Abstract
Objective
We previously reported a 2×2 randomized clinical trial of individual and combined treatment with atomoxetine (ATX) and parent training (PT) for attention-deficit/hyperactivity disorder (ADHD) symptoms and behavioral noncompliance in 128 children with autism spectrum disorder, ages 5–14 years. We now describe a 24-week extension of treatment responders and non-responders.
Method
One-hundred seventeen participants from the acute trial (91%) entered the extension; 84 of these were in two subgroups: (1) “treatment responders” (n=43) from all four groups in the acute trial, seen monthly for 24 weeks, and (2) “placebo nonresponders” (n=41), treated with open-label ATX for 10 weeks. Participants originally assigned to PT continued in PT during the extension; the remainder served as controls. Primary outcome measures were parent-rated Swanson, Nolan and Pelham ADHD scale and Home Situations Questionnaire.
Results
Sixty percent (26/43) of treatment responders in the acute trial, including 68% of responders originally assigned to ATX, still met response criteria at the end of extension. The response rate of placebo nonresponders treated with 10-week open-label ATX was 37% (15/41), similar to the acute trial. Children receiving open-label ATX+PT were significantly more likely to be ADHD responders (53% vs 23%) and noncompliance responders (58% vs 14%) than those receiving open-label ATX alone.
Conclusion
Most ATX responders maintained their responses during the extension. PT combined with ATX in the open-label trial appeared to improve ADHD and noncompliance outcomes more than ATX alone.
Keywords: autism, attention-deficit/hyperactivity disorder, atomoxetine, combined modality therapy, outcome assessment
INTRODUCTION
Co-occurring behavioral concerns are common in children with autism spectrum disorder (ASD). Diagnosable attention-deficit/hyperactivity disorder (ADHD) occurs in about one-third;1 clinically significant noncompliance or oppositional behavior (e.g., ignoring, refusing, defying or arguing with requests) occurs in one-quarter or more.2 Both are associated with impaired child and family functioning.3–4
Stimulant medication can be effective for ADHD symptoms in children with ASD.5 However, response rates (>25% symptom reduction plus clinician impression of substantial improvement) are much lower than in typically developing (TD) children with ADHD.5 Barely half of children with ASD are “responders,”5 compared to 70% or more of TD children.6–7 Moreover, children with ASD are about four times as likely as TD youth to experience intolerable side effects.5,8
Procedures based on applied behavior analysis (ABA) have been shown to ameliorate behavioral noncompliance in some children with ASD in single-subject studies.9 A randomized clinical trial (RCT) of 124 children with ASD, age 4–13 years, found that parent training in ABA strategies, combined with risperidone, was more effective than risperidone alone for reducing irritability.10 The same parent training (PT) program, adapted for younger children, surpassed didactic parent education for reducing behavioral noncompliance and other disruptive behavior in an RCT of 180 children with ASD, age 3–6 years.11
To address the need for additional treatment options for children with ASD and co-occurring ADHD, the Children with Hyperactivity and Autism Research Treatment Study (CHARTS) examined the single and combined effects of: (1) atomoxetine (ATX), a non-stimulant medication approved by the Food and Drug Administration for ADHD, and (2) parent training (PT) in ABA strategies to reduce disruptive behavior. ATX is classified as a norepinephrine reuptake inhibitor. It is effective for reducing ADHD symptoms in TD children12 but has limited evidence in children with ASD.13 PT involves teaching parents to use strategies such as reinforcement systems to encourage socially desirable behaviors and withholding of reinforcement or implementing timeout to reduce behavioral problems. PT for children with behavioral concerns uncomplicated by ASD usually involves 6–16 sessions (often about 10) and is well-established.14–15 However, it has not been well studied in children with ASD and ADHD.16
CHARTS began with a 10-week RCT that examined the single and combined effects of PT and ATX in a 2 × 2 factorial design (ATX+PT, ATX alone, PT+Placebo, and Placebo alone). Week-10 data revealed that, although ATX and PT did not appear to have additive effects, each significantly improved ADHD symptoms while ATX (both alone and combined with PT) significantly decreased noncompliance.17
At week 10, all participants were invited into an extension for an additional 24 weeks (a total of 34 weeks). The extension was intended to test three questions relevant to clinical practice: First, did PT groups surpass no-PT groups on behavioral noncompliance? Second, did responders maintain their responder status at the end of the extension (week 34)? Third, did placebo nonresponders improve when given open-label ATX?
