Abstract
Purpose
To evaluate the long-term outcomes after femtosecond laser (FSL)-assisted mushroom-configuration keratoplasty in advanced keratoconus.
Patients and methods
Thirteen eyes with Amsler–Krumeich stage IV keratoconus underwent FSL-assisted mushroom-configuration penetrating keratoplasty (PKP) and deep anterior lamellar keratoplasty (DALK) at a tertiary referral centre. Preoperative risk factors included low orneal thickness, high keratometry measurements, previous hydrops, and central stromal scarring. Main outcome measures were visual acuity and refractive outcome.
Results
The median follow-up was 33 months (range: 4–43). Preoperatively, the mean corrected distance visual acuity (CDVA) was 1.22±0.47 LogMAR (range: 0.5–1.9 LogMAR), mean minimum corneal thickness was 282±100.8 μm (range: 147–478 μm), and mean average keratometric (K) value was 63.4±7.63 dioptre (D; range: 57.0–75.7 D). Four patients underwent PKP and nine underwent DALK (two converted to PKP). Five patients subsequently underwent a modified arcuate mushroom interface dissection (AMID) procedure for astigmatic correction. At the final follow-up, the mean CDVA was 0.05±0.13 LogMAR (range: −0.10 to 0.20 LogMAR), mean spherical equivalent was −3.21±3.21D, mean cylindrical refractive error was 3.23±2.20 D, and mean average K was 43.1±1.53 D. Complications included early graft dehiscence, corneal vascularisation, stromal rejection, and sclerokeratitis. Sutures were completely removed at the mean 18.4 months for PKP and 9.1 months for DALK postoperatively.
Conclusion
FSL-assisted mushroom-configuration keratoplasty is feasible and safe in patients with stage IV keratoconus. AMID could further enhance the refractive outcome safely.
Introduction
Corneal transplantation, both penetrating keratoplasty (PKP) and deep anterior lamellar keratoplasty (DALK) are pivotal in the treatment of keratoconus.1, 2, 3 Visual recovery of PKP is often slow because of prolonged wound-healing and irregular astigmatism, and patients are at risk of graft endothelial rejection or failure.3, 4 Despite its advantages, DALK has been slow to gain its popularity until the introduction of big-bubble Descemet membrane (DM) dissection.5 Stepped corneal trephination was first conceived in 1950s;6 however, it only became technically possible when it was reintroduced by Busin.7 Nevertheless, the complexity and the lack of reproducibility prevented the technique from becoming popular.
Femtosecond laser (FSL) simplifies this by its ability in swiftly making precise and customised trephination. Farid et al first reported FSL-assisted zig-zag PKP producing faster visual recovery with significantly less astigmatism, although these were limited to early postoperative period.8 In addition, mushroom PKP has been shown to offer better wound approximation and mechanical stability over conventional PKP.9, 10 In DALKs, FSL-assisted mushroom-shaped keratoplasty also produces faster visual recovery, allowing earlier suture removal and refractive outcome comparable to manual PKP or FSL-assisted lamellar or PKP.11, 12, 13, 14, 15 Lu et al16 indicated that FSL-assisted DALK could improve uncorrected and best spectacle corrected visual acuity in patients with keratoconus because of the precise cutting of the donor corneal edge profile and the recipient bed. This approach, although in a small cohort, showed a safe and effective surgical choice in the treatment of keratoconus.
However, patients with Amsler–Krumeich stage IV keratoconus,17 such as those with marked central corneal scarring, mean central keratometry (K) readings >55.0 dioptre (D), minimum corneal thickness ≤200 μm and those with unmeasurable refraction, pose challenges to corneal transplantation. The effect of FSL-assisted keratoplasties in these patients is currently unclear, as previous reports included patients with heterogeneous diagnoses, or those with low-risk characteristics. Here we report our experience with FSL-assisted mushroom-configuration PKP and DALK for patients with stage IV keratoconus.
Materials and methods
Patient selection
Approval by the local ethics committee was obtained, and this study adheres to the Declaration of Helsinki. Thirteen eyes from 13 patients underwent FSL-assisted mushroom-shaped PKP or DALK between April 2010 and December 2012. The inclusion criteria were the presence of one or more of the following conditions: the mean central keratometric readings >55.00 D, corneal stroma scarring, thinnest corneal pachimetry <200 µ, previous hydrops, previous laser in situ keratomileusis (LASIK). All patients underwent preoperative and postoperative evaluation, including uncorrected and corrected distance visual acuity (UCVA and CDVA, respectively), optical coherence tomography-based corneal pachymetry (RTVue SD OCT, Optovue Inc., Fremont, CA, USA), and Scheimpflug-based corneal tomography (Pentacam, Oculus, Lynnwood, WA, USA). Preoperative refraction was attempted but failed in all patients because of the advanced disease status. Four patients underwent planned PKP because of previous hydrops with severe Descemet split, central deep stromal scarring, and low central corneal thickness, while the other patients underwent DALK.
