Abstract
Purpose
The purpose of this study was to describe clinical features, risk factors, causative organisms, treatment options, and outcomes of post-traumatic endophthalmitis in children and adolescents.
Methods
Retrospective interventional case series. Case records of 143 consecutive eyes presenting with post-traumatic endophthalmitis between 1997 and 2007 were reviewed. Univariate and multivariate analysis were done to analyze factors associated with adverse outcomes.
Results
Mean age at presentation was 9.2 years (median 8 years, range: 2 months to 18 years). Broomstick and hypodermic needle were most common causes for injuries. Common presenting features were cataract (n=51), hypopyon (n=45) and retinal detachment (n=29). Corneal abscess (n=21; OR: 5, CI: 1.4–18.7) and retinal detachment (n=29, OR: 5, CI: 1.6–11.3) were independent risk factors for poor outcome (P=0.04 and 0.012, respectively). Gram-positive bacteria were isolated in 54% (n=31) of culture-positive cases. Forty-nine (34%) patients had ambulatory vision at final visit. Patients who received treatment within 24 h were 3.6 and 9 times more likely to have better anatomical outcome than those treated at 2–7 days, or >7 days, respectively (P=0.0001). Patients undergoing early vitrectomy were 27 times more likely to have better outcome (P=0.0001).
Conclusion
Post-traumatic endophthalmitis in children is more common in boys <10 years of age and most often caused by injury with organic matter. Corneal abscess and retinal detachment are associated with poor outcome. E. fecalis is the most common causative organism. Early vitrectomy results in better outcomes.
Introduction
Children are known to be prone to trauma and open globe injuries.1 Post-traumatic endophthalmitis (PTE) is a rare but sight-threatening complication that leads to blindness in large number of patients. The incidence of PTE in subjects in pediatric age group was estimated to be 2.8% of all patients with ocular trauma.2 Several clinical features like male sex, size of the corneal tear, lens rupture, presence of intraocular foreign body, time taken for intervention, and rural background have been identified as risk factors for development of PTE in adults.3, 4, 5
Children are not ‘small adults' and management strategies used for adults cannot be directly applied to pediatric cases. Even the clinical features, microbiological profile, and response to treatment are known to differ from adults.2 This necessitates studies with focused attention on this special age group for better understanding of clinical features and development of optimal-treatment strategies. There are very few reports in the literature about PTE in pediatric patients. PubMed search using the keywords ‘post traumatic', ‘endophthalmitis', ‘children', and ‘paediatric' revealed only nine relevant reports. The purpose of this study was to describe the presenting clinical features, microbiological profile and management of PTE in 143 eyes of pediatric patients and to evaluate these features in terms of anatomical and functional outcomes.
Patients and methods
This was a retrospective, single centre, interventional case series of 214 eyes of 214 children presenting with endophthalmitis at a tertiary eye care centre in south India from December 1997 to December 2007. All patients ≤18 years with PTE were included. Of 214 children presenting with endophthalmitis, 143 children presented with PTE. Children with history of any ocular surgery up to 3 months prior to trauma, sepsis, or any systemic foci of infection were excluded. Prior institution review board approval was obtained for this study. A written informed consent was obtained from parent/guardian of all participating subjects. All the tenets of the declaration of Helsinki were followed.
The epidemiological data of each patient was noted. A detailed history pertaining to type and mode of trauma was elicited from patients/guardians. Visual acuity in pre-school and younger patient was noted with Teller/Cardiff acuity cards. Snellen's charts were used for school-going children. All patients underwent complete ophthalmic examination including slit lamp biomicroscopy and indirect ophthalmoscopy. Ultrasound was performed in all cases where primary repair was already done and in cases with suspected occult globe rupture. The ultrasound probe was disinfected with Bacillocid 1% solution (Raman and Weil, Mumbai, India)and placed with gentle pressure over the globe. Ultrasound was deferred in cases with open globe injury at presentation. The interval between onset of clinical features of endophthalmitis and first treatment for endophthalmitis was noted and was graded as immediate (<24 h), early (2–7 days), and delayed (>7 days).
