]. Thus, based on their localization dictated by CXCR5 expression and functions, these distinct CD4+ T cells were named as Tfh cells.
It has been suspected that a subset of Th1 and Th2 cells can function as Tfh cells, rather than Tfh cells stemming from a specialized lineage for follicular helper function. However, gene expression analysis of human Tfh cells (CD57+CXCR5hi) revealed a unique expression profile that is distinct from that of Th1 and Th2 cells [8, 21]. This finding was further supported by the notion that in vivo generated mouse Tfh cells do not express Th1-, Th2- and Th17-specific genes [4, 22]. More importantly, the generation of CXCR5+ Tfh cells is independent of that of other Th cell lineages (Th1 (IFN-γ, STAT1), Th2 (IL-4, STAT4), Th17 (IL-17, IL-17F, RORα and RORγ) and Tregs (TGF-β) [4, 21, 22]. Genetic analysis further identified molecules highly expressed by both mouse and human Tfh cells that are crucial for their development and function, including: ICOS, CD40L, PD-1, BTLA, SAP, IL-21, IL-6 receptor (R), IL-21R and Bcl6 [4, 5, 8, 21, 22]. These molecules play a critical role in promoting both the development and maintenance of Tfh cells and regulating their ability to provide help to B cells.
In 2009, three independent groups including our own demonstrated that mice lacking Bcl6 are impaired in germinal-center formation and Tfh cell development, but not required for differentiation of other T helper subsets including Th1, Th2, Th17 and Treg cells [11, 23, 24]. Taken together, these findings clearly identify Tfh cells as a distinct lineage of Th cells, with Bcl6 as a master regulator of their differentiation.
However, recent data suggest that Tfh cells can express cytokines characteristic of other Th effector cells, including IL-4 and IFN-γ, consistent with their pivotal role in providing help for cytokine-driven patterns of immunoglobulin class switching [25–27]. Thus, Tfh cells exhibit overlapping characteristics with multiple Th effector subsets, raising the possibility of flexibility between Tfh cells and other Th effector populations. Later in this review, we discuss in more detail the mechanism underlining expression of Th2 cytokine IL-4 by Tfh cells, with the perspective of understanding the function of IL-4-expressing Tfh cells towards Th2-mediated immunity.
3. Differentiation of T follicular helper cells
Tfh cell differentiation is a tightly regulated complex process. This starts during T cell priming by DCs in T cell zone of secondary lymphoid tissues, followed by further differentiation and maturation with help from antigen specific B cells in B cell follicles. Each stage of Tfh cell development requires signaling pathways downstream of cell surface molecules (T cells receptor and costimulatory molecules) and cytokines, leading to activation of specific transcription factors.
Costimulatory molecules
CD28 and ICOS
CD28 and ICOS are two structurally and functionally closely related co-stimulators that are important for T cell-dependent B cell responses [28]. Mice deficient in CD28 showed absence of Tfh cells and blocking of CD28 inhibits up-regulation of Bcl6 and CXCR5 and thus migration to the follicle [28, 29]. It is likely that CD28 is required at early stage of Tfh cell differentiation, since blockade of the CD28 pathway starting 6 days post protein immunization did not have any effects on the frequency of CXCR5+PD-1+ cells [30]. Recent studies, however, reported that B7.2 expression on B cells is required for GC formation, suggesting the role for B7-CD28 interaction between T and B cells in B cell follicles [31].
Another CD28 family member, namely ICOS has been known for many years as an important regulator of Tfh cells [32]. Mice in which ICOS-ICOSL interactions are disrupted, or patients with mutations in ICOS, have decreased Tfh cell generation and defect in GC formation [4, 33–37]. However, mechanism(s) responsible for this defect were unknown until recently. Using a viral infection model for Tfh differentiation in 2011 Choi et al. demonstrated that ICOS signaling is required firstly for Bcl6 induction during DC priming and secondly for Tfh maintenance of Bcl6 during T cell – B cell cognate interactions [30]. However, later studies in 2015 by Jan Weber et al. have shown that primary defect in ICOS deficient mice is impaired upregulation of CXCR5, not of Bcl6, which is independent of Bcl-6 and achaete-scute family bHLH transcription factor (Ascl)2 expression [38]. ICOS exerts its costimulatory function via activation of PI3K-AKT signaling leading to phosphorylation and deactivation of Foxo1 and its downstream target Kruppel like factor (Klf2) that directly binds to the promoter region of CXCR5 [38]. Klf2 has to be expressed at low level to maintain high expression of CXCR5 on Tfh cells and keep them in B cell follicles [38]. This means that ICOS costimulation does not directly regulate lineage specific factors like Bcl6 and Ascl2, but is important for maintenance of Tfh cell phenotype by controlling their localization in B cell follicle within secondary lymphoid organs [38]. The important role of ICOS for Tfh cell maintenance during T-B cell interaction was also emphasized by the fact that B-cell specific knockout of ICOSL showed significantly reduced Tfh cell frequencies [4]. ICOSL blockade at effector stage of Tfh development leads to reversion of their phenotype to non-Tfh and re-location back from B cell follicle to T cell zone [38]. Subsequently, Tfh cells lose expression of Bcl6 which can be explained by osteopontin-dependent degradation of Bcl6 protein [39].