Surgical technique
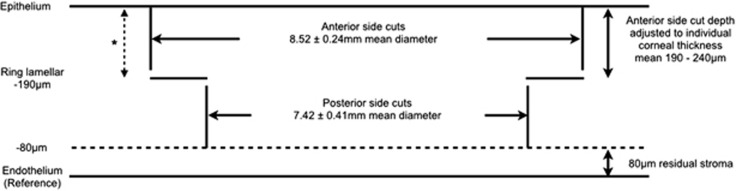
All surgeries were performed by one surgeon (VM) at Moorfields Eye Hospital, London, UK. The dimensions and design of the corneal graft donor and host bed were predetermined based on central and peripheral corneal thickness measurements. Figure 1 summarises the parameters of the host bed. The thinnest point within an 8-mm zone of the host cornea was first determined using OCT pachymetry. From 80 μm anterior to this level, the host bed posterior side cuts, which were 100 μm in length, were then made in a posterior–anterior direction. The ring lamellar cuts were 0.5–1.2 mm wide, with the anterior side cuts connecting the peripheral ends of the lamellar cuts to the anterior corneal surface. Stromal bridges of 10 μm were preserved between all cuts to minimise any risk of inadvertent corneal perforation, thus ensuring safe transfer of the patient from the laser suite to the operating room. An angulation of 90° was used in all cuts. For the donor, the anterior lamellar diameter was oversized by 0.25 mm, with the posterior lamellar diameter being identical as the host, and the depth of posterior side cuts were set at the maximum value of 1200 μm to ensure full perforation of the donor corneal button.
Figure 1.
Settings on FSL to create a host bed for mushroom-shaped deep anterior lamellar graft. With pachymetry measurement, the lowest corneal thickness was used as the reference plane and the depth of the other incisions was calculated preoperatively. All side cuts were placed at 90-degree angulations. Stromal bridges (10 μm) were preserved between incisions for safe transfer of patient. Asterisk illustrates the depth of arcuate interface dissection in selected patients.
The donor button was first prepared as per the Moorfields technique18 as summarised here: the donor cornea (Moorfields Lions Eye Bank, London, UK) was mounted carefully on a Barron artificial anterior chamber (Katena Inc., Denville, NJ, USA) primed with viscoelastic (2% HPMC, Moorfields Pharmaceutical, London, UK). The tissue retainer and locking ring were placed and secured. The corneal epithelium was removed and the apex was marked. FSL (150 kHz; IntraLase Enabled Keratoplasty software and IntraLaser FS Laser; IntraLase Corp., Irvine, CA, USA) was programmed with customised parameters, and a full-thickness mushroom-shaped cut was made upon complete cone applanation. The corneal button was removed from its corneoscleral rim and replaced in storage media.
The host bed was constructed after instillation of topical tetracaine 1% and povidone–iodine 5%. Cornea was measured and the geometrical centre marked. Suction ring was applied, and the applanation cone positioned with the FSL centred on the central corneal mark. A mushroom-shaped cut was made according to predetermined dimensions, as detailed above.
The patient was transferred to the operating room. Surgery was performed either under general anaesthesia or with a subtenon block consisting a 5-ml mixture of 1% lidocaine and 1 : 80 000 adrenaline. The eye was prepared with 5% povidone–iodine solution, sterile draped, and a lid speculum inserted. The side cut and lamellar plane were identified and bluntly dissected using plane forceps.
For DALK cases, big-bubble DM dissection was attempted. A peripheral paracentesis was made and air was injected into the anterior chamber to check for the big-bubble sign as described by Fontana et al.19 A 25-gauge needle attached with a 5-ml syringe filled with air was inserted through the posterior side cut towards the cone apex. Air was forcefully injected into the stroma until a big bubble was seen across the posterior corneal surface. Viscoelastic (ProVisc, Alcon Laboratories, Fort Worth, TX, USA) was then applied to protect the anterior DM surface after the corneal stroma was incised with a sharp blade.2 The corneal stroma was excised at the posterior side cut using corneal scissors, and viscoelastic was irrigated away. The donor DM and endothelium were removed using dry microsponges; the button was then placed onto the host bed and secured with 16 interrupted 10-0 nylon sutures placed at the depth of the ring lamellar dissection.
In cases of large DM perforations during DALK and in planned PKP, fullthickness corneal button was excised from the host using corneal scissors. The donor cornea was positioned and secured with 16 interrupted 10-0 nylon sutures placed at the depth of the ring lamellar cut. The graft–host interface was checked watertight at the end of the procedure.