Aqueous/vitreous tap was performed in all cases undergoing treatment under general anesthesia. The decision for aqueous or vitreous tap was based on clinical judgment of predominant focus of involvement. Eviscerated material was sent for microbiological analysis in cases where primary evisceration was advised. Aspirates were sent for microbiological analysis which included smear (Grams and KOH), culture sensitivity, and PCR for Eubacteria, P. acne, and fungi. Empirical treatment consisting of topical antibiotics and mydriatic agents along with intravitreal antibiotic injections with/without steroids was started based on initial smear and PCR reports and was individualized in accordance with culture results, and severity of signs and symptoms.
Treatment options included topical and systemic medications, intravitreal medications, and surgical management. All patients with evidence of bacterial infection or clinically suspected bacterial infection were started on topical steroids and received intravitreal Vancomycin (1 mg/0.1 ml), Ceftazidime (2.25 mg/0.1 ml), and dexamethasone (0.4 mg/0.1 ml). Patients were treated with systemic steroids (1 mg/kg body weight) wherever the clinician felt inflammation to be significant. All patients with bacterial infections received parenteral Cefotaxime (50 mg/kg body weight in divided doses) and Gentamicin (5–7 mg/kg body weight/day). Patients with proven fungal infections were treated with parenteral fluconazole (6–12 mg/kg body weight/day) with monitoring of liver enzymes.
All surgeries were performed under general anesthesia with proper aseptic conditions. All patients were reviewed on day 1, day 6 and then 6 weeks after the treatment. Follow-up intervals were individualized at the discretion of the treating surgeon. The details of the last follow-up visit were noted. Treatment outcomes were analyzed in terms of predetermined criteria as follows:
Optimal anatomical outcome (OAO): it was defined as status of an eye with no signs of inflammation at final visit with no complications like retinal detachment or phthisis bulbi.
Adverse anatomical outcome (AAO): was defined as status of an eye with either retinal detachment/phthisis bulbi or eyes that underwent enucleation/evisceration.
Optimal functional outcome (OFO): was defined as best corrected visual acuity better than 3/60 with OAO.
Adverse functional outcome (AFO): was defined as best corrected visual acuity equal to or <3/60 with OAO.
Univariate and multivariate analysis using logistic regression was done for age, sex, visual acuity at presentation, type of trauma, source of trauma, corneal tear, corneal abscess, hypopyon, hyphema, exudates in anterior chamber, lens injury, retinal status, culture positivity, microbiological profile, interval between injury and treatment, intravitreal steroids, and vitrectomy to assess the risk factors associated with AAO. Statistical analysis was performed using SPSS 20 software (SPSS Inc, Chicago, IL, USA).
Results
Baseline characteristics
143 eyes of 143 patients met the inclusion criteria. The mean age at presentation was 9.2 years (median: 8 years; range: 2 months to 18 years) and average follow-up period was 23 months (median: 20, range: 9–53 months). Baseline characteristics of the patients are listed in Table 1. The most common organic source of trauma was broom stick (n=59), followed by pencil-tip injury (n=29); whereas hypodermic needle was most common source in inorganic group (n=14). Sixty-five (46%) eyes had primary repair done within 24 h of injury, 68 (48%) eyes had self-sealed corneal tear, whereas 10 (6%) eyes had occult globe rupture. The average interval between injury and presentation was 17 days (median 12; range 0–198 days). Visual acuity at the time of presentation was light perception or worse in 125 (87%) eyes, whereas only 4 (3%) eyes had visual acuity >6/60. Presenting clinical features are listed in Table 1.