SAP
SAP expression in CD4+ T cells, specifically in GC Tfh cells is important for formation of GCs, long-lived plasma cells and memory B cells [40, 41]. Bcl6 promotes SAP expression in GC Tfh cells [42]. SAP mediates responses to members of the SLAM family by binding to a conserved domain on those receptors, four of which are highly expressed on both Tfh cells and B cells: SLAM/SLAMF1, Ly9/SLAMF3, CD84/SLAMF5 and Ly108/SLAMF6 [40, 43, 44]. SAP regulates GC responses by modulating T-B cell conjugates, cytokine secretion and TCR signal strength [45]. Mutation of SAP in humans, as well as studies involving SAP-deficient mice, suggested a critical role for SAP in Tfh cell help to B cells [5]. While SAP-deficient CD4+ T cells express normal levels of CXCR5 and Bcl6, they failed to stably interact with cognate B cells and induce B-cell clonal expansion [43, 46–48]. Thus, SAP function is to sustain adhesion between T cells and B cells that is necessary for the delivery of adequate Tfh cell help to B cells [43]. Recent work defined the two potential molecular mechanisms underlying SAP-mediated regulation of T:B cell adhesion: 1) SAP binds cytoplasmic tails of SLAM family receptors and mediates positive signaling by recruiting the Src family kinase Fyn [49]; 2) SAP competes with Src homology region 2 domain-containing phosphatase (SHP)-1 for binding to Ly108 and abrogates Ly108 signaling that shorten Tfh:B cell contact [43]. In addition to prolonging adhesion, SLAM family receptors regulate the secretion of cytokines [50, 51]. SAP specifically regulates IL-4 secretion by GC Tfh cells [52]. Production of IL-4 by Tfh cells is dependent on SLAM and on positive signaling through SAP and PKC-θ [52, 53]. Moreover, SAP through SLAM family receptors can modulate strength of TCR signaling in Tfh cells [53, 54]. For example, positive signaling through Ly108 and SAP sustains TCR signaling through Extracellular Signal-regulated Kinase (ERK) activation and results in higher quality interactions between Tfh:B cells within the GCs [54].
BTLA
BTLA is an inhibitory receptor expressed on T and B cells that binds Herpes Virus Entry Mediator (HVEM) and inhibits T cell proliferation [55]. BTLA deficient mice exhibit increased antigen-specific IgG responses and gradually developed hyper-y-globulinemia and autoantibody production [56]. BTLA is highly expressed on Tfh cells compared to CD4+CXCR5+T cells [4, 57]. Percentage of CXCR5+PD1+ Tfh cells was not affected in BTLA knockout mice suggesting that BTLA does not control Tfh development [57]. However, mice deficient in BTLA show an increase in percentage of GC B cells and antigen-specific IgG2a and IgG2b after protein immunization, suggesting that BTLA may control Tfh cell function [57]. In fact, increased IL-21 production by BTLA deficient Tfh cells is essential for enhanced IgG production by B cells, suggesting that BTLA controls Tfh-mediated Ig responses by inhibiting IL-21 production [57].
Cytotoxic T-Lymphocyte-Associated Protein (CTLA)-4
CTLA4 is an inhibitory receptor that acts in opposition to the CD28 to limit T cell immune responses [32]. CD28 and CTLA4 share same ligands CD80 and CD86 that are expressed by antigen-presenting cells and B cells, but CTLA-4 binds to them with higher affinity than CD28, limiting CD28 dependent activation [32]. CTLA4 is a key mediator of Treg function and also controls conventional T cells [32]. Deletion of CTLA4 in mice results in fatal multiorgan inflammation within 2 to 4 weeks of age, as well as increased antibody levels, suggesting CTLA4 role in controlling B cell responses [58–61]. Treg-specific deletion of CTLA4 recapitulates increase in antibody production, pointing the essential role of CTLA4 on Tregs in limiting B cell responses [62]. In 2014, two independent groups determined cellular mechanisms by which CTLA4 regulates B cell responses [63, 64]. These studies demonstrated that preventing CTLA4 signaling results in increased GC responses, with corresponding increase in number of Tfh cells and follicular regulatory T cells (Tfr) cells, a specialized effector subset of Tregs that suppress B cell responses [63, 64]. CTLA-4 functions to inhibit Tfh differentiation, maintenance and effector function. In addition, while CTLA4 controls Tfr development and maintenance, it is required for Tfr cells to suppress antigen-specific B cell responses in GCs [63, 64]. Wang et al. also reported that abundance in Tfh cell formation in the absence of CTLA4 is dependent on graded down-regulation of CD28 engagement. Thus, T-cell dependent humoral immunity is tightly controlled by CTLA4 [65].
PD-1
PD-1 and its ligands (PD-L1 and PD-L2) are highly expressed on GC Tfh cells and GC B cells, correspondingly [66]. PD-1 was initially expected to be an inhibitory signal to GC Tfh cells, as mice with impaired PD-1 signaling showed increased Tfh cell numbers after protein immunization [66]. However unexpectedly, these mice showed a decrease in B cells responses and lower number of plasma cells. This outcome was explained by reduced production of B-cell-helping cytokines IL-4 and IL-21 by Tfh cells in the absence of PD-1 signaling [66]. A recent study by Peter Sage et al., explains the previous observation on the role PD-1 in regulation of humoral immunity [67]. Mice deficient in PD-1 and its ligand PD-L1 had increased number of Tfr in lymph nodes and those Tfr cells had enhanced suppressive function [67]. This study has been the first to indicate the immunoregulatory role for PD-1-PD-L1 pathway in limiting differentiation and function of Tfr cells and for further control of the humoral immune responses through migration of circulating Tfr cells into the lymph nodes and suppression of B-cell responses.
Cytokines
Generation of the different T helper subsets is associated with the action of particular cytokines. IL-6 and IL-21 play a central role in Tfh development. IL-6 is produced by a wide range of cells including antigen-presenting cells and B cells [68]. Type I interferons (IFNs) can induce IL-6 production in DCs, thus leading to Tfh cell development [69]. IL-6 was shown to be involved in the early differentiation stage of Tfh cells by inducing Bcl6 and IL-21 expression [10, 70, 71]. Mice deficient in IL-6 or IL-6R show reduced or delayed Tfh cell formation due to impairment in STAT3 and STAT1 signaling [68, 72].
IL-21 is the main cytokine that is produced by Tfh cells; however, IL-21 could also drive IL-21 production in an autocrine manner and Tfh cell differentiation in a STAT3 dependent manner [4]. IL-21 deficient mice display reduced number of Tfh cells after protein immunization, and T cells deficient in IL-21R show intrinsic defect in Tfh cell formation, indicating the autocrine regulation of Tfh cells by IL-21 [4, 73]. There is evidence of some redundancy between IL-6 and IL-21 in Tfh cell differentiation. These cytokines are likely to collaborate, as concomitant blockade of both cytokines significantly reduced the number of Tfh cells in vivo [68, 70]. However, Tfh cells are present at reduced number in the absence of both IL-6 and IL-21, indicating that additional factors contribute to Tfh cell development [68, 70]. In fact, IL-27 by signaling through STAT3 can also induce IL-21 production and Tfh generation [74]. Besides IL-6, IL-21 and IL-27, in mouse and human CD4+ T cells, IL-12 can also induce IL-21, CXCR5, ICOS and Bcl6 expression in a STAT3 dependent manner, which are features of Tfh cells [75–78]. Interestingly, Nakayamada et al. determined the direct role of Type I IFNs in Tfh differentiation by inducing Bcl6, CXCR5 and PD-1 expression in a STAT1 dependent manner [79], but not IL-21. Also, upon IFNα/β ligation STAT1 was found to bind directly to the Bcl6 locus [79].