All patients received subconjunctival cefuroxime and dexamethasone injection. Eyes were patched and shielded. Subsequently, all patients received standard post-graft regime treatment consisting of chloramphenicol 0.5% eyedrops four times a day for 14 days and dexamethasone 0.1% eyedrops 2 hourly for the first 2 weeks, and then tapered to four times a day for 2 months, three times a day for 2 months, twice a day for 2 months, and once a day for 6 months. Afterwards, dexamethasone was switched to fluorometholone 0.1% eyedrops once a day for another 6 months. The above regimen was shortened for DALK to only 6–8 months postoperatively.
Patients were reviewed on postoperative day 1, week 1, month 1, month 3, month 6, and then 6 monthly, with additional reviews when/if required.
In patients with post-keratoplasty astigmatism ≥4 D after the removal of all corneal sutures, a modified technique of arcuate mushroom interface dissection (AMID) was performed. We described this procedure as dissection because no sharp blades were used and no new incisions were made. AMID was performed at a minimum of 6 weeks from complete suture removal to allow time for corneal astigmatism stabilisation. Positions and lengths of the interface dissections were determined by manifest refraction, corneal topography and Lindstrom normogram was followed to determine the size of dissection.20 Vertical and horizontal meridians and the proposed AMID were marked with patient upright during slit-lamp examination with a Thornton arcuate marker (Duckworth & Kent, Baldock, UK). The graft–host junction along the pre-marked site was then re-opened from the epithelial surface down to the ring lamellar cut only (Figure 1) with Weiss LASIK flap lifter (John Weiss International, Milton Keynes, UK). Seidel's negativity was confirmed at the end of the procedure. Patients were given chloramphenicol 1% and dexamethasone 0.1% eyedrops four times daily for 2 weeks post procedure and were reviewed 4 weeks later.
Data were analysed with Microsoft Excel 2011. Student's t-test was used, with P≤0.05 considered statistically significant.
Results
Patient demographics and follow-up
This study included 13 eyes from 13 patients (7 male, 64%), and none were excluded in the analysis (Table 1). The mean age was 30±9.4 years (range: 26–51 years) at the time of surgery. One patient had previous LASIK, three had central corneal scarring, and three had corneal hydrops. The mean minimum corneal pachymetry in the central 8-mm zone was 282±100.8 μm (range: 147–478 μm). The median follow-up was 31±12.8 months (range: 4–43 months). Two patients were repatriated after initial postoperative reviews. Number of subjects available for analysis were 13 at 3 months, 11 at 6 months, 9 at 12 months, 11 at 18 months, 4 at 24 months, 6 at 30 and 36 months, and 2 at 43 months. Duration of follow-up was not significantly different between PKP and DALK (P=0.37).
Table 1. Patient baseline characteristics.
| No. | Age/sex | Risk factors | Corneal pachymetry (μm) | Mean K value (D) | Preoperative CDVA | Treatment | Intraoperative complications | Follow-up (mo) | Final CDVA | Final refraction | Time of complete ROS (mo) | Postoperative complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28/M | Previous hydrops | 456 | 52 | 0.60 | PKP | — | 33 | 0.10 | Plano/−5.00 × 70 | 23 | |
| 2 | 51/F | Previous hydrops | 297 | 68 | 1.60 | PKP | — | 42 | 0.00 | −8.50/−1.25 × 180 | 18 | Resuturing (day 1) |
| 3 | 29/M | 284 | 70 | 0.80 | PKP | — | 43 | 0.00 | +1.00/−4.00 × 170 | 19 | ||
| 4 | 26/F | Previous hydrops | 238 | 57 | 0.50 | PKP | — | 36 | −0.10 | −0.25/−3.25 × 80 | 20 | |
| 5 | 31/M | Previous LASIK | 147 | 67 | 1.90 | PKPa | DM perforation | 12b | 0.20 | +1.00/−2.25 × 63 | n/a | |
| 6 | 30/M | Stromal scarring | 181 | 60 | 1.60 | DALK | DM perforation | 37 | 0.00 | −5.75/−4.50 × 76 | 13 | Corneal vascularisation; stromal rejection (3 mo) |
| 7 | 28/F | 216 | 60 | 1.80 | PKPa | DM perforation | 37 | −0.10 | +1.00/−3.00 × 63 | 13 | ||
| 8 | 21/M | 205 | 65 | 1.60 | DALK | — | 4b | 0.30 | Plano/−8.00 × 180 | 4 | Stromal rejection (4 mo); mucus-fishing syndrome; sclerokeratitis | |
| 9 | 24/M | 478 | 57 | 0.20 | DALK | — | 32 | 0.20 | −1.00/−1.00 × 145 | 12 | Resuturing (week 1) | |
| 10 | 25/M | 206 | 67 | 0.50 | DALK | — | 31 | 0.20 | −2.25/−1.75 × 170 | 12 | ||
| 11 | 50/F | 356 | 60 | 0.60 | DALK | — | 36 | 0.20 | −1.00/−1.25 × 45 | 4 | Corneal vascularisation | |
| 12 | 26/M | Stromal scarring | 290 | 72 | 1.00 | DALK | — | 18 | 0.00 | +1.25/−1.25 × 125 | n/a | |
| 13 | 24/M | Stromal scarring | 315 | 60 | 1.00 | DALK | — | 12 | 0.00 | +0.25/−3.75 × 45 | n/a |
Abbreviations: CDVA, corrected distance visual acuity (in LogMAR); DALK, deep anterior lamellar keratoplasty; DM, Descemet membrane; K, keratometric; mo, months; PKP, penetrating keratoplasty; ROS, removal of sutures.