METHOD
Procedures
We previously described the background, methods, and week 10 results.16–17 Briefly, CHARTS was a parallel-groups, placebo-controlled trial conducted at three sites: University of Pittsburgh, Ohio State University, and University of Rochester. All procedures were approved by each institution's institutional review board. Randomization was stratified by site in equal numbers to ATX+PT, ATX-only, PT+placebo, or placebo-only and balanced by mental age (MA < 6 years vs. > 6 years). As noted, the two phases included an acute, 10-week randomized trial and a 24-week extension (34 weeks total). During the acute trial, ATX assignment was double-blind and PT assignment was single-blind: known by only the family, behavior therapist, and study coordinator, while other study personnel, including raters, remained blinded. Visits initially occurred weekly to assess medication response, monitor adverse events (AEs) and adjust doses. Final dose adjustments were made at week 6, with subsequent monitoring at weeks 8 and 10. Families assigned to PT met weekly for up to 10 1:1 sessions with a PT clinician for 60 to 90 minutes. Variations of the PT manual, which three of the authors of this article helped develop, were used in two prior RCTs of children with ASD and behavioral problems, as described in the Introduction.10–11 The manual can be obtained at www.rubinetwork.org.
At week 10, participants were considered ADHD responders (having a favorable ADHD outcome) if they received a Clinical Global Impressions (CGI) – Improvement18 rating of 1 or 2 for ADHD symptoms by a blinded evaluator and >30% decrease on the ADHD subscale of the parent-rated Swanson, Nolan, and Pelham (SNAP) Fourth Edition (SNAP-IV, http://www.forabrighterfuture.com/pdf/SNAP-IVTeacherParetnRatingScale.pdf). Participants were classified as noncompliance responders (achieving a favorable noncompliance outcome) if they received a CGI-Improvement rating of 1 or 2 for Noncompliance by the blinded evaluator and >30% decrease on the mean severity score on the Home Situations Questionnaire – Pervasive Developmental Disorder (HSQ).19–20 Responders (either ADHD or noncompliance) remained in their assigned treatments during the 24-week double-blind extension. Nonresponders who had been taking placebo (placebo nonresponders) began open-label ATX. All other participants were treated clinically; outcomes for these participants are reported in Supplement 1, available online. All participants who were originally assigned to PT during the acute trial continued in PT during the 24-week extension; this included responders and nonresponders in both the ATX+PT and PT+placebo groups. Thus, depending on clinical response, participants could change medication but not PT assignment in the extension. Placebo nonresponders were seen weekly for the first 6 weeks of the extension for open-label ATX, with subsequent monitoring 2 and 4 weeks later; they were then seen every 4 weeks until the end of the extension (for a total of 4 additional visits). Responders were seen every 4 weeks throughout the extension (6 visits total). In addition, families assigned to PT had additional parent training sessions every 4 weeks (6 visits total), plus one home visit.
Participants
Participants were children, age 5.0–14.11 years, with MA > 24 months, based upon either the Stanford-Binet–5th Edition (SB5)21 or Mullen Scales of Early Learning.22 All participants had an ASD diagnosis (autistic disorder, Asperger's disorder, pervasive developmental disorder not otherwise specified [PDD-NOS]), based upon the Autism Diagnostic Interview–Revised23 and expert clinical evaluation that included interview, observation, and DSM-IV-TR checklist. Participants had to exhibit problematic overactivity and/or inattention at both home and school, defined as mean item score > 1.50 on both the parent- and teacher-completed SNAP-IV and CGI-Severity score > 4. Participants were enrolled irrespective of severity of noncompliance scores. Consequently, not all displayed clinically significant noncompliance. Participants had to be free of all psychotropic medications for two weeks prior to randomization. We excluded children with significant psychiatric disorders, medical conditions, or abnormalities on routine laboratory tests and electrocardiogram (ECG).