While the above described cytokines are promoting the formation of Tfh cells, certain factors are known to inhibit the differentiation of Tfh cells. IL-2 is shown to be inhibitory for the formation of Tfh cells through the activation of STAT5, which enhances the Blimp1 expression and prevents the binding of STAT3 to the Bcl6 locus [80]. Aside from IL-2, IL-10 signaling is also shown to be inhibitory for Tfh cell formation as IL-10Rβ signaling deficiency in Th cells leads to enhanced IL-21 and IL-17 producing Tfh cells [81].
Transcriptional regulation of Tfh cells
There is a growing list of transcription factors known to be involved in controlling follicular T helper cell development [71, 82]. These factors usually function downstream of signaling cascades induced by cytokine and cytokine receptor interactions and both positively and negatively affect Tfh cell differentiation and function.
Bcl6
The B-cell lymphoma 6 protein (Bcl6) is well recognized as a key factor for Tfh cell development and function and for efficient germinal center responses [11, 23, 24]. Expression of Bcl6 is majorly driven by IL-6 and IL-21 and the downstream transcription factors STAT1 and STAT3 in Tfh cells [38]. CD4+ T cells upon Bcl6 overexpression show up-regulation of PD1, CXCR5, CXCR4, the key components of Tfh cell development along with various other factors critical for Tfh cell function in T and B cell interaction [30, 71]. Bcl6 mainly promotes Tfh cells by suppressing genes crucial for other T helper lineages such as IFN-γ receptor, T-bet, STAT4, GATA3, ROR-γt, IL-17 and its receptor and several microRNAs responsible for suppressing Tfh cells [11, 23, 24, 83, 84]. Bcl6 expression in both B and T cells is required for germinal center reactions and Bcl6 deficiency in T cells leads to impaired Tfh development both in vitro and in vivo [11, 23, 24]. A recent study by Liu et al. has analyzed genome wide Bcl6 occupancy together with transcriptome profiling and identified Bcl6 target genes in Tfh cells thus providing important insights into the biological functions of Bcl6 in Tfh cells [85]. Bcl6 binds mainly to introns and intergenic regions in Tfh cells and promotes Tfh cell differentiation through antagonizing IL-7R (CD127)/STAT5 axis. Bcl6 also plays a critical role in inhibiting Tet1 recruitment and 5-hydroxymethylated Cytosine modification in Tfh cells [85].
c-MAF
ICOS signaling induces the transcription factor c-MAF which in turn induces IL-21, a crucial determinant of Tfh cell differentiation and maintenance [34]. The absence of c-MAF reduces the number of Tfh cells due to defective induction of IL-4 and IL-21 [34]. Furthermore, co expression of Bcl6 and c-MAF cooperate in the induction of crucial Tfh associated genes such as CXCR4, PD-1, and ICOS [42].
Batf
Batf is a multifunctional transcription factor required for differentiation of various Th effector subtypes and also GC and B cell generation. It is known to directly induce transcription of ROR-γt, IL-17, IL-21, and IL-22 in Th17 cells, as well as Bcl6 and c-MAF in Tfh cells [86–88]. Recently it was shown that Batf mainly exerts its function through the Batf-Jun complex in association with IRF4 in Th17, Th2 and in cells stimulated with IL-21 [89]. Our group has recently identified a novel role of Batf in controlling the IL-4 expression in pro-allergic Tfh cells during asthma pathogenesis. Batf-IRF4 complex in cooperation with STAT3 and STAT6 controls IL-4 expression in Tfh cells though the CNS2 element in the IL-4 locus [90]. Whether IL-4 produced by these Tfh cells contributes to the development and maintenance of Tfh cells is an open question.
IRF4
Apart from its role as a multifaceted transcription factor involved in the regulation of Th2, Th17, and Th9, and the repressive ability of Treg cells, IRF4 is also essential for the differentiation of Tfh cells [91]. IRF4 exerts its function by forming physical complexes with Batf and Jun transcription factors of the AP-1 family and binding to its target sequences [89, 92]. Genome wide analysis revealed that most IL-21-regulated genes were associated with combined STAT3-IRF4 sites and corresponding ChIP-seq results revealed decreased STAT3 binding to the target genes after IL-21 treatment in IRF4 deficient T cells. IRF4 deficient mice showed impaired IL-21-induced Tfh cell differentiation in vivo suggesting a functional cooperation between STAT3 and IRF4 [92, 93].
STATs
Various members of the STAT family of transcription factors are involved in the regulation of Tfh cells either positively or negatively [71, 82, 94]. Among them STAT1, STAT3 and STAT4 are known to positively influence Tfh cell development [4, 71, 82]. STAT3 is mainly required for IL-21 production and deficiency of STAT3 in both mice and humans results in a great reduction of Tfh cells [4, 78, 95]. STAT1 is known to exert Tfh cell control by partnering with STAT3. Similar to STAT3, STAT4 deficiency leads to a vast decrease in Tfh cell development: Tfh, GC B cells and IgG2b titers were significantly reduced following immunization of STAT4 KO mice [96]. In contrast, studies show that STAT5 functions to limit Tfh cell development and humoral immunity [94, 97]. Deficiency of STAT5 enhances Bcl6 expression and subsequent Tfh gene expression and Tfh and GC formation in vivo [94, 97]. IL-2 mediated STAT5 positively influences BLIMP-1 expression which is known to suppress Bcl6 and Tfh-associated genes [94, 97].
Ascl2
Achaete-scute homologue 2 (Ascl2) is a basic helix–loop–helix (bHLH) transcription factor which is selectively up-regulated in Tfh cells and directs early Tfh cell differentiation [95]. Ectopic overexpression of Ascl2 up-regulates CXCR5 and down-regulates CCR7 and P-selectin ligand 1 (PSGL1) expression in T cells in vitro, as well as accelerates T-cell migration to the follicles and Tfh-cell development in vivo in mice [95]. Ascl2 deletion as well as blockade of its function with the Id3 protein in CD4+ T cells results in impaired Tfh cell development and GC response. Genome-wide analysis indicates that Ascl2 directly regulates Tfh-related genes whereas it inhibits expression of Th1 and Th17 signature genes [95].