Patients 5 and 7 were converted from DALK to PKP intraoperatively.
Patients 5 and 8 were repatriated and were lost to further follow-up.
Refractive outcome
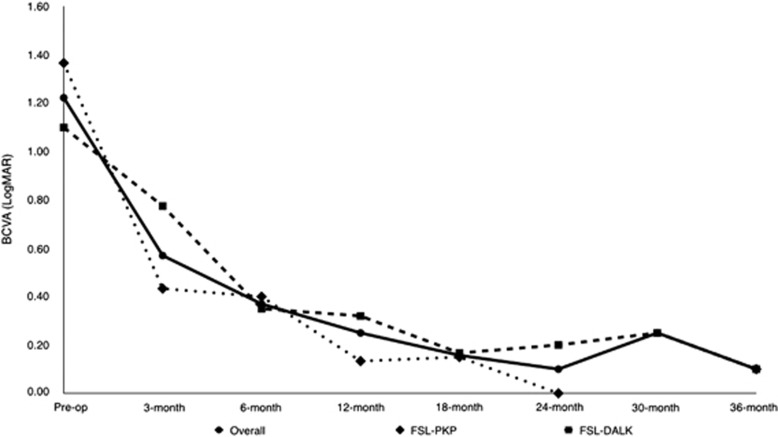
The mean preoperative contact lens CDVA±SD in LogMAR was 1.22±0.47 (range: 0.5–1.9), whereas the mean pin-hole visual acuity was 0.90±0.56 (range: 0.2–1.0). The postoperative CDVA was tested with spectacle correction, and the mean postoperative CDVA (including data from subjects before AMID) at 3 months was 0.57±0.47 LogMAR (range: 0.0–1.50); 0.37±0.16 LogMAR (range: 0.20–0.60) at 6 months; 0.25±0.21 LogMAR (range: 0.0–0.60) at 12 months; 0.16±0.15 LogMAR (range: 0.0–0.30) at 18 months; 0.10±0.14 LogMAR (0.0–0.20) at 24 months; 0.25±0.07 LogMAR (range: 0.20–0.30) at 30 months; and 0.05±0.13 LogMAR (range: −0.10 to 0.20) at 36 months. The mean CDVA did not differ significantly between PKP and DALK patients at any point of follow-up (Figure 2).
Figure 2.
CDVA (LogMAR) after FSL-assisted PKP and FSL-assisted DALK. Data in patients who had astigmatic corrections by AMID were excluded from the point of the procedure. Statistical comparison between DALK and PKP groups at different follow-up: preoperative, P=0.35; at 3 months P=0.29; at 6 months P=0.67; at 12 months P=0.26; at 18 months P=0.82; at 24 months, not available; at 30 months, not available; last follow-up, P=0.41.
Preoperative refractions were unavailable because of advanced keratoconus. Postoperatively, the mean cylindrical error and refractive spherical equivalent (RSE) in dioptres (D), respectively (including data from subjects before AMID) were 5.46±3.04 D (range: 9.00 to 2.00 D) and −4.50±3.98 D (range: −10.0 to −0.75 D) at 3 months; 5.67±3.34 D (range: 10.0 to 1.50 D) and −5.81±3.72 D (range: −9.00 to −2.00 D) at 6 months; 3.00±2.24 D (range: −5.50 to 0 D) and −5.10±3.36 D (range: −9.50 to −1.50 D) at 12 months; 4.20±2.75 D (range: 8.0 to 1.50 D) and −3.8±4.08 (range: −8.50 to −1.0 D) at 18 months; 4.00±3.18 D (range: 6.25 to 1.75 D) and −2.88±0.35 (range: −3.13 to −2.63) at 24 months; 1.75 and −3.13 (n=1) at 30 months; and 3.50±1.67 (range: 5.0 to 1.25 D) and −3.50±3.02 (range: −8.0 to −1.63 D) at 36 months. The mean postoperative RSE did not differ significantly between PKP and DALK patients at any point of follow-up.