Outcome Measures
Primary Outcome for ADHD
The SNAP-IV Parent Rating Scale contains an ADHD section with items for each of the 18 DSM-IV symptoms of ADHD rated from 0 (not at all) to 3 (very much). For inclusion, a mean item score >1.5 on both Parent and Teacher SNAP-IV was required for all 18 ADHD symptoms, the 9 hyperactive-impulsive symptoms, or the 9 inattentive symptoms.
Primary Outcome for Noncompliance
The HSQ is a 25-item parent rating scale assessing noncompliance. Parents indicate whether each item is a problem and, if so, rate its severity from 1 (mild) to 9 (severe). Originally developed for TD children with disruptive behavior,19 it was adapted by the RUPP Autism Network for children with ASD.20
Secondary Outcomes
The parent-completed Aberrant Behavior Checklist (ABC)24–25 contains 58 items rated from 0 (not a problem) to 3 (severe). The ABC is reliable, valid, and sensitive to treatment effects.26 The raw score of the Irritability subscale (15 items) and Hyperactivity/Noncompliance subscale (16 items) were secondary outcomes.
The CGI17 includes scales for severity and improvement. The CGI-Severity (CGI-S) is scored from “1” (normal) to “7” (extremely ill). The CGI-Improvement (CGI-I) score ranges from “1” (very much improved), through “4” (no change), to “7” (very much worse). The CGI was completed by a blinded rater, based on parent/child interview, observation, and review of parent and teacher ratings. Separate CGI ratings were obtained for ADHD and noncompliance because we hypothesized that PT would have a greater impact on noncompliance and ATX would have a greater impact on ADHD.
The parent ratings (SNAP-IV, HSQ, ABC) were completed at each study visit during the extension (every two weeks for placebo nonresponders in the open trial to adjust ATX dose, every four weeks for all other participants). The CGI was completed by a blinded rater every four weeks for all participants. Teacher ratings were obtained at weeks 22 and 34. However, they are not reported here because many participants started a new school year during the trial or were enrolled in the trial during summer recess, which disrupted collection of these measures. Teacher data were available for only 46 of 128 participants (36%) at week 22 and 83 of 128 (65%) at week 34.
Data Analysis
Descriptive statistics were derived for baseline demographic and clinical characteristics, as well as therapist fidelity and parent adherence to PT, ATX dose, side effects, and concomitant medications. For Question 1 (did the PT groups surpass the no-PT groups on behavioral noncompliance?), we analyzed data through week 22 of the 34-week study (10 weeks of RCT plus 12 weeks of extension). This time point was chosen prior to the onset of the trial16 based on a previous trial showing that outcomes in PT did not separate from outcomes in no-PT until 20 weeks.10 Because of this finding, we decided to set the primary assessment of PT effect after 20 weeks (i.e., week 12 of the extension, week 22 of the study) and perform a secondary test of PT at the end of the acute trial (i.e., week 10 of the study; see 16, Section 6.1). Linear mixed models for repeated measures contrasted treatment effects for each continuous outcome with site, treatment, time, and time-by-treatment interaction as independent variables. Data analyses for PT effect were based on initial treatment assignment and intent-to-treat (ITT) principle, regardless of changes in drug treatment at extension. Primary endpoints were the changes from baseline to week 22 (or week of dropout before week 22, using last observation carried forward [LOCF]).
For Question 2 (did responders maintain their responder status at week 34?), we calculated how many responders maintained responder status at week 34, as well as how many of these responders were in the PT and no-PT groups. For Question 3 (did placebo nonresponders improve when given open-label ATX?), mixed models for repeated measures were employed to examine (a) changes from the beginning of the 10-week open-label trial at week 10 to the end of the open-label trial at week 20, (b) changes from baseline (week 0) to end of the open trial (week 20), and (c) comparisons between open-trial ATX with and without PT.
All analyses used the MIXED procedure in SAS (SAS Institute, Inc., Cary, NC), with the default missing-at-random (MAR) assumption. Model assumptions were assessed by examination of residuals. Missing data due to earlier exit of study were handled using LOCF. Sensitivity analyses were carried out (without LOCF) to confirm earlier conclusions based on the same mixed models using LOCF. Sensitivity analyses results are not reported when conclusions are not different from the primary analysis.