Foxo1 and FoxP1
Foxo1 negatively regulates Bcl6 expression by directing binding to the Bcl6 genomic locus and subsequently Tfh cell differentiation [98]. Specific deletion of the Foxo1 gene in T cells leads to enhanced Bcl6 expression and excessive Tfh cell differentiation in the absence of ICOS ligand signals [99]. FoxP1 is another suppressor of Tfh cell differentiation. It mainly exerts its effects by suppressing ICOS signaling and IL-21 production [100].
Blimp1
Blimp-1 is a well-known antagonist of Bcl6, and is known to inhibit Bcl6 expression in B and T cells and vice versa. Forced expression of Blimp1 blocks Bcl6, CXCR5, ICOS, and PD-1 and increases SLAM expression [23].
4. Cytokine production by Tfh cells
Cytokine signaling is one of the means by which Tfh cells provide help to GC B cells. Although Tfh cells have distinct phenotypic and functional attributes compared to other effector Th cells, there is growing evidence demonstrating that Tfh cells can express cytokines such as IFN-γ, IL-17 and IL-4 characteristic of Th1, Th17 and Th2 effector populations as well [101]. In fact, Tfh cells isolated ex vivo can be induced to express these effector cytokines depending on the cytokine environment under polarizing conditions in vitro [102]. Thus, it is possible that Tfh cells produce multiple factors that are required for effective GC responses. In this section, we will review the role of different cytokines in regulating humoral immunity with particular emphasis on IL-4 production and regulation by Tfh cells.
IL-21
Tfh function is mainly driven by IL-21 which is the major effector cytokine secreted by Tfh cells and is required for extra-follicular antibody-producing cell formation and for proliferation of GC B cells [4, 73]. IL-21 also leads to enhanced B cell proliferation through the help from CD40L ligation [103]. Beyond this, Tfh cell-produced IL-21 maintains the expression of Bcl6 in GC B cells and affinity maturation of the GC responses [104]. Transcriptionally, IL-21 is known to be regulated by the c-MAF transcription factor in an ICOS dependent manner in Tfh cells [34].
IFN-γ and IL-17
Following LCMV infection in mice, effector and memory CD4+ T cells with Tfh-like characteristics were shown to produce IFN-γ [105]. Some Tfh cell traits were also observed in the memory CD4+ T cell pool post infection [105]. IL-17 was identified to be produced by cells displaying a Tfh phenotype in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) [34]. This IL-17 production in Tfh cells is ICOS dependent.
IL-4
Recent data suggest that Tfh cells are a major cellular source of IL-4 which is a Th2 signature cytokine [25]. Although IL-4 appears to be unimportant for Tfh cell generation, it is critical for B cell maturation and class switching to IgG1 in response to parasitic infections [25, 106]. Moreover, IL-4 is required for memory B cells to effectively differentiate into plasmablasts during secondary immunization [106]. Studies in a parasitic infection model of 4get/KN2 mice showed that IL-4 producing Tfh cells express Tfh cell markers such as CXCR5, ICOS, PD-1, BTLA, Bcl6 and IL-21 specifically in B cell follicles and germinal centers of the reactive lymph nodes [25]. However, within the non-lymphoid effector site, huCD2+ IL-4 producing cells do not express the Tfh characteristics [107], suggesting that IL-4 producing cells in the lymph nodes are phenotypically different from peripheral Th2 cells and have distinct characteristics of Tfh cells. Interestingly, Tfh cells are absent in µMT/4get mice (B-cell deficient mice) immunized with serum schistosome egg antigen (SEA) compared to B-cell sufficient 4get mice, suggesting that Tfh population could be derived from the Th2 lineages in the presence of B cells [107]. Moreover, the transcription of GATA3, master regulator of Th2 differentiation, was comparable between Tfh cells found in B cell follicles and IL-4-competent cells (PD1-IL-4/GFP+CD4+) from outside the B cells follicles from SEA immunized mice [107], further suggesting that Th2 cells located within lymph nodes have the potential to become either Tfh cells within GCs of lymph nodes or migrate to peripheral tissues to perform Th2 effector functions. The importance of IL-4 produced by Tfh cells was further shown by the evidence of co-production of IL-4 and IL-21 in Tfh cells which is required for optimal IgG1 production [106]. Memory B cells also require IL-4 for efficient differentiation to plasmablasts, indicating an important role for IL-4 in the cross-talk between Tfh and B cells during secondary immune responses.
Recently, the transcriptional regulation of IL-4 expression in Tfh cells is an area of active research and various costimulatory molecules and transcription factors along with coordinated chromatin remodeling and epigenetic changes occurring at the IL-4 locus have been shown to play a crucial role in IL-4 production by Tfh cells. Loss of SAP is shown to have a moderate effect on non-GC Tfh cell differentiation, but mice deficient in SAP display an absence of GC Tfh cells [52]. SAP deficient Tfh cells are found to be defective in IL-4 and IL-21 production. Moreover, SLAM (Slamf1, CD150), a surface receptor that utilizes SAP signaling is specifically required for IL-4 production by GC Tfh cells [52]. Blockade of ICOSL in L. major infected 4get/KN2 reporter mice also leads to reduction in GC formation, fewer hCD2+ IL-4-producing Tfh cells within B cell follicles and reduced GC B cells without affecting IL-4 expressing Th2 cells [108], suggesting that ICOSL is not required for Th2 cell development but is needed for IL-4 producing Tfh cells within GCs of reactive lymph nodes.