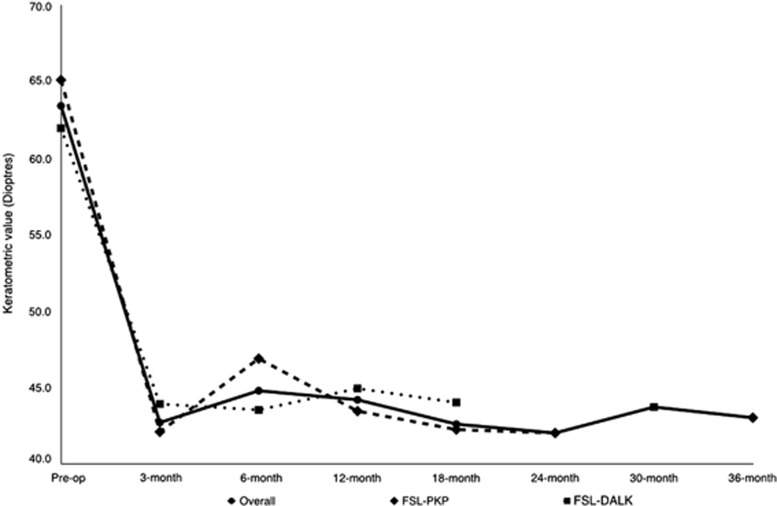
Figure 3 shows the changes in average K values before and after surgery. Preoperative topographic average K value was 63.4±7.63 D (range: 57.0–75.7 D). The values (D) subsequently (including data from subjects before AMID) were 42.7±1.08 (range: 41.9–43.9 D) at 3 months; 44.8±3.81 (range: 40.6–50.3 D) at 6 months; 44.2±1.0 D (range: 42.9–45.1 D) at 12 months; 42.6±1.37 D (range: 40.3–44.0 D) at 18 months; and 42.0, 43.7, and 43.0 at 24, 30, and 36 months, respectively (n=1). These values did not significantly differ between PKP and DALK patients at any point of follow-up (Figure 3).
Figure 3.
Keratometry measurements after FSL-assisted PKP and FSL-assisted DALK. Data in patients who had astigmatic corrections by AMID were excluded from the point of the procedure. Statistical comparison between DALK and PKP groups at different follow-up: preoperative, P=0.50; at 3 months, not available; at 6 months, P=0.26; at 12 months, P=0.10; at 18 months, P=0.09; at 24 months, not available; at 30 months, not available; last follow-up, not available.
Sutures were completely removed in PKP and DALK patients at a mean of 18.6±3.26 (range: 13–23 months) and 9.1±4.09 months (range: 4–13 months), respectively (P=0.006). AMID was performed in five patients to reduce post-keratoplasty astigmatism after suture removal (Table 2). The median time of AMID after complete suture removal was 10 weeks (range: 6–15 weeks), and the median follow-up after AMID was 19.4 months (range: 13.5–23 months). The mean pre-AMID UCVA was 0.82±0.31 LogMAR (range: 0.5–1.3 LogMAR) and the mean cylindrical refractive error was 7.55±4.28 D (range: 4–15 D). Six weeks after AMID, the mean UCVA was 0.53±0.05 LogMAR (range: 0.50–0.60, 36% improvement) and the mean cylindrical refractive error was 2.44±1.14 D (range: 1.25–4 D, 68% improvement). At the last follow-up, the mean UCVA was 0.46±0.32 LogMAR (range: 0.2–1.0 LogMAR) and the mean cylindrical refractive error was 2.35±1.28 D (range: −1.0 to −4.0 D).
Table 2. Characteristics of patient subgroup undergone arcuate mushroom interface dissection.