RESULTS
Participant Characteristics and Group Assignments
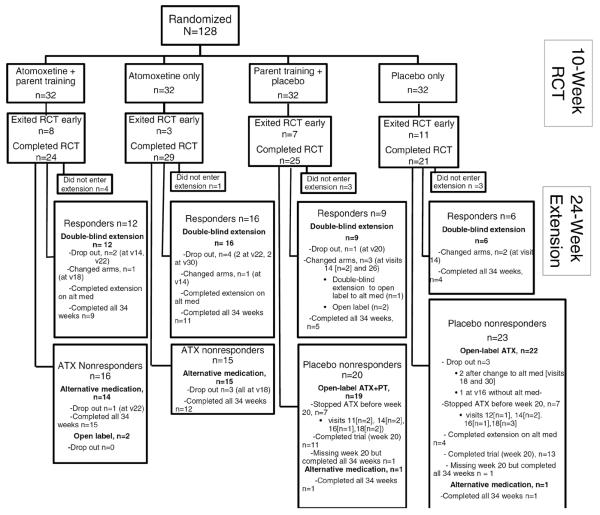
Handen et al.17 described recruitment and retention in the 10-week acute trial. The consolidated standards of reporting trials (CONSORT) diagram in Figure 1 shows group assignments during the 24-week extension. Altogether, 117 of the 128 randomized participants (91%) entered the extension. The extension contained two subgroups that we examine in the current report (n=84): (a) responders who remained in the double-blind condition (n=43), including 16 who had been in ATX-only during the acute trial, 12 in ATX+PT, 9 in PT+placebo, and 6 in placebo-only), and (b) placebo nonresponders, who received open-label ATX for 10 weeks (n=41, 19 who had been in PT+placebo and 22 in placebo only). Data from responders are shown in the second-to-last row of Figure 1; data from placebo nonresponders are shown in the two right-most boxes in the last row of Figure 1. Supplement 1 (available online) presents data on a third subgroup: ATX nonresponders, who were treated by study clinicians with other medications during the extension (n = 29, 14 in ATX-only and 15 in ATX+PT). The remaining 4 participants in the extension were not in any of these subgroups (2 ATX nonresponders in the acute trial continued ATX in the extension at family request, and 1 PT+placebo and 1 placebo only nonresponders in the acute trial continued on alternative medication).
Figure 1.
Flow of participants through the study. Note: ATX = atomoxetine; RCT = randomized clinical trial.
Table 1 summarizes characteristics of participants in each subgroup in the extension. The overall sample was M(SD) = 8.1 (2.1) years old, predominately male (85%), and mostly Caucasian (81%). Most had full-scale IQs above 70, M(SD) = 82.2(23.9), lived in families with income > $60,000 (56.4%), and attended special education classes (53.9%). Differences among the 3 subgroups or between participants receiving PT or no-PT were not significant.
Table 1.
Participant and Family Demographics at Baseline (Week 0) by Subgroup in the Extension
| Responders at Week 10 (continued blinded treatment in extension) | Placebo Nonresponders at Week 10 (received 10-weeks open-label ATX in extension) | All in Extension N=117a | |||
|---|---|---|---|---|---|
| Variable | ATX n=28 | PBO n=15 | OL/ATX + PT n=19 | OL/ATX n=22 | |
| Age(years) mean±SD | 8.0 ± 1.8 | 8.1 ± 2.0 | 7.7 ± 1.3 | 8.4 ± 2.5 | 8.1 ± 2.1 |
| IQ, mean ± SD | 85.4 ± 22.9 | 74.3± 26.6 | 82.9 ± 24.1 | 89.4 ± 22.9 | 82.2 ± 23.9 |
| Diagnosis | |||||
| Autistic Disorder | 9 (32.1%) | 9 (60.0%) | 7 (36.8%) | 11 (50.0%) | 52 (44.4%) |
| Asperger's | 6 (21.4%) | 2 (13.3%) | 3 (15.8%) | 3 (13.6%) | 19 (16.2%) |
| PDD-NOS | 13 (46.4%) | 4 (26.7%) | 9 (47.4%) | 8 (36.4%) | 46 (39.3%) |
| Male | 23 (82.1%) | 10(66.7%) | 18 (94.7%) | 17 (77.3%) | 99 (84.6%) |
| Race | |||||
| Caucasian | 24 (85.7%) | 9 (60.0%) | 15 (79.0%) | 18 (81.8%) | 95 (81.2%) |
| African American | 1 (3.6%) | 3 (20.0%) | 1 (5.3%) | 3 (13.6%) | 10 (8.5%) |
| Other | 0 (0%) | 1 (6.7%) | 1 (5.3%) | 0 (0%) | 3 (2.6%) |
| Multiracial | 3 (10.7%) | 2 (13.3%) | 2 (10.5%) | 1 (4.6%) | 9 (7.7%) |
| Income | |||||
| <$60,000 | 16 (57.1%) | 8 (53.3%) | 6 (31.6%) | 8 (36.4%) | 51 (43.6%) |
| >$60,000 | 12 (42.9%) | 7 (46.7%) | 13 (68.4%) | 14 (63.6%) | 66 (56.4%) |
| School Placement | |||||
| Regular Ed | 12 (42.9%) | 8 (53.3%) | 10 (52.6%) | 14 (63.6%) | 54 (46.2%) |
| Special Ed | 16 (57.1%) | 7 (46.7%) | 9 (47.4%) | 8 (36.4%) | 63 (53.9%) |
| ADHD CGI-S | |||||
| Moderate | 9 (32.1%) | 3 (20.0%) | 4 (21.1%) | 7 (31.8%) | 29 (24.8%) |
| Marked | 16 (57.1%) | 11 (73.3%) | 9 (47.4%) | 6 (27.3%) | 61 (52.1%) |
| Severe/Extreme | 3 (10.7%) | 1 (6.7%) | 6 (31.6%) | 9 (40.9%) | 27 (23.1%) |
| Noncompliance CGI-S | |||||
| Mild | 2 (7.1%) | 0 (0%) | 1 (5.3%) | 1 (4.6%) | 5 (4.3%) |
| Moderate | 14(50.0%) | 9(60.0%) | 9 (47.4%) | 10(45.5%) | 61 (52.1%) |
| Marked/Severe | 12(42.9%) | 6(40.0%) | 9(47.4%) | 11(50.0%) | 51 (43.4%) |
Note. ADHD = attention-deficit/hyperactivity disorder; ATX = atomoxetine; CGI-S = Clinical Global Impressions–Severity; OL = open label; PBO = placebo; PDD-NOS = pervasive developmental disorder not otherwise specified; PT = parent training.
29 participants in the extension were ATX nonresponders; their data are summarized in the supplemental materials. Four participants were not in any subgroup (2 ATX+PT nonresponders continued ATX; 1 PT and 1 Placebo only Nonresponders took alternative medication).
Effects of PT vs. no-PT on Behavioral Noncompliance
The 64 PT participants attended a mean of 11.9± 2.6 sessions from baseline (week 0) to week 22. Fidelity of PT clinicians was >80% in 90% of 81 sessions rated by study investigators (B.H. and T.S.); parent adherence averaged 86%. Seven PT participants and 4 no-PT participants did not enter the extension; 2 PT participants and 5 no-PT participants exited the extension before week 22 (Figure 1). LOCF was used to analyze data from these 18 participants; thus, their data were retained in the analysis even though they dropped out prior to week 10 or week 22. Table 2 displays the mean and standard deviation for each outcome measure for the PT and no-PT groups at baseline (week 0), end of acute trial (week 10 or last visit in the acute trial), and primary endpoint (week 22 or LOCF). As shown in the table, improvement was greater for PT than no-PT on all outcome measures, but not statistically significant on any measure based on the LOCF analysis or the mixed model for repeated measures without LOCF.
Table 2.