Extensive chromatin immunoprecipitation (ChIP) analysis of the IL-4 locus revealed an abundance of acetylated H3K9/14 at the HS2, HS5a, and CNS2 sites and H3K4me2 methylation at the CNS2 locus of the IL-4 locus was observed in Tfh cells [109, 110]. Analyses of either HSV or CNS2 deficient mice demonstrated non-redundant function of conserved cis-element CNS2 in Tfh-specific regulation of IL-4 gene [109, 110]. Deletion of CNS2 led to profound reduction in IgG1 and IgE post-immunization, and this defect was mainly due to loss of IL-4 expression by Tfh cells indicating CNS2 as an active and specific enhancer for IL-4 producing Tfh cells and crucial for IgG1 and IgE class switching [110]. In naïve T cells, along with enriched H3K4me2 methylation, the CNS2 region is in a highly demethylated state indicating towards its importance in early IL-4 expression during in vivo immune response [110]. CNS2 deficiency also leads to retained H3k27me3 (silencing marker) recruitment particularly in Tfh cells further suggesting that IL-4 production in Tfh cells is solely CNS2 dependent [110]. Further, CNS2 is known to exert its enhancer effects by the SLAM-SAP pathway and Notch and Recombining Binding Protein suppressor of hairless (RBP-J) signaling for IL-4 production [52, 111]. Epigenetic marks on CNS2 site were slightly affected in the absence of SLAM and were not altered in the absence of RBP-J, indicating marginal participation of SLAM but not Notch pathway for CNS2 activity in Tfh cells [112]. In contrast to Tfh cells, Th2 cells, basophils and eosinophils are less dependent on HSV and CNS2 for IL-4 production [110].
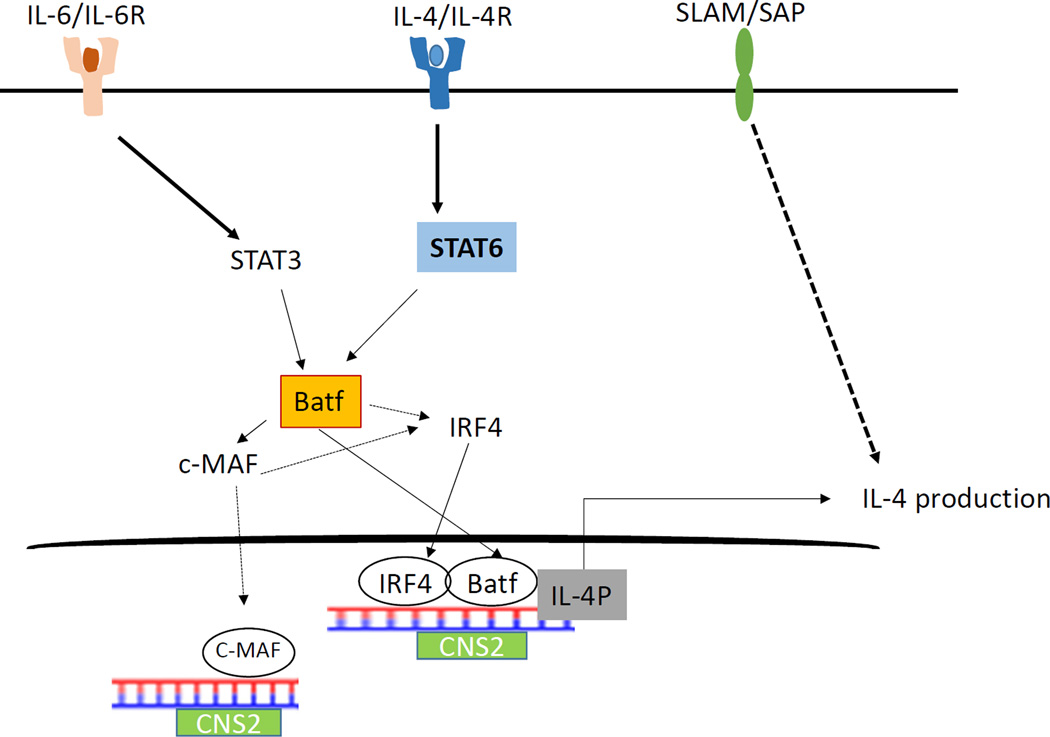
Although the regulation of IL-4 in Th2 cells is multifactorial and has been extensively studied, there is a dearth of information on the transcriptional factor mediated control of IL-4 in Tfh cells. Our recent study identifies a novel and crucial role of the transcription factor Batf in controlling IL-4 expression in Tfh cells in vivo [90] (Fig. 2). We observed direct binding of Batf to the CNS2 region in the IL-4 locus in Tfh cells. Furthermore Batf deficiency led to decrease in the abundance of active histone proteins such as AcH3 and H3k4 to the CNS2 locus suggesting that Batf regulates IL-4 expression in Tfh cells through direct binding and controlling access of the chromatin machinery in the CNS2 region. Batf also physically interacts with IRF4 leading to transactivation of the CNS2 region in the IL-4 locus. In addition, Batf-IRF4 interaction towards induction of IL-4 expression in Tfh cells is dependent on both STAT3 and STAT6 transcription factors.
Figure 2. IL-4 regulation in Tfh cells.
i) IL-4/STAT6 signalling leads to upregulation of Batf that in turn cooperates with IRF4 to bind to CNS2 region promoting IL-4 production. ii) Batf is also upregulated by IL-6/STAT3 signalling further activating c-MAF which binds to CNS locus leading to IL-4 production.
Apart from Batf and IRF4, we identified the function of c-MAF in the regulation of IL-4 in Tfh cells. c-MAF is known to be a target of Batf in Tfh cells, which is required for regulation of IL-21 expression and consequently for Tfh expansion [90]. c-MAF is also known to control IL-4 expression in cooperation with IRF4 and NFAT in Th2 cells [90]. We also observed increased binding of c-MAF to the CNS2 locus in Tfh cells as well as c-MAF-induced transactivation of the CNS2 region indicating that c-Maf aids in IL-4 transcription in Tfh cells [90].
5. Tfh cells in disease setting
Tfh cells have been implicated in various disorders such as autoimmunity, inflammation and viral infections [5, 9]. Generation of protective antibodies against infections is an important aspect of Tfh cells, whereas a dysregulation in Tfh cell generation has been implicated in a range of primary immune-deficiencies. In this section of the review, we will discuss the known functions of Tfh cells in various disease settings.