| Patient no. | ROS to AMID (week) |
Pre-AMID |
Post-AMID |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UCVA | CDVA | Refraction | First post-AMID review (weeks) | UCVA | CDVA | Refraction | Final post-AMID review (months) | UCVA | CDVA | Refraction | ||
| 2 | 15 | 1.30 | 0.20 | −3.00/−15.00 × 77 | 11 | 0.60 | 0.20 | −8.50/−1.25 × 70 | 20 | 1.00 | 0.20 | −8.50/−1.25 × 180 |
| 3 | 6 | 0.60 | 0.00 | +4.25/−6.00 × 170 | 5 | 0.50 | −0.10 | +0.50/−4.00 × 170 | 23 | 0.30 | −0.10 | +0.50/−4.00 × 170 |
| 4 | 12 | 0.50 | 0.00 | +0.50/−6.25 × 68 | 4 | 0.20 | −0.10 | −0.25/−3.25 × 80 | 14 | 0.20 | −0.10 | −0.25/−3.25 × 80 |
| 7 | 8 | 0.80 | 0.00 | +3.00/− 6.50 × 75 | 2 | 0.50 | 0.00 | +1.00/−2.25 × 57 | 23 | 0.30 | −0.10 | +1.00/−2.25 × 63 |
| 9 | 10 | 0.90 | 0.30 | −1.00/−4.00 × 122 | 4 | 0.50 | 0.20 | −0.75/−2.25 × 160 | 17 | 0.50 | 0.00 | −1.00/−1.00 × 145 |
Abbreviations: AMID, arcuate mushrom interface dissection; CDVA, corrected distance visual acuity (in LogMAR); K, keratometric value (D); ROS, removal of sutures; UCVA, uncorrected visual acuity (LogMAR).
Complications
There were three cases of DM perforation during big-bubble dissection in DALK surgery, two were converted to mushroom-configuration PKP (patients 5 and 7). There were two cases of early suture loosening without graft dehiscence (patients 2 and 9) requiring re-suturing. Two patients had stromal rejection due to poor medication compliance (patients 6 and 8) that resolved with intensive topical dexamethasone eyedrops. There were three cases of peripheral corneal superficial neovascularization related to corneal sutures (patients 6, 8, and 11). All were treated successfully with selective suture removal and/or subconjunctival bevacizumab injections. Patient 8 also had inflammatory sclerokeratitis. The condition resolved with sutures' removal and steroid treatment with dexamethasone 0.1% eyedrops four times a day for 2 weeks. No patients suffered graft failures or elevated intraocular pressures. In addition, graft interface haze was not observed in any of the DALK patients.
Discussion
In this series, we report the medium-term follow-up of FSL-assisted mushroom-shaped keratoplasties in Amsler–Krumeich stage IV keratoconus. Despite the high-risk characteristics, visual acuity and refractive outcomes at the final follow-up were comparable to previous studies.11, 12, 14 The mean time to complete suture removal was different between PKP and DALK, but is comparable to previous studies of FSL-assisted PKP and DALK.11, 14
Stepped corneal trephination has the advantage of creating a larger surface area at the graft–host interface, leading to improved wound apposition, faster wound-healing, and allowing earlier suture removal.7, 8, 11, 13 With the advent of FSL in keratoplasty, customised configurations could be created with speed and with great precision. Several different configurations have been studied for their refractive and mechanical attributes. Zig-zag PKP and DALK have been reported to significantly reduce postoperative corneal astigmatism, although it has no effect on mechanical stability of the corneal graft.8, 9, 21, 22 Top-hat configuration significantly enhanced mechanical stability in the laboratory model;9 however, it has a large posterior lamellar that could be challenging to handle intraoperatively.
Our report has several key differences compared with previous studies. To our knowledge, this study is the longest follow-up report on FSL-assisted mushroom-shaped keratoplasty in a homogenous cohort of patients diagnosed with advanced, often end-stage keratoconus with high K readings (mean K: 63.4±7.63 D), very low corneal thickness (mean: 282±100.8 μm), and stromal scarring from hydrops. Second, the dimension of the mushroom configuration was individualised according to the patients' corneal profile, with the length of the posterior side cut kept at 100 μm to provide sufficient graft–host interface for suturing. Third, the big-bubble DM dissection technique was attempted in all appropriate cases. Finally, to further reduce residual high astigmatism after complete suture removal, AMID was performed in five patients.
Mushroom-configuration keratoplasty was preferred in this series. In PKP, it has the advantage of enhancing mechanical stability similar to that of top-hat keratoplasty9 while producing better visual and refractive outcome. Levinger et al23 compared the outcomes of 26 eyes that underwent mushroom FSL-enabled PKP with 33 eyes receiving conventional PKP for keratoconus disease and the FSL group demonstrating significantly less post-keratoplasty astigmatism, even though the final postoperative CDVAs were similar in the two groups after suture removal. Birnbaum et al studied 123 FSL-assisted PKP, among which 32 were of mushroom configuration in keratoconus.11 At a mean of 14.1-month follow-up, they reported significantly better visual acuity and corneal surface irregularity index compared with that of top-hat PKP.
In DALK, mushroom-configuration trephination allows easy access for big-bubble DM dissection. Moreover, in our experience, mushroom configuration allows simpler intraoperative conversion from DALK to PKP; therefore, we prefer this configuration for cases of advanced keratoconus, where the risk of intraoperative conversion would be higher. Outcomes of FSL-assisted mushroom-configuration DALK were previously reported by Rootman et al.13, 14 Nineteen patients underwent FSL-assisted mushroom DALK, with eight completing follow-up for more than a year. The mean minimum preoperative pachymetry was 542 μm (range: 441–628 μm). The mean best correct visual acuity (BCVA) improved from 20/108 (range: 20/30–20/400) to 20/35 (range: 20/15–20/200) after the mean follow-up of 13 months. The mean spherical equivalent and mean keratometric cylinder greatly improved to approximately −4 and 4 D at the end of the follow-up period, respectively. Recently, Shehadeh-Mashor et al15 compared these cases with 19 eyes that underwent manual straight edge configuration DALK. Although they did not find significant difference in the two groups for mean spherical equivalence, postoperative cylinder astigmatism, and BCVA at the final follow-up, BCVA in the DALK group was significantly better at 3 months postoperatively (P=0.00002). Importantly, in contrast to our study, the patient cohort in these reports was not those of advanced keratoconus.
In the subgroup of patients who underwent AMID, the mean UCVA improved from 0.82±0.31 to 0.46±0.32 LogMAR and the mean cylindrical error improved by over 4 D. Busin previously described the idea of near-full-thickness blunt dissection of graft–host interface to reduce post-keratoplasty astigmatism.24 By selectively re-opening the anterior lamellar interface of the mushroom-configuration corneal graft, while keeping the lamellar ring and posterior lamellar graft–host interface intact, we believe our modified AMID is safer than Busin's method and than conventional arcuate keratotomy.25, 26 As our technique is unlikely to inadvertently enter the anterior chamber, AMID might also be more predictable as the incision depth is pre-set by the graft construction, and could be applied to other stepped configuration keratoplasty. However, we are aware that further studies with larger cohorts of patients are needed to confirm the advantages of AMID.
In our series, intraoperative DM perforation occurred in three patients and two of those (22.2%) required conversion to PKP. The rate of DM perforation was similar to previous reports (0–39.2%),27, 28 and all the patients with perforations had advanced keratoconus with minimal corneal thickness less than 250 μm.
No endothelial rejection was observed in patients who had PKP; however, there were two cases of stromal rejections in DALK patients. In one patient there was also concurrent sclerokeratitis. Both cases had wide anterior lamella (9.3 and 8.5 mm) to avoid suturing into ectatic corneal tissue and pre-existing corneal neovascularizations. The close proximity between the limbus and the wider anterior lamella of the mushroom-shaped corneal graft was speculated to be reason behind the higher rate of stromal rejection as previously observed by Feizi et al29 and Shehadeh-Mashor et al.14
In summary, our study showed the potential of FSL-assisted mushroom-configuration keratoplasty in the management of patients with stage IV advanced keratoconus, and the described AMID could further enhance the post-keratoplasty refractive and astigmatic outcomes. Future studies with larger multicentre cohorts would help to determine the preferred surgical technique in this particular subgroup of patients.
Footnotes
VM received £30 000 unrestricted research grant from Abbott Medical Optics. The remaining authors declare no conflict of interests.
References
- Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: visual outcome and success. Ophthalmology 2000; 107(6): 1125–1131. [DOI] [PubMed] [Google Scholar]
- Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology 2004; 111(9): 1676–1682. [DOI] [PubMed] [Google Scholar]
- Han DC, Mehta JS, Por YM, Htoon HM, Tan DT. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol 2009; 148(5): 744–751 e741. [DOI] [PubMed] [Google Scholar]
- Panda A, Bageshwar LM, Ray M, Singh JP, Kumar A. Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea 1999; 18(2): 172–175. [DOI] [PubMed] [Google Scholar]
- Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 2002; 28(3): 398–403. [DOI] [PubMed] [Google Scholar]
- Franceschetti A. [Combined lamellar and perforant keratoplasty (mushroom graft)]. Bull Schweiz Akad Med Wiss 1951; 7(2): 134–145. [PubMed] [Google Scholar]
- Busin M. A new lamellar wound configuration for penetrating keratoplasty surgery. Arch Ophthalmol 2003; 121(2): 260–265. [DOI] [PubMed] [Google Scholar]
- Farid M, Steinert RF, Gaster RN, Chamberlain W, Lin A. Comparison of penetrating keratoplasty performed with a femtosecond laser zig-zag incision versus conventional blade trephination. Ophthalmology 2009; 116(9): 1638–1643. [DOI] [PubMed] [Google Scholar]
- Bahar I, Kaiserman I, McAllum P, Rootman D. Femtosecond laser-assisted penetrating keratoplasty: stability evaluation of different wound configurations. Cornea 2008; 27(2): 209–211. [DOI] [PubMed] [Google Scholar]
- Malta JB, Soong HK, Shtein R, Banitt M, Musch DC, Sugar A et al. Femtosecond laser-assisted keratoplasty: laboratory studies in eye bank eyes. Curr Eye Res 2009; 34(1): 18–25. [DOI] [PubMed] [Google Scholar]
- Birnbaum F, Wiggermann A, Maier PC, Bohringer D, Reinhard T. Clinical results of 123 femtosecond laser-assisted penetrating keratoplasties. Graefes Arch Clin Exp Ophthalmol 2013; 251(1): 95–103. [DOI] [PubMed] [Google Scholar]
- Buzzonetti L, Laborante A, Petrocelli G. Standardized big-bubble technique in deep anterior lamellar keratoplasty assisted by the femtosecond laser. J Cataract Refract Surg 2010; 36(10): 1631–1636. [DOI] [PubMed] [Google Scholar]
- Chan CC, Ritenour RJ, Kumar NL, Sansanayudh W, Rootman DS. Femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea 2010; 29(3): 290–295. [DOI] [PubMed] [Google Scholar]
- Shehadeh-Mashor R, Chan C, Yeung SN, Lichtinger A, Amiran M, Rootman DS. Long-term outcomes of femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea 2013; 32(4): 390–395. [DOI] [PubMed] [Google Scholar]
- Shehadeh-Mashor R, Chan CC, Bahar I, Lichtinger A, Yeung SN, Rootman DS. Comparison between femtosecond laser mushroom configuration and manual trephine straight-edge configuration deep anterior lamellar keratoplasty. Br J Ophthalmol 2014; 98(1): 35–39. [DOI] [PubMed] [Google Scholar]
- Lu Y, Shi YH, Yang LP, Ge YR, Chen XF, Wu Y et al. Femtosecond laser-assisted deep anterior lamellar keratoplasty for keratoconus and keratectasia. Int J Ophthalmol 2014; 7(4): 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alio JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg 2006; 22(6): 539–545. [DOI] [PubMed] [Google Scholar]
- Iovieno A, Chowdhury V, Stevens JD, Maurino V. Moorfields technique of donor cornea mounting for femtosecond-assisted keratoplasty: use of viscoelastic in the artificial anterior chamber. Ophthalmic Surg Lasers Imaging 2012; 43(4): 348–350. [DOI] [PubMed] [Google Scholar]
- Fontana L, Parente G, Tassinari G. Simple test to confirm cleavage with air between Descemet's membrane and stroma during big-bubble deep anterior lamellar keratoplasty. J Cataract Refract Surg 2007; 33(4): 570–572. [DOI] [PubMed] [Google Scholar]
- Lindstrom RL. The surgical correction of astigmatism: a clinician's perspective. Refract Corneal Surg 1990; 6(6): 441–454. [PubMed] [Google Scholar]
- Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg 2009; 35(5): 809–813. [DOI] [PubMed] [Google Scholar]
- Price FW Jr, Price MO, Grandin JC, Kwon R. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J Cataract Refract Surg 2009; 35(5): 804–808. [DOI] [PubMed] [Google Scholar]
- Levinger E, Trivizki O, Levinger S, Kremer I. Outcome of "mushroom" pattern femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty in patients with keratoconus. Cornea 2014; 33(5): 481–485. [DOI] [PubMed] [Google Scholar]
- Incisional surgery for correction of high-degree astigmatism in post-keratoplasty eyes with shaped wound. ASCRS annual meeting; Chicago, 2012. abstract 1240127.
- Poole TR, Ficker LA. Astigmatic keratotomy for post-keratoplasty astigmatism. J Cataract Refract Surg 2006; 32(7): 1175–1179. [DOI] [PubMed] [Google Scholar]
- Hoffart L, Touzeau O, Borderie V, Laroche L. Mechanized astigmatic arcuate keratotomy with the Hanna arcitome for astigmatism after keratoplasty. J Cataract Refract Surg 2007; 33(5): 862–868. [DOI] [PubMed] [Google Scholar]
- Mosca L, Fasciani R, Tamburelli C, Buzzonetti L, Guccione L, Mandara E et al. Femtosecond laser-assisted lamellar keratoplasty: early results. Cornea 2008; 27(6): 668–672. [DOI] [PubMed] [Google Scholar]
- Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol 1997; 81(3): 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi S, Javadi MA, Jamali H, Mirbabaee F. Deep anterior lamellar keratoplasty in patients with keratoconus: big-bubble technique. Cornea 2010; 29(2): 177–182. [DOI] [PubMed] [Google Scholar]