Abstract
Purpose
To investigate the choroidal thickness in patients with scleroderma and to compare them with healthy control subjects.
Methods
Forty-six patients with scleroderma (3 male and 43 female) and 31 healthy controls (6 male and 25 female) were included in the study. Twenty-five patients had limited-type and 21 patients had diffuse-type scleroderma. Only left eyes of the patients and control subjects were used in the analysis. Demographic features of all the patients and control subjects were recorded. Each subject underwent ophthalmological examinations including refraction, visual acuity, intraocular pressure, axial length (AXL) measurement, slit-lamp biomicroscopy, and fundus examination. Body mass index (BMI) was estimated for all participants.
Results
There were no significant differences between the patients with scleroderma and the control subjects in terms of age, gender, BMI, mean AXL, and mean spherical equivalent refractive error (SE) (P=0.1, P=0.086, P=0.37, P=0.55, and P=0.072 respectively). The patients with scleroderma had significantly thinner nasal, temporal, and subfoveal choroid than the healthy control subjects (P1=0.012, P2=0.046, and P3<0.001, respectively). There were no significant differences between the patients with limited-type and diffuse-type scleroderma in terms of age, gender, BMI, mean AXL, mean SE, nasal, temporal, and subfoveal choroidal thicknesses (all P>0.05).
Conclusions
Choroidal thickness in patients with scleroderma was significantly less than healthy control subjects. Vasculopathy in scleroderma is characterized by obliteration of arterioles and reduced capillary density may cause atrophy of choroid in patients with scleroderma.
Introduction
Scleroderma is a chronic, multisystem, autoimmune disease of unknown etiology. The most common affected tissue is skin but other structures such as kidney, heart, and small vessels may be affected in patients with scleroderma. The pathophysiology of scleroderma is characterized by immune activation, vasculopathy, and widespread fibrosis.1 Vasculopathy in the scleroderma mainly affects small arteries and capillaries is characterized by obliteration of vessels and reduced capillary density.2 The most common ocular findings are keratoconjunktivitis sicca, eyelid skin fibrosis, anterior uveitis, normal tension glaucoma, optic neuropathy, and choroidopathy.3, 4, 5, 6
Choroid is a vascular structure of the eye that supplies oxygen and metabolites to the outer segment of the retina.7 Enhanced depth imaging optical coherence tomography (EDI-OCT) technologies provide accurate quantitative analysis of the choroid tissue. Choroidal thickness has been investigated in healthy individuals, patients with high myopia, polypoidal choroidal vasculopathy, age-related macular degeneration, central serous chorioretinopathy, glaucoma, pseudoexfoliation syndrome, sarcoid, tuberculosis-related granulomatous uveitis, and Behcet disease-related posterior uveitis.8, 9, 10, 11, 12, 13, 14, 15 Investigation of choroidal morphology in patients with scleroderma may help better understanding of choroidal involvement in patients with scleroderma. The aim of the present study was to investigate the choroidal thickness in patients with scleroderma and to compare them with healthy control subjects.
Materials and methods
In this prospective study, we evaluated patients with scleroderma diagnosed according to diagnostic criteria of American College of Rheumatology for scleroderma and healthy control subjects as a control group between June 2015 and August 2015.16 Only left eyes of the patients and control subjects were used in the analysis. Exclusion criteria included diabetes mellitus, a history of intraocular surgery or laser treatment, uveitis, age-related macular degeneration, central serous chorioretinopathy, amblyopia, high myopia, and poor image quality. Demographic features of the all the patients and control subjects were recorded. Each subject underwent ophthalmological examinations including refraction, best spectacle corrected visual acuity, intraocular pressure, axial length (AXL) measurement, slit-lamp biomicroscopy, and fundus examination. Body weight and height of the scleroderma patients and control subjects were recorded; body mass index (BMI) was estimated with the formula of weight/height2 (kg/m2). Gaziantep University ethics committee approval and informed consents from all patients in accordance with the Declaration of Helsinki were obtained.
EDI-OCT measurement protocol
All EDI-OCT measurements were accomplished between 1430 and 1600 hours using Heidelberg Spectralis equipment (Heidelberg Engineering Inc., Heidelberg, Germany). The EDI-OCT images of seven sections, each comprising 100 scan using the automatic averaging and eye tracking features were obtained. The horizontal section going directly through the center of the fovea was selected. The choroidal thickness was described vertical distance from the outer portion of the hyperreflective line corresponding to the retina pigment epithelium (RPE) to the inner surface of the sclera (Figures 1 and 2). The choroidal thicknesses were determined under the fovea, 3 mm nasal to the fovea, and 3 mm temporal to the fovea. Two examiners who were masked to diagnosis of the patients (EC and SK) measured the choroidal thickness in all subjects and the arithmetic mean of the measurements was calculated. The eyes having >15% difference of measurement between the two clinicians were excluded from the study.
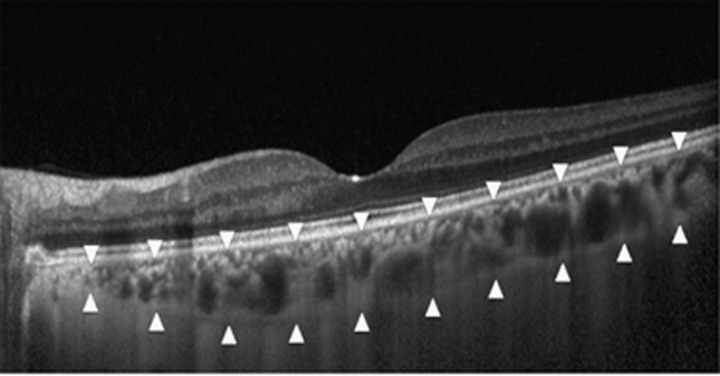
Figure 1.
An enhanced depth imaging optical coherence tomography scan obtained from the left eye of a 36-year-old female patients with scleroderma. The choroidal thickness was measured from the retina pigment epithelium to the inner surface of the sclera (arrowheads).
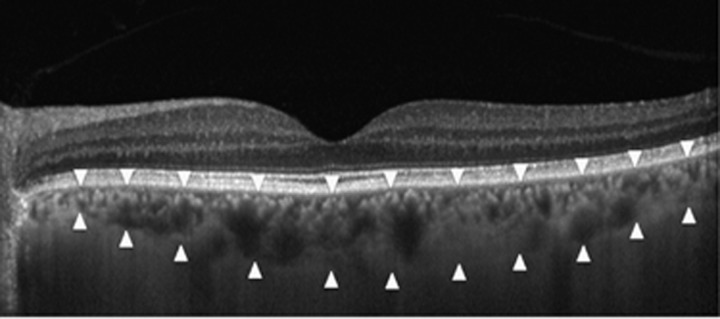
Figure 2.
An enhanced depth imaging optical coherence tomography scan obtained from the left eye of a 60-year-old female control subjects. The choroidal thickness was measured from the retina pigment epithelium to the inner surface of the sclera (arrowheads).
Statistical analysis
SPSS software version 22.0 (SPSS for Windows software; SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data were recorded as the means±SD. Pearson's correlation coefficient was used to evaluate the correlation between the choroidal thicknesses measurements of the two examiners. Differences between patients with scleroderma and control subjects were tested for significance using the t-test. Differences between the patients with diffuse-type scleroderma and patients with limited-type scleroderma were tested for significance using the Mann–Whitney U-test. Categorical variables were compared with the χ2-test. For all comparisons, P<0.05 was considered statistically significant.
Results
Forty-six patients with scleroderma (3 male and 43 female) and 31 healthy control subjects (6 male and 25 female) were included in the study. Twenty-five patients had limited-type scleroderma and 21 patients had diffuse-type scleroderma. One patient with scleroderma was excluded from the study because of dry-type age-related macular degeneration. There were no significant differences between the patients with scleroderma and control subjects in terms of age, gender, and BMI (P=0.1, P=0.086, P=0.37). The fundus examinations of the patients with scleroderma revealed local RPE changes in seven eyes (15%), on the other hand, the fundus examinations of control subjects were unremarkable.
The demographic and clinical features of the patients with scleroderma and control subjects are shown in Table 1. Although there were no significant differences between the groups in terms of mean AXL, and mean spherical equivalent refractive error (SE; P=0.55 and P=0.072), the patients with scleroderma had significantly thinner nasal, temporal, and subfoveal choroid than the healthy control subjects (P1=0.012, P2=0.046 and P3<0.001, respectively). There were no significant differences between the patients with limited-type scleroderma and diffuse-type scleroderma in terms of age, gender, BMI, mean AXL, and mean SE and nasal, temporal, and subfoveal choroidal thicknesses (all P>0.05). Demographic features and ophthalmic findings of the patients with diffuse-type scleroderma and limited-type scleroderma are shown in Table 2.
Table 1. Demographic and clinical features of the scleroderma patients and control subjects.
| Scleroderma group (n=46) | Control subjects (n=31) | P-value | |
|---|---|---|---|
| Age (year) | 48.2±9.2 | 52.4±12 | 0.1 |
| Gender (male/female) | 3/43 | 6/25 | 0.086 |
| BMI (kg/m2) | 26.1±3.6 | 26.8±3.6 | 0.37 |
| ANA positivity, n (%) | 35 (76) | ||
| Anti-centromer Ab, n (%) | 10 (21.7) | ||
| Anti-SCL-70 Ab, n (%) | 9 (19.5) | ||
| AXL (mm) | 22.80±0.71 | 22.71±0.64 | 0.55 |
| SE (diopter) | −0.77±1.93 | 0.06±1.16 | 0.072 |
| Nasal choroidal thickness (μm) | 148±51.5 | 261.9±66.9 | 0.012* |
| Temporal choroidal thickness (μm) | 181.8±62.7 | 276.5±70.2 | 0.046* |
| Subfoveal choroidal thickness (μm) | 230.5±66.3 | 363.2±83.16 | < 0.001* |
Abbreviations: ANA, anti-nuclear antibody; Ab, antibody; AXL, axial length; BMI, body mass index; SE, spherical equivalent refractive error.
*P<0.05.
Table 2. Demographic and clinical features of the patients with diffuse-type scleroderma and limited-type scleroderma.
| Patients with limited-type scleroderma (n=25) | Patients with diffuse-type scleroderma (n=21) | P-value | |
|---|---|---|---|
| Age (year) | 54.3±11.9 | 50.2±12.1 | 0.26 |
| Gender (male/female) | 1/24 | 2/19 | 0.45 |
| BMI (kg/m2) | 26±3.3 | 26.13±3.99 | 0.97 |
| AXL (mm) | 22.85±0.82 | 22.75±0.70 | 0.52 |
| SE (diopter) | −0.70±2.15 | −0.86±1.69 | 0.72 |
| Nasal choroidal thickness (μm) | 141.2±54 | 156.1±48.5 | 0.13 |
| Temporal choroidal thickness (μm) | 224.9±72.3 | 237.5±59.5 | 0.38 |
| Subfoveal choroidal thickness (μm) | 272.3±61.2 | 280.8±80.4 | 0.65 |
Abbreviations: AXL, axial length; BMI, body mass index; SE, spherical equivalent refractive error.
Discussion
Scleroderma is characterized by vascular insufficiency due to the decrease in the number of capillaries, stenosis of small arteries and arterioles, and abnormality in capillaries.2 Choroid contains small arterioles and capillaries, and as a result, abnormality in choroidal vasculature may occur in patients with scleroderma. However, there are few studies and case presentations, which have investigated the pathophysiology of choroid and given information about changes in choroid in patients with scleroderma.5, 6, 17, 18, 19
It was established that 50% of patients with scleroderma had choroidal hypoperfusion affecting the choriocapillaries in fundus fluorescein angiography (FFA).20 Choroidal vessel leakage in both eyes of a patient with scleroderma was determined in indocyanine green angiography (ICGA).18 Although both FFA and ICGA give information about choroidal circulation, these methods cannot give information about cross-sectional anatomy of choroidal tissue. Histological studies have revealed that there were endothelial cell damage, thickening of basement membrane, and loss of pericytes in choroidal vessels in patients with scleroderma.6, 21 However, histological studies have some limitations. First, histological studies are invasive methods. Second, choroid is highly vascular structure and there are many factors affecting choroid thickness including endogenous nitric oxide production, endogenous circulating catecholamines, and its intrinsic vasomotricity.22, 23 All these factors affect postmortem evaluation of choroid tissue and measurement of the choroidal thickness.
It has been reported that age, gender, refractive error, AXL, and BMI affect choroidal thickness and, further, there is a diurnal variation in choroidal thickness.24 Our study demonstrated that the nasal, temporal, and subfoveal choroidal thicknesses were significantly thinner in the patients with scleroderma than in the healthy control subjects after adjusting age, gender, refractive error, AXL, and BMI. In addition, all choroidal thickness measurements were done at the same time of the day. To our best knowledge, there is only one study that has investigated choroidal thickness in patients with scleroderma.19 In this previous study, choroidal thickness was measured at 9 points (at the subfoveal, superior, inferior, nasal, and temporal regions, with intervals of 0.5–1 mm from the fovea). Choroidal thickness was significantly thinner in patients with scleroderma than in healthy control subjects at 7 regions (central, inner inferior, inner nasal, inner superior, outer temporal, outer inferior, and outer nasal). In addition, choroidal thicknesses at the inner temporal and outer superior regions were also thinner in patients with scleroderma than in healthy control subjects; however, the difference did not reach statistical significance. Our sample size (46 eyes of 46 patients) is larger than that included in the study of Ingegnoli et al19 (24 eyes of 12 patients) and the different results of these studies might be due to the different sample sizes.
In our study, fundus examination revealed that 15% of the eyes had local RPE changes. Serup et al found that RPE changes were seen in 14% of patients with scleroderma. In the same study, FFA revealed that 33% of the patients had hyperfluorescence in the late phase of angiography due to pigment epitheliopathy of RPE.25 Kraus et al26 demonstrated that 26.3% of the patients with scleroderma had atrophy of RPE in FFA. It was suggested that atrophy of RPE was likely to be because of an underlying abnormality and damage of the choroidal vasculature at the choriocapillary level.25, 26
In our study, there were no detectable retinal changes in our patients with scleroderma. It has been reported that retinal arterioles are relatively unaffected in patients with scleroderma.20, 25, 26 Pericytes of nerves are important structures in pathophysiology of scleroderma and retinal arterioles are less sensitive to scleroderma due to the fact that retinal arterioles do not have nerve supply.25 On the other hand, choroid vessels have nerve supply and pericytes. Accordingly, although scleroderma involves choroid, it does not affect retina.
Some limitations of the current study need to be declared. First, using FFA and ICGA to show angiographic changes in retina, RPE, and choroid and to compare the angiography results with the EDI-OCT results could make additional contributions. Second, we measured choroidal thickness manually as there is no commercially available automated software.
In conclusion, thinning of nasal, subfoveal, and temporal choroid was detected in patients with scleroderma. Vasculopathy in the scleroderma is characterized by obliteration of arterioles and reduced capillary density may cause atrophy of choroid in patients with scleroderma. EDI-OCT give beneficial information about choroidal tissue in patients with scleroderma.
Acknowledgments
We acknowledge the kind assistant of associate professor Veysi Öner in reviewing the article.
The authors declare no conflict of interest.
References
- Sakkas LI. New developments in the pathogenesis of systemic sclerosis. Autoimmunity 2005; 38(2): 113–116. [DOI] [PubMed] [Google Scholar]
- Rabquer BJ, Koch AE. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep 2012; 14(1): 56–63. [DOI] [PubMed] [Google Scholar]
- Gomes Bde A, Santhiago MR, Magalhaes P, Kara-Junior N, Azevedo MN, Moraes HV Jr. Ocular findings in patients with systemic sclerosis. Clinics 2011; 66(3): 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor R, Gupta A, Herrick A, Kwartz J. Ocular manifestations of scleroderma. Surv Ophthalmol 2009; 54(2): 292–304. [DOI] [PubMed] [Google Scholar]
- Hesse RJ, Slagle DF 2nd. Scleroderma choroidopathy: report of an unusual case. Ann Ophthalmol 1982; 14(6): 524–525. [PubMed] [Google Scholar]
- Farkas TG, Sylvester V, Archer D. The choroidopathy of progressive systemic sclerosis (scleroderma). Am J Ophthalmol 1972; 74(5): 875–886. [DOI] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol 2003; 121(4): 547–557. [DOI] [PubMed] [Google Scholar]
- Coskun E, Okumus S, Gurler B, Yayuspayi R, Oren B, Kaydu E et al. Choroidal thickness in healthy Turkish subjects. Turk J Med Sci 2014; 44(1): 56–61. [DOI] [PubMed] [Google Scholar]
- Gupta P, Saw SM, Cheung CY, Girard MJ, Mari JM, Bhargava M et al. Choroidal thickness and high myopia: a case-control study of young Chinese men in Singapore. Acta Ophthalmol 2014; 93(7): e585–e592. [DOI] [PubMed] [Google Scholar]
- Rishi P, Rishi E, Mathur G, Raval V. Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normals. Eye 2013; 27(9): 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang NH, Kim YT. Change in subfoveal choroidal thickness in central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy. Eye 2013; 27(3): 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang W, Zhou M, Huang W, Chen S, Li X et al. Enhanced depth imaging-optical coherence tomography of the choroid in moderate and severe primary angle-closure glaucoma. Acta Ophthalmol 2015; 93(5): e349–e355. [DOI] [PubMed] [Google Scholar]
- Eroglu FC, Asena L, Simsek C, Kal A, Yilmaz G. Evaluation of choroidal thickness using enhanced depth imaging by spectral-domain optical coherence tomography in patients with pseudoexfoliation syndrome. Eye 2015; 29(6): 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H, Sim DA, Keane PA, Zarranz-Ventura J, Gallagher K, Egan CA et al. Structural changes of the choroid in sarcoid- and tuberculosis-related granulomatous uveitis. Eye 2015; 29(8): 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun E, Gurler B, Pehlivan Y, Kisacik B, Okumus S, Yayuspayi R et al. Enhanced depth imaging optical coherence tomography findings in Behcet disease. Ocul Immunol Inflamm 2013; 21(6): 440–445. [DOI] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23(5): 581–590. [DOI] [PubMed] [Google Scholar]
- Milenkovic S, Petrovic L, Risimic D, Kosanovic-Jakovic N, Jaksic V, Djakovic Z et al. Choroidal sclerosis in localized scleroderma (morphea en plaque). Ophthalmic Res 2008; 40(2): 101–104. [DOI] [PubMed] [Google Scholar]
- Abdellatief A, Balasubramaniam SC, Grube TJ, Gonzalez Santiago TM, Osborn TG, Pulido JS. Indocyanine green angiographic evidence of choroiditis in scleroderma. Retin Cases Brief Rep 2015; 9(3): 231–234. [DOI] [PubMed] [Google Scholar]
- Ingegnoli F, Gualtierotti R, Pierro L, Del Turco C, Miserocchi E, Schioppo T et al. Choroidal impairment and macular thinning in patients with systemic sclerosis: the acute study. Microvasc Res 2015; 97: 31–36. [DOI] [PubMed] [Google Scholar]
- Grennan DM, Forrester J. Involvement of the eye in SLE and scleroderma. A study using fluorescein angiography in addition to clinical ophthalmic assessment. Ann Rheum Dis 1977; 36(2): 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean H, Guthrie W. Retinopathy in scleroderma. Trans Ophthalmol Soc UK 1970; 89: 209–220. [PubMed] [Google Scholar]
- Polak K, Luksch A, Berisha F, Fuchsjaeger-Mayrl G, Dallinger S, Schmetterer L. Altered nitric oxide system in patients with open-angle glaucoma. Arch Ophthalmol 2007; 125(4): 494–498. [DOI] [PubMed] [Google Scholar]
- Delgado E, Marques-Neves C, Rocha I, Sales-Luis J, Silva-Carvalho L. Intrinsic vasomotricity and adrenergic effects in a model of isolated rabbit eye. Acta Ophthalmol 2009; 87(4): 443–449. [DOI] [PubMed] [Google Scholar]
- Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci 2012; 53(4): 2300–2307. [DOI] [PubMed] [Google Scholar]
- Serup L, Serup J, Hagdrup H. Fundus fluorescein angiography in generalized scleroderma. Ophthalmic Res 1987; 19(5): 303–308. [DOI] [PubMed] [Google Scholar]
- Kraus A, Guerra-Bautista G, Espinoza G, Barojas E, Quiroz-Mercado H, Sanchez-Echeverri G et al. Defects of the retinal pigment epithelium in scleroderma. Br J Rheumatol 1991; 30(2): 112–114. [DOI] [PubMed] [Google Scholar]