Several viral infections elicit a Th1 and/or Tfh cell response. In mice with LCMV infections, the initial Th1 cell programming is shown to be redirected to a more Tfh phenotype [113] especially in the chronic phase of viral infection, GC Tfh cells are seen to increase in number. In the case of HIV, huge numbers of GC Tfh cells (defined by CXCR5+PD1+CD4+ T cells in the blood) were found in HIV infected patients and in macaques infected with SIV [114, 115]. These numbers increase if patients are not treated implicating that HIV viral persistence is associated with the high numbers of Tfh cells present. In addition, humans infected with HIV for several years are found to generate broadly neutralizing antibodies indicating towards a correlation between Tfh cell number and the serum antibody response in HIV patients and the possibility of Tfh cell involvement in hyper-γ-globulinemia [116]. However, recent studies on Tfh cells isolated from HIV infected individuals showed a defective function of Tfh cells as seen by the decrease in production of IL-21 and the inefficiency in providing B cell help compared to non-infected controls [117]. B cells analyzed from these patients showed that although the frequency of GC B cells and plasma cells is increased, memory B cells are significantly depleted. Because IL-21 promotes GC B cell maintenance and affinity maturation, this decreased IL-21 production in HIV patients may reduce the mutation rate and selection in B cells that are necessary to produce broadly neutralizing antibodies against HIV [118]. Taken together, Tfh cells can potentially be used an indicator of viral persistence in HIV patients and can be potentially targeted for improved vaccine development against HIV.
Uncontrolled Tfh cells are reported to be responsible for providing excess help to B cells to generate reactive auto-antibodies; thus, Tfh cells play a significant role in the pathogenesis of various autoimmune diseases as well such as rheumatoid arthritis, SLE, Sjogren’s Syndrome [119]. The strongest evidence for Tfh cell role in autoimmunity comes from studies utilizing the Sanroque mice that have a mutated roquin gene encoding the RING-type ubiquitin ligase that dysregulates ICOS expression [22]. This leads to spontaneous GC formation, SLE-like symptoms such as anti-double stranded antibodies and excessive Tfh cells with increased ICOS and IL-21 [120]. Also, an increase in CXCR5+ICOS+, CXCR5+PD-1+, ICOS+CD4+ cells have been detected in patients with SLE [121, 122]. Consistent with this observation, an increase in serum IL-21 and CXCL13, which also correlated with disease scores, have been detected in Systemic lupus erythematosus (SLE) patients [123]. Interestingly, CXCR5+IL-21+CD4+ T cells in SLE patients correlated with presence of memory B cells [120]. Also, in SLE patients treated with corticosteroids, a decrease in the frequency of CXCR5+PD-1+Tfh cells was noted [122].
Studies on characterization of Tfh cells in Rheumatoid Arthritis (RA) patients have shown an increase in CD4+ICOS+CXCR5+ T cells in the rheumatoid synovial joint and in circulation with increased CD19+ B cells and high serum IL-21 which is positively associated with disease scores and presence of anti-citrullinated antibodies [124–126]. Interestingly, early stage RA patients displayed elevated levels of serum IL-21, but serum IL-21 in chronic RA patients was not very different from normal donors [124, 125]. Tfh cells have also been implicated in other autoimmune conditions such as Sjogren’s Syndrome, a systemic autoimmune disorder characterized by dysregulation of the exocrine glands such as salivary and lacrimal glands. CXCL13 and CXCR5+ CD4+ T cells have been detected in the salivary gland and CXCR5+PD-1+Tfh cells in peripheral blood of Sjogrens Syndrome patients with increase in IL-21 levels [121]. Further analysis of Th1-, Th2- and Th17-like Tfh cells revealed an increase in Th17-like Tfh cells that correlated with the presence of serum autoantibodies [127].
Multiple sclerosis (MS) is an autoimmune neurodegenerative disorder that causes damage to myelin and axons in the brain. Ectopic lymphoid follicle-like structures containing GC have been observed in the meninges of some MS patients [128]. Furthermore, presence of ectopic GCs has been associated with more rapid disease progression and generally a poor prognosis [128]. Recent studies have shown an increase in peripheral blood ICOS+CXCR5+ Tfh cells in patients with MS at all stages of disease – relapsing-remitting or progressive compared to healthy controls [129]. Furthermore, this increase in Tfh population correlated with increase in plasmablasts in the peripheral blood and disease scores [129]. Also, analysis Tfh cells in MS patients revealed decrease in Tfh1 cells in relapsing-remitting and progressive MS patients and an increase in Th17-like Tfh cells in progressive MS patients compared with normal donors [129]. Moreover, IL-21+CD4+T cells have been detected in active lesions of MS patients [130]. These data indicate that circulated Tfh cells to be used as an indicator of the progression of disease in MS.
Tfh cells have been implicated in type 2 immune responses as evidenced by the production of IL-4 [25, 106, 107]. Our group has recently shown that Batf deficiency impairs the generation of IL-4-committed Tfh cells which leads to protection against asthma [90] indicating a more pro-allergic function of IL-4 producing Tfh cells. Also, a recent study showed the frequency of circulating Tfh cells to be higher in patients with severe asthma with increase in Bcl6 expression and plasma IL-21 levels in comparison with mildly asthmatic patients [131]. However, Tato AB et al., show that initial exposure to house dust mites (HDM) did not lead to Th2 cell development but instead promoted the formation of IL-4 producing Tfh cells. Following challenge exposure to HDM, Tfh cells differentiated into IL-4 and IL-13 double-producing Th2 cells that accumulated in the lung and recruited eosinophils [132]. Thus, Tfh cells are precursors of HDM-specific Th2 cells after secondary exposure indicating their plasticity depending on the antigenic exposure [132].
There has now been increasing evidence on the association of Tfh cells and tumor immunity. Neoplastic cells in angioimmunoblastic T cell lymphoma (AITL) display Tfh cell features such as expression of Bcl6, CXCL13 and PD-1 and these cells arise in the follicular sites of lymph nodes [133–135]. Further, AITL is also associated with B cell expansion, hyper-γ-globulinemia and production of autoantibodies, which may be suggestive of Tfh cell functions in AITL [133, 135]. Non-neoplastic T cells exhibit features of Tfh cells in B cell lymphoma which are GC derived such as follicular lymphoma and Hodgkin’s disease [136]. Also, an increase in CD4+CXCR5+ T cells in the blood is found in patients with chronic lymphocytic leukemia [137]. However, whether the expression of Tfh cells among neoplastic cells is indicative of survival and proliferation of neoplastic cells needs to be further investigated. Tfh cells may also be involved in anti-tumor responses in certain cancer types such as breast cancer which involves expression of high level of lymphocytes including Tfh cells [138]. Also, tumors displaying higher levels of Tfh cell infiltrates are found to be associated with increased survival [138]. However, the exact function of these Tfh cells in inhibiting tumor survival remains to be determined.