Mean and Standard Deviation on Outcome Measures for Participants in Parent Training (PT; n = 64) and No-PT (n = 64)
| Outcome | Treatment | Baseline | Week 10a | Week 22a | PT vs no PT est (SE); p-valueb |
|---|---|---|---|---|---|
| HSQ Severity | No PT | 3.84 (1.70) | 2.54 (1.77) | 2.03 (1.65) | 0.18 (0.25); p=.47 |
| PT | 3.91 (1.59) | 2.52 (1.83) | 1.97 (1.56) | ||
| Parent-completed SNAP-IV | |||||
| ADHD | No PT | 2.19 (0.48) | 1.59 (0.74) | 1.30 (0.65) | 0.12 (0.11); p=.28 |
| PT | 2.21 (0.37) | 1.46 (0.69) | 1.30 (0.66) | ||
| Inattention | No PT | 2.28 (0.47) | 1.68 (0.75) | 1.35 (0.65) | 0.09 (0.12); p=.43 |
| PT | 2.25 (0.47) | 1.47 (0.72) | 1.30 (0.65) | ||
| Hyperactivity | No PT | 2.10 (0.66) | 1.50 (0.86) | 1.26 (0.77) | 0.14 (0.12); p=.24 |
| PT | 2.17 (0.48) | 1.44 (0.78) | 1.30 (0.79) | ||
| Parent-completed ABC | |||||
| Irritability | No PT | 16.48 (9.02) | 12.48 (8.84) | 10.97 (8.72) | 2.58 (1.46); p=.08 |
| PT | 18.02 (9.17) | 12.36 (9.21) | 9.91 (8.01) | ||
| Hyperactivity | No PT | 30.28 (9.79) | 21.44 (12.99) | 17.63 (11.46) | 1.42 (1.74); p=.42 |
| PT | 30.63 (8.81) | 20.92 (12.06) | 16.91 (10.49) | ||
Note. ABC = Aberrant Behavior Checklist; ADHD = attention-deficit/hyperactivity disorder; ATX = atomoxetine; CGI-S = Clinical Global Impressions – Severity; HSQ = Home Situations Questionnaire; PDD-NOS = pervasive developmental disorder not otherwise specified; SE = standard error; SNAP-IV = Swanson, Nolan, and Pelham Fourth Edition.
Week 10 mean and standard deviation were estimated based on last observation carried forward (LOCF) for all 128 participants treated at acute trial, and Week 22 data was estimated based on LOCF for 117 participants treated at extension.
Estimated mean (est.) and SE of the difference between PT and no-PT groups in amount of change from baseline to week 22 (or last visit before week 22, LOCF); p-value for the pairwise comparison of change in PT vs no-PT groups based on the mixed model with LOCF. Positive value of estimate suggests greater improvement in PT than no-PT.
Status of Acute Trial Responders at Week 34
Of the 43 Responders at the end of the acute trial (week 10), 26 (60%) continued to meet responder criteria at the end of the double-blind extension (week 34). Table 3 shows responder status at week 34 for participants in each of the four originally assigned groups. Although not significant, the two ATX groups were more likely than the two placebo groups to maintain responder status (67%, 69% vs. 44%, 50%). The PT groups were slightly less likely than the no-PT groups to maintain responder status (67%, 44% vs. 69%, 50%). Table S1 (available online) summarizes change from baseline (week 0) to endpoint (week 34) on the primary outcome measures for ADHD (SNAP-IV) and noncompliance (HSQ) for all 43 responders in each of the four originally assigned groups. Across groups and measures, the mean improvement in scores was 52–74%.
Table 3.
Outcomes of Treatment Responders at Week 10 (n = 43) in the 24-Week Extension
| Treatment Group | Non-Responders | Responders at Week 34 | |
|---|---|---|---|
| Dropped Out/Changed Txa | Nonresponders at Week 34 | ||
| ATX+PT (n=12) | 3 | 1 | 8 (67%) |
| ATX (n=16) | 5 | 0 | 11 (69%) |
| PT+PBO (n=9) | 4 | 1 | 4 (44%) |
| PBO (n=6) | 2 | 1 | 3 (50%) |
Note: ATX = atomoxetine; PBO = placebo; PT = parent treatment; Tx = treatment.
Reasons for dropping out or changing treatments: behavioral deterioration (n = 4), side-effects (n = 8), or parent request (n = 2); 6 at week 14, 1 at week 18, 1 at week 20, 3 at week 22, 1 at week 26, and 2 at week 30.
Open-Label ATX for Placebo Nonresponders
Of the 41 placebo nonresponders at the end of the acute trial (week 10) who entered the 10-week open-label trial, 24 (59%) completed it. Seventeen participants exited the open trial early because of non-response or side-effects; 16 of these remained in the extension, receiving alternative medications. The data obtained after exiting open-label ATX treatment were used only in sensitivity analyses. Primary analyses are based on data obtained while participants were receiving ATX without additional medications. The mean open-label/ATX dose at exit from the trial was 37.7 ± 17.4 (1.16 ± 0.44mg/kg).
Fifteen of the 41 placebo nonresponders (37%) had favorable ADHD outcomes at endpoint of the open-label trial (week 20). Ten of 19 (53%) who had received PT in the acute trial and continued PT in the open label (open-label ATX+PT) and 5 of 22 (23%) who received open-label ATX-only (Fisher exact p = .06) were ADHD responders. Fourteen of 41 (34%) met criteria for noncompliance response. The rate of favorable noncompliance outcome was significantly higher (p=.01) for open-label ATX+PT (11 of 19, 58%) than open-label ATX (3 of 22, 14%).
Table 4 summarizes data at entry into the study (week 0), open-label baseline (week 10), and open-label endpoint (week 20 or LOCF) for the primary ADHD and noncompliance outcome measures (SNAP-ADHD and HSQ, respectively). The table shows that both open-label ATX+PT and open-label ATX-only significantly reduced scores on SNAP-ADHD, SNAP-Inattention, SNAP-Hyperactivity, HSQ, and ABC-Hyperactivity. Pre-post effect sizes were 0.33–0.82. Improvements in open-label ATX+PT were slightly larger than in open-label ATX-only from week 10 to week 20, although not statistically significant. However, open-label ATX+PT was statistically better than open-label ATX from baseline to week 20 in both SNAP-ADHD and HSQ. Figures S1 and S2 (available online) display the data at each time point from week 0 to week 22.
Table 4.
Means and Standard Deviations (SDs) for Primary and Secondary Variables at Baseline (Week 10 or Last Observation Carried Forward [LOCF]) and Endpoint (Week 20 or LOCF) for Participants in Open-Label Atomoxetine (ATX) Alone or Open-Label ATX Combined With Parent Training (PT)
| Baseline (Week 0) | Baseline (Week 10) | Week 20 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | ATX+PT (n=19) | ATX (n=22) | ATX+PT (n=19) | ATX (n=22) | ATX+PT (n=19) | ATX (n=22) | Source of Significance and ES |
| (1) | (2) | (3) | (4) | (5) | (6) | ||
|
| |||||||
| SNAP Parent | |||||||
|
| |||||||
| ADHD | 2.22 (0.38) | 2.21 (0.53) | 1.78 (0.65) | 2.18 (0.56) | 1.42 (0.73) | 1.85 (0.78) | 1:5***, 2:6**, 1:5 vs 2:6*, 3:5*a, 4:6*b |
|
| |||||||
| Inattention | 2.22 (0.51) | 2.31 (0.48) | 1.75 (0.75) | 2.27 (0.50) | 1.42 (0.77) | 1.93 (0.77) | 1:5***, 2:6**, 1:5 vs 2:6*, 3:5*c, 4:6*d |
|
| |||||||
| Hyperactivity | 2.21 (0.46) | 2.11 (0.76) | 1.80 (0.73) | 2.08 (0.79) | 1.43 (0.81) | 1.76 (0.89) | 1:5***, 2:6*, 1:5 vs 2:6*, 3:5*e, 4:6*f |
|
| |||||||
| ODD | 1.34 (0.71) | 1.19 (0.73) | 0.97 (0.72) | 1.02 (0.76) | 0.91 (0.87) | 1.05 (0.72) | 1:5* |
|
| |||||||
| HSQ Severity | 3.78 (1.48) | 4.00 (1.77) | 2.96 (1.94) | 3.73 (1.78) | 1.98 (1.51) | 3.11 (2.00) | 1:5***, 2:6**, 1:5 vs 2:6*, 3:5**g, 4:6*h |
|
| |||||||
| ABC Parent | |||||||
|
| |||||||
| Irritability | 17.00 (9.10) | 17.45 (8.89) | 13.84 (10.30) | 15.64 (9.38) | 12.72 (10.52) | 15.33 (11.61) | 1:5* |
|
| |||||||
| Hyperactivity | 30.89 (10.35) | 31.23 (10.80) | 28.26 (11.66) | 30.23 (12.37) | 18.83 (11.50) | 25.14 (13.73) | 1:5***, 2:6**, 1:5 vs 2:6*, 3:5***i, 4:6*j |
Note. For teacher rated outcomes, true baseline (week 0) sample sizes were as follows: ATX+PT n=18; ATX n=21. At Baseline of the extension (week 10) sample sizes were: ATX+PT n=16; ATX n=21. Sample sizes at week 20 or LOCF were: ATX+PT n=18; ATX n=21. Two participants did not have data for these scales to LOCF after week 10 to the week 20 endpoint. In the Source of Significance column, numbers refer to columns. For example, 1:5 refers to a comparison of the data in column 1 versus the data in column 5; 1:5 vs 2:6 refers to a comparison of the data in columns 1 and 5 versus the data in columns 2 and 6. ABC = Aberrant Behavior Checklist; ADHD = attention-deficit/hyperactivity disorder; ES= effect size; HSQ = Home Situations Questionnaire; ODD = oppositional defiant disorder; SNAP-IV = Swanson, Nolan, and Pelham Fourth Edition.
ES=0.54;
ES=0.59;
ES=0.44;
ES=0.68;
ES=0.52;
ES=0.41;
ES=0.53;
ES=0.33;
ES=0.82;
ES=0.39
p< .05,
p< .01,
p< .001
Regarding side effects in the open trial, gastrointestinal concerns were reported for most participants, including appetite decrease (54%), nausea (32%), vomiting (32%), constipation (20%), abdominal pain (17%), and diarrhea (15%). Headache (39%), labile mood (32%), and sleep difficulties (24%) or fatigue (27%) were also common. Aggression and irritability were each reported for 12% of participants. Side effects will be examined in detail in a future report.
DISCUSSION
This article reports a 24-week extension of only the second RCT to examine combined drug and psychosocial treatment in ASD, in this case ATX and PT. Retention was high, with 117 of 128 acute-trial participants (91%) continuing in the extension. Responders in the 10-week RCT were maintained in the double-blinded treatment during the extension, providing the longest blinded evaluation of ATX in ASD to date.12,27 In addition, nonresponders to placebo in the acute trial received open-label ATX. All participants who received PT in the acute trial continued in PT during the extension, allowing for tests of longer-term effects of PT. The resolution to our three questions was as follows.
Effects of PT vs. no-PT on Behavioral Noncompliance
Our ITT analyses, based on randomization at baseline for all participants who entered the study, did not show a significant advantage of PT over no-PT. The analyses were designed to be adequately powered to compare PT and no-PT16 and used LOCF to include data from all participants. Nevertheless, the ITT analyses were compromised because most participants changed medications at least once during the extension. Although clinically appropriate, the changes prevented a test of the effects of PT by itself or in combination with a constant, single medication during the extension.
Given the limitations of the ITT analysis, we examined PT in subgroups of the extension (responders, placebo nonresponders, and ATX nonresponders). Although we did not see an effect for PT in responders or ATX nonresponders, there appeared to be benefits of PT for placebo nonresponders who received open-label ATX during the extension. Participants receiving open-label ATX+PT were significantly more likely to be noncompliance responders (from original baseline) than participants receiving open-label ATX alone. They also made nominally larger improvements on parent rating scales. Thus, PT may have augmented ATX in the placebo nonresponder subgroup. This subgroup provided the cleanest test of PT effects in the extension because medications were held constant and PT continued long enough, even though the test had low statistical power because of the small sample in the open-label trial.
Despite evidence of benefit for PT in some participants, it must be acknowledged that PT effects were inconsistent. In contrast, two other RCTs, which enrolled a total of 304 participants and used variations of the same PT manual that we used, indicated that PT is efficacious in reducing noncompliance.10–11 A possible reason for the discrepancy is that participants were eligible for the current study regardless of their baseline level of noncompliance. Accordingly, we enrolled some participants who had minor issues with compliance and thus had little room for improvement, leading to ceiling effects. Another possible reason is