Table 1. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India: baseline characteristics.
| Clinical characteristics (n=143) | Number of patients (%) |
|---|---|
| Age (years) | |
| 0–1 | 01 (0.7) |
| >1–3 | 27 (19) |
| >3–12 | 82 (57) |
| >12–16 | 33 (23) |
| Sex | |
| Male | 103 (72) |
| Female | 40 (28) |
| Type of infective source | |
| Organic | 79 (55) |
| Inorganic | 64 (45) |
| Type of trauma | |
| Penetrating | 133 (93) |
| Occult rupture | 10 (7) |
| Lens rupture | |
| Yes | 51 (36) |
| No | 92 (64) |
| Presenting VA | |
| ≥6/60 | 04 (03) |
| <6/60 | 14 (10) |
| PL or worse | 125 (87) |
| Clinical features at presentation | |
| Cataract | 51 (35) |
| Hypopyon | 45 (31) |
| Retinal detachment | 29 (20) |
| Hyphema | 23 (16) |
| Corneal abscess | 21 (15) |
| IOFB | 06 (04) |
| Scleral abscess | 05 (03) |
| Panophthalmitis | 01 (0.7) |
Abbreviations: IOFB, intra-ocular foreign body; PL, perception of light; VA, visual acuity.
Microbiology
Specimen collected for microbiological examination included aqueous tap (n=4), vitreous tap (n=102), and eviscerated material (n=28; primary evisceration=25, secondary evisceration=3). No sample was collected from 12 eyes; either owing to non-compliance (n=9) or owing to pre-phthisical changes with no signs of active inflammation (n=3). Of the 134 specimen, culture was positive only in 55 (41%) eyes. Gram-positive bacteria were isolated in 31 (23%) eyes and Gram-negative in 21 (16%) eyes. Organisms identified as causative agents are listed in Table 2. Fungi were isolated in 3 (2%) eyes. Four eyes (3%) had mixed infection. Enterococcus fecalis was the most common Gram-positive organism, whereas Klebsiella serratia was the most common Gram-negative organism isolated in 8 (6%) and 6 (4%) eyes, respectively. Among fungi, Aspergillus fumigatus was isolated in two (1.5%) eyes.
Table 2. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India: microbiological profile.
| Organism | Number of eyes (%) |
|---|---|
| Gram positive (n=31) | |
| Enterococcus faecalis | 08 (26) |
| Bacillus cereus | 06 (19) |
| Streptococcus pneumoniae | 05 (16) |
| Staphylococcus aureus | 05 (16) |
| Corynebacterium | 03 (10) |
| Micrococcus | 02 (06) |
| Psuedomonas acnes | 02 (06) |
| Gram negative (n=21) | |
| Klebsiella serratia | 06 (29) |
| Bacteroides | 03 (14) |
| Acinetobacter | 03 (14) |
| Aeromonas | 02 (10) |
| Escherichia coli | 02 (10) |
| Proteus vulgaris | 02 (10) |
| Citrobacter | 02 (10) |
| Serratia | 01 (5) |
| Fungus (n=3) | |
| Aspergillus fumigatus | 02 (67) |
| Candida albicans | 01 (33) |
Management
Table 3 depicts the distribution of various treatment modalities used in the management of PTE along with the respective outcomes. Intravitreal antibiotics were initially given according to the Endophthalmitis vitrectomy study (EVS) protocol6 and then subsequently based on culture-sensitivity reports, if required. Intravitreal steroid was given in 57 eyes along with standard antibiotics. Adjunct procedures included corneal/scleral tear repair (n=21), penetrating keratoplasty (n=4), IOFB removal (n=6), and retinal-detachment surgery (n=15).
Table 3. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India: treatment received.
| Intervention | Adverse AO | Optimal AO | Optimal VO |
|---|---|---|---|
| Nil (n=15) | 15 | 0 | 0 |
| L+V+IV (n=71) | 27 | 44 | 30 |
| V+AP (n=27) | 15 | 12 | 08 |
| Evisceration (n=28) | 28 | 00 | 00 |
| Enucleation (n=2) | 02 | 00 | 00 |
Abbreviations: AO, anatomical outcome; AP, adjunct procedure; IV, intravitreal drugs; L, lensectomy; V, vitrectomy; VO, visual outcome.
Treatment outcomes
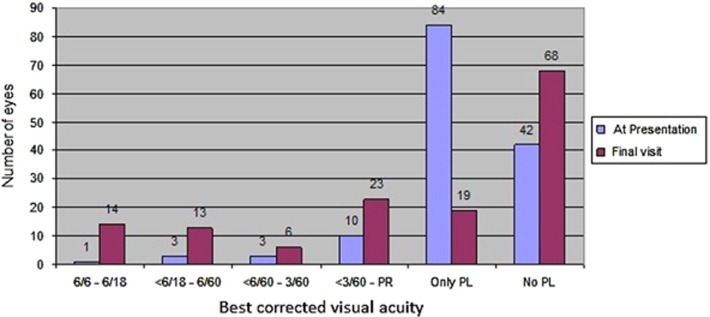
Of 143 eyes at final visit (mean 23 months), 56 (39%) eyes achieved OAO and 87 (61%) eyes had AAO, with retinal detachment in 27 (19%) eyes and phthisis bulbi in 30 (21%) eyes. OFO was seen in 38 (27%) eyes with 14 eyes having best corrected VA of 6/18 or more. AFO was seen in 18 (13%) eyes in which VA deteriorated due to corneal, macular or optic nerve pathologies. Figure 1 shows the detailed distribution of visual acuity at presentation and at final visit in study subjects.
Figure 1.
Comparison of number of patients with best corrected vision at presentation and at final follow-up.
Table 4 shows the results of the multivariate analysis done for AAO. Fourteen of 23 patients who were immediately treated (within 24 h) had optimal anatomical and functional outcome, 16 of 42 patients who received early treatment (2–7 days) and 15 of 78 patients who received delayed treatment (>7 days) had OAOs, respectively. Patients who received treatment for endophthalmitis within 24 h were 3.6 and 9 times more likely to have better anatomical outcome than those who were treated at an interval of 2–7 days and >7 days, respectively (P=0.0001). Patients with corneal abscess and/or retinal detachment were 5 times more likely to have an adverse outcome, (P=0.04) and (P=0.012), respectively. Vitrectomy had a protective role and patients undergoing vitrectomy were 27 times more likely to have good anatomical outcome (P=0.0001). Age, sex, visual acuity at presentation, type of trauma, source of trauma, corneal tear, hypopyon, hyphema, lens injury, culture positivity, and use of intravitreal steroids did not have any impact on functional or anatomical outcomes measured (Table 4).
Table 4. Post-traumatic endophthalmitis in 143 eyes of children and adolescents from India: multivariate analysis of risk factors for AAO.
| Clinical feature | Odds ratio |
95% CI |
P-value | |
|---|---|---|---|---|
| Upper | Lower | |||
| Age | 0.422 | 0.130 | 1.369 | 0.151 |
| Sex | 0.618 | 0.217 | 1.760 | 0.368 |
| VA | 1.023 | 0.546 | 1.918 | 0.944 |
| Type of injury | 0.996 | 0.408 | 2.428 | 0.992 |
| Type of trauma | 0.965 | 0.409 | 2.274 | 0.934 |
| Corneal tear | 1.276 | 0.704 | 2.311 | 0.422 |
| Lens injury | 1.552 | 0.547 | 4.406 | 0.409 |
| Hypopyon | 0.367 | 0.130 | 1.037 | 0.058 |
| Corneal abscess | 5.211 | 1.081 | 25.125 | 0.040 |
| AC exudates | 0.517 | 0.196 | 1.361 | 0.182 |
| Hyphema | 0.765 | 0.159 | 3.679 | 0.739 |
| Retinal detachment | 5.122 | 1.437 | 18.259 | 0.012 |
| Culture | 0.784 | 0.090 | 6.838 | 0.826 |
| Gram staining | 0.822 | 0.216 | 3.137 | 0.775 |
| Fungal | 0.244 | 0.007 | 8.593 | 0.437 |
| Time interval | ||||
| Within 24 h | 00 | — | — | — |
| 2–7 days | 3.6 | 1.12 | 11.4 | 0.03 |
| >7 days | 9.0 | 3.2 | 29.6 | 0.0001 |
| Intravitrael steroid | 0.714 | 0.254 | 2.006 | 0.522 |
| Vitrectomy | 27.620 | 5.683 | 134.235 | 0.0001 |
Abbreviations: AAO, adverse anatomical outcome; AO, anatomical outcome; VO, visual outcome.
P<0.05 is considered as significant.
Discussion
There is limited literature available on PTE among children and adolescents. Paediatric cases accounted for nearly 17% of all cases of PTE in western population.5 The same was 51% in a series of PTE from India.7 PTE constituted 69% of all endophthalmitis in children in the present study. The higher incidence of traumatic ocular injuries in boys as compared with girls puts them at a higher risk of developing endophthalmitis.8 In our series, 72% of patients were males. Similar rates (75%) were reported by Alfaro et al2 and Junejo et al1 (67%) in their series. Injuries with ‘broomstick' or with ‘bow and arrow' were the most common source of injury accounting for 41% of cases in our series. Previous studies from India have also reported a higher incidence of penetrating trauma caused by ‘bow and arrow' injuries, which is peculiar to the subcontinent.8, 9 The mean age at presentation has been reported to be 10 years,2 whereas the same was 9.2 years in our series.
Culture-positive rates have been reported to range from 44 to 75% in western studies.2, 9, 10 Narang et al8 reported of 27% culture positivity from ocular specimen from India. In our study, culture positivity was 41%. The lower culture-positivity rates in our population could be because of the easy ‘over-the-counter' availability of antibiotics. This study enrolled patients from a tertiary-care centre and most of the patients had received prior antibiotic treatment elsewhere. Gram-positive organisms account for 57–67% of all microbes in PTE.2, 9, 11 In the present study, 56% (n=31) of patients had Gram-positive infections and E. fecalis was the most common organism isolated in 8 (15%) eyes, whereas Staphylococcus and Streptococcus species were isolated in 5 (9%) eyes, each. Staphylococcus aureus, Streptococcus epidermidis and Bacillus cereus were identified as the most common causative organisms in previous studies.2, 9, 11 In our literature search by PubMed, we came across no other reports with E. faecalis as the most common causative organism in PTE in children. We earlier published our series on E. faecalis endophthalmitis in 7 children with PTE.12 It carries a poor prognosis and the EVS revealed E. faecalis in only 1.23% of all microbial isolates in culture.13, 14
Risk factors for endophthalmitis after penetrating trauma have been identified previously.5, 7, 10 We analyzed these risk factors in terms of anatomical and functional outcomes in our series. We did not find any impact of age on functional or anatomical outcome following treatment. This is in contrast to Alfaro et al2 who reported poor visual outcomes in children younger than 10 years. This may be owing to the fact that their report came in 1995 and since then many advances have been made in managing endophthalmitis cases in terms of availability of potent antibiotics and improvements in surgical instrumentation. Further, their sample size was very small in comparison with our study. Lens rupture has been reported to have poor outcome,3, 4, 5, 11 but Jonas et al4 in their study did not find any association between the two. Likewise, we also did not find any significant relation between lens rupture and final visual and/or anatomical outcomes.
However, corneal abscess and retinal detachment were identified as independent risk factors for poor outcome in our study. Patients with corneal abscess and/or retinal detachment were five times more likely to have AAO. Presence of either of these warrants an early and aggressive treatment.
Therapeutic vitrectomy was performed in 69% of cases in our study. This is in concordance with the recommendations of previous reports.12, 13 Early vitrectomy drastically decreases the microbiological load and helps in diffusion of intravitreal and systemic antibiotics within the eye.14, 15, 16, 17 In this study, >30% patients gained ambulatory vision (VA>3/60) after vitrectomy. Patients undergoing vitrectomy were 27 times more likely to have good anatomical outcome than those that did not have vitrectomy.
In conclusion, PTE among children and adolescents is more common in school-age boys and most often caused by injury with organic matter. It is caused by Gram-positive bacteria in more than half the cases with E. fecalis being the most common causative organism. Corneal abscess and retinal detachment are independent risk factors associated with poor outcome. Early treatment and therapeutic vitrectomy helps in attaining significant improvement in functional and anatomical outcomes.

Acknowledgments
We acknowledge the contributions of our colleagues involved in patient care and technical support: Drs Lingam Gopal, Tarun Sharma, Muna Bhende, Parveen Sen, Rajiv Raman, Chetan Rao, Pradeep Susvar, Vikas Khetan from the Shri Bhagwan Mahavir Vitreoretinal Services, and J Malathi, K Lily Therese, HN Madhavan from L&T Microbiology department at Sankara Nethralaya, Chennai.
The authors declare no conflict of interest.
References
- Junejo SA, Ahmed M, Alam M. Endophthalmitis in pediatric penetrating ocular injuries in Hyderabad. J Pak Med Assoc 2010; 60(7): 532–535. [PubMed] [Google Scholar]
- Alfaro DV, Roth DB, Laughlin RM, Goyal M, Liggett PE. Pediatric post-traumatic endophthalmitis. Br J Ophthalmol 1995; 79: 888–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M, Faghihi H, Hajizadeh F, Rasoulinejad SA, Rajabi MT, Tabatabaey A et al. Epidemiology of open-globe injuries in Iran: analysis of 2,340 cases in 5 years (report no. 1). Retina 2009; 29: 1141–1149. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology 2000; 107: 823–828. [DOI] [PubMed] [Google Scholar]
- Essex RW, Yi Q, Charles PG, Allen PJ. Post-traumatic endophthalmitis. Ophthalmology 2004; 111: 2015–2022. [DOI] [PubMed] [Google Scholar]
- Rishi E, Rishi P, Nandi K, Shroff D, Therese KL. Endophthalmitis caused by Enterococcus faecalis—a case series. Retina 2009; 29: 214–217. [DOI] [PubMed] [Google Scholar]
- Das T, Kunimoto DY, Sharma S, Jalali S, Majji AB, Nagaraja Rao T et al. Relationship between clinical presentation and visual outcome in postoperative and posttraumatic endophthalmitis in South Central India. Indian J Ophthalmol 2005; 53: 5–16. [DOI] [PubMed] [Google Scholar]
- Narang S, Gupta V, Simalandhi P, Gupta A, Raj S, Dogra MR. Pediatric open globe injuries. Visual outcome and risk factors for endophthalmitis. Indian J Ophthalmol 2004; 52: 29–34. [PubMed] [Google Scholar]
- Dasgupta S, Mukerjee R, Ladi DS, Gandhi VH. Pediatric ocular trauma. A clinical presentation. J Postgrad Med 1990; 36: 20–22. [PubMed] [Google Scholar]
- Chhabra S, Kunimoto DY, Kazi L, Regillo CD, Ho AC, Belmont J et al. Endophthalmitis after open globe injury. Microbiologic spectrum and susceptibilities of isolates. Am J Ophthalmol 2006; 142: 852–854. [DOI] [PubMed] [Google Scholar]
- Mieler WF, Ellis MK, Williams DF, Han DP. Retained intraocular foreign bodies and endophthalmitis. Ophthalmology 1990; 97: 1532–1538. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Flynn HW Jr. Endophthalmitis after penetrating ocular trauma. Curr Opin Ophthalmol 1997; 8: 32–38. [DOI] [PubMed] [Google Scholar]
- Sternberg Jr P, Martin DF. Management of endophthalmitis in the post-endophthalmitis vitrectomy study era. Arch Ophthalmol 2001; 119: 754–755. [DOI] [PubMed] [Google Scholar]
- Thordsen JE, Harris L, Hubbard GB. Pediatric endophthalmitis: A 10 year consecutive series. Retina 2008; 28: S3–S7. [DOI] [PubMed] [Google Scholar]
- Weinstein GS, Mondino BJ, Weinberg RJ, Biglan AW. Endophthalmitis in a pediatric population. Ann Ophthalmol 1979; 11: 935–943. [PubMed] [Google Scholar]
- Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH et al. Spectrum and susceptibility ofmicrobiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol 1996: Jul; 122(1): 1–17. [DOI] [PubMed] [Google Scholar]
- Driebe WT, Mandelbaum S, Forster RK, Schwartz LK, Culbertson WW. Pseudophakic endophthalmitis. Diagnosis and management. Ophthalmology 1986; 93: 442–448. [DOI] [PubMed] [Google Scholar]