6. Conclusion
Since the discovery of Tfh cells as a distinct subset that is responsible for mediating B cell responses, various groups have identified several important molecular and cellular factors that are necessary for the development and function of Tfh cells. Although Tfh cells are mainly defined by expression of the chemokine receptor CXCR5, transcription factor Bcl6 and the cytokine IL-21 depending on the microenvironment, Tfh cells could also produce Th1, Th2 or Th17 related cytokines. The production of these cytokines could possibly be regulated by common factors or may also be differentially regulated during the transition of Th/Tfh subsets. We have recently identified the role of Tfh cells and the IL-4 produced by them in the pathogenicity of type II allergic responses. IL-4 producing Tfh cells contribute to promotion of allergic airway inflammation as well as retain a stable phenotype during the course of disease progression. The regulation of IL-4 in Tfh cells is controlled by the transcriptional factor Batf through permissive epigenetic modifications and cooperation of other transcription factors like IRF4/STAT3/STAT6 mainly through direct binding to the CNS2 locus. Further studies are necessary to determine the exact nature of relationship between Tfh/Th2 cells and the molecular controls driving this plasticity and effector gene expression during disease progression. The exact role of IL-4 derived from Tfh cells in shaping up type 2 immunity in physiological and disease settings should also be further clarified by extensive studies. Tfh cells are emerging as central players in a number of autoimmune diseases as well as in cancer and recent discoveries about the complex biology and plasticity of Tfh cells has opened new avenues for the manipulation of Tfh cells for the development of new therapeutic and vaccine strategies. It is hoped that a complete mechanistic understanding of Tfh cell development and progression can result in new therapeutic approaches against major autoimmune and inflammatory diseases and cancer.
Highlights.
Tfh cells are a distinct T helper lineage that provides help to B cells.
Tfh cell differentiation is a multistage, multifactorial process with significant heterogeneity.
Tfh cells can acquire characteristic overlapping with other Th effector cells.
Tfh cells are a major source of IL-4 which is differentially regulated compared to Th2 cells.
Tfh cells are implicated in a majority of diseases including immune-mediated disorders.
Acknowledgments
This work is supported by NIH research grants (A1R03AI120027 and 1R21AI20012 (RN)), Institutional Research Grant (RN), start-up grant (RN), MD Anderson CIC seed grant (RN).
Abbreviations
- Th
T helper
- Tfh
T follicular helper
- IL
Interleukin
- Ig
Immunoglobulin
- STAT
signal transduction and activator of transcription
- ICOS
Inducible co-stimulator
- SLAM
signaling lymphocytic activation molecule
- SAP
slam associated protein
- GC
germinal center
- Bcl6
B cell lymphoma 6
- SEA
schistosome egg antigen
- Batf
basic leucine zipper transcription factor ATF
- Ig
Immunoglobulin
- BTLA
B and T-lymphocyte attenuator
- IRF4
interferon regulatory factor 4
- PD-1
programmed death-1
- Blimp-1
B lymphocyte-induced maturation protein-1
- ERK
extracellular signal regulated kinase
- HVEM
Herpes virus entry mediator
- MAF
musculoaponeurotic fibrosarcoma
- Ascl2
Achaete-scute homologue 2
- CCR
C-C chemokine receptor 7
- PSG-1
P-selectin ligand −1
- CNS
conserved non-coding sequence
- ChIP
Chromatin Immunoprecipitation
- CTLA-4
Cytotoxic T-Lymphocyte-Associated Protein
- RBP-J
Recombining binding protein suppressor of hairless
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 2.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annual review of immunology. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. Journal of immunology. 2005;175:5–14. [PubMed] [Google Scholar]
- 4.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 6.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. Journal of immunology. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. The Journal of experimental medicine. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nurieva RI, Chung Y. Understanding the development and function of T follicular helper cells. Cellular & molecular immunology. 2010;7:190–197. doi: 10.1038/cmi.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith KM, Pottage L, Thomas ER, Leishman AJ, Doig TN, Xu D, et al. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. Journal of immunology. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 13.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobner T, Wolf I, Emrich T, Lipp M. Differentiation-specific expression of a novel G protein-coupled receptor from Burkitt’s lymphoma. European journal of immunology. 1992;22:2795–2799. doi: 10.1002/eji.1830221107. [DOI] [PubMed] [Google Scholar]
- 15.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. The Journal of experimental medicine. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 18.Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, et al. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. Journal of immunology. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 19.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nature immunology. 2001;2:876–881. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 21.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 22.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular he l per T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 23.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. The Journal of experimental medicine. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, et al. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. Journal of immunology. 2011;186:5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nature reviews Immunology. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 33.Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. Journal of immunology. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 34.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 37.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. Journal of immunology. 2001;166:3659–3662. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 38.Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. The Journal of experimental medicine. 2015;212:217–233. doi: 10.1084/jem.20141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nature immunology. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annual review of immunology. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 41.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. Journal of immunology. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kageyama R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M, et al. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity. 2012;36:986–1002. doi: 10.1016/j.immuni.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J, Havenar-Daughton C, Crotty S. Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination. Current opinion in virology. 2013;3:363–370. doi: 10.1016/j.coviro.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. The Journal of experimental medicine. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 48.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nature cell biology. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 50.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nature immunology. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- 52.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) Journal of immunology. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, et al. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. Journal of immunology. 2010;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao F, Cannons JL, Dutta M, Griffiths GM, Schwartzberg PL. Positive and negative signaling through SLAM receptors regulate synapse organization and thresholds of cytolysis. Immunity. 2012;36:1003–1016. doi: 10.1016/j.immuni.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annual review of immunology. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 56.Oya Y, Watanabe N, Owada T, Oki M, Hirose K, Suto A, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis and rheumatism. 2008;58:2498–2510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashiwakuma D, Suto A, Hiramatsu Y, Ikeda K, Takatori H, Suzuki K, et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. Journal of immunology. 2010;185:2730–2736. doi: 10.4049/jimmunol.0903839. [DOI] [PubMed] [Google Scholar]
- 58.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 59.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 60.Bour-Jordan H, Grogan JL, Tang Q, Auger JA, Locksley RM, Bluestone JA. CTLA-4 regulates the requirement for cytokine-induced signals in T(H)2 lineage commitment. Nature immunology. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- 61.Walker LS, Wiggett HE, Gaspal FM, Raykundalia CR, Goodall MD, Toellner KM, et al. Established T cell-driven germinal center B cell proliferation is independent of CD28 signaling but is tightly regulated through CTLA-4. Journal of immunology. 2003;170:91–98. doi: 10.4049/jimmunol.170.1.91. [DOI] [PubMed] [Google Scholar]
- 62.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 63.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature immunology. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nature immunology. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. The Journal of experimental medicine. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS one. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunological reviews. 2013;252:139–145. doi: 10.1111/imr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. Journal of immunology. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A funda m ental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. The Journal of experimental medicine. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 76.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, et al. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakayamada S, Poholek AC, Lu KT, Takahashi H, Kato M, Iwata S, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. Journal of immunology. 2014;192:2156–2166. doi: 10.4049/jimmunol.1300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai G, Nie X, Zhang W, Wu B, Lin J, Wang H, et al. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. Journal of immunology. 2012;189:1294–1302. doi: 10.4049/jimmunol.1102948. [DOI] [PubMed] [Google Scholar]
- 82.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annual review of immunology. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 83.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. The Journal of experimental medicine. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kusam S, Toney LM, Sato H, Dent AL. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. Journal of immunology. 2003;170:2435–2441. doi: 10.4049/jimmunol.170.5.2435. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Lu H, Chen T, Nallaparaju KC, Yan X, Tanaka S, et al. Genome-wide Analysis Identifies Bcl6-Controlled Regulatory Networks during T Follicular Helper Cell Differentiation. Cell reports. 2016;14:1735–1747. doi: 10.1016/j.celrep.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betz BC, Jordan-Williams KL, Wang C, Kang SG, Liao J, Logan MR, et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. The Journal of experimental medicine. 2010;207:933–942. doi: 10.1084/jem.20091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature immunology. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sahoo A, Alekseev A, Tanaka K, Obertas L, Lerman B, Haymaker C, et al. Batf is important for IL-4 expression in T follicular helper cells. Nature comm unications. 2015;6:7997. doi: 10.1038/ncomms8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bollig N, Brustle A, Kellner K, Ackermann W, Abass E, Raifer H, et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8664–8669. doi: 10.1073/pnas.1205834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glasmacher E, Agrawal S, Chang AB, Murphy TL, Zeng W, Vander Lugt B, et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science. 2012;338:975–980. doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. The Journal of biological chemistry. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nature immunology. 2014;15:856–865. doi: 10.1038/ni.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42:239–251. doi: 10.1016/j.immuni.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nature immunology. 2014;15:657–666. doi: 10.1038/ni.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, Geng J, Wen X, Bi E, Kossenkov AV, Wolf AI, et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nature immunology. 2014;15:667–675. doi: 10.1038/ni.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu KT, Kanno Y, Cannons JL, Handon R, Bible P, Elkahloun AG, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cannons JL, Lu KT, Schwartzberg PL. T follicular helper cell diversity and plasticity. Trends in immunology. 2013;34:200–207. doi: 10.1016/j.it.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suan D, Nguyen A, Moran I, Bourne K, Hermes JR, Arshi M, et al. T follicular helper cells have distinct modes of migration and molecular signatures in naive and memory immune responses. Immunity. 2015;42:704–718. doi: 10.1016/j.immuni.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. The Journal of experimental medicine. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, et al. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. Journal of immunology. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glatman Zaretsky A, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harada Y, Tanaka S, Motomura Y, Harada Y, Ohno S, Ohno S, et al. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hale JS, Ahmed R. Memory T follicular helper CD4 T cells. Frontiers in immunology. 2015;6:16. doi: 10.3389/fimmu.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moir S, Fauci AS. B cells in HIV infection and disease. Nature reviews Immunology. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunology and cell biology. 2014;92:64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 120.Terrier B, Costedoat-Chalumeau N, Garrido M, Geri G, Rosenzwajg M, Musset L, et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. The Journal of rheumatology. 2012;39:1819–1828. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 121.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 122.Feng X, Wang D, Chen J, Lu L, Hua B, Li X, et al. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PloS one. 2012;7:e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawamoto M, Harigai M, Hara M, Kawaguchi Y, Tezuka K, Tanaka M, et al. Expression and function of inducible co-stimulator in patients with systemic lupus erythematosus: possible involvement in excessive interferon-gamma and anti-double-stranded DNA antibody production. Arthritis research & therapy. 2006;8:R62. doi: 10.1186/ar1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu R, Wu Q, Su D, Che N, Chen H, Geng L, et al. A regulatory effect of I L-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis research & therapy. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clinical & developmental immunology. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma L, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clinical and experimental immunology. 2013;174:212–220. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjogren’s syndrome. Biochemical and biophysical research communications. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 128.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain : a journal of neurology. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 129.Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PloS one. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tzartos JS, Craner MJ, Friese MA, Jakobsen KB, Newcombe J, Esiri MM, et al. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. The American journal of pathology. 2011;178:794–802. doi: 10.1016/j.ajpath.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gong F, Su Q, Jiang D, Chen J, Pan Y, Huang X. High frequency of circulating follicular helper T cells in patients with bronchial asthma. Clinical laboratory. 2014;60:963–968. doi: 10.7754/clin.lab.2013.130427. [DOI] [PubMed] [Google Scholar]
- 132.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 134.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. The American journal of surgical pathology. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dupuis J, Boye K, Martin N, Copie-Bergman C, Plonquet A, Fabiani B, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. The American journal of surgical pathology. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 136.Gaulard P, de Leval L. Follicular helper T cells: implications in neoplastic hematopathology. Seminars in diagnostic pathology. 2011;28:202–213. doi: 10.1053/j.semdp.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahearne MJ, Willimott S, Pinon L, Kennedy DB, Miall F, Dyer MJ, et al. Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. British journal of haematology. 2013;162:360–370. doi: 10.1111/bjh.12401. [DOI] [PubMed] [Google Scholar]
- 138.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. The Journal of clinical investigation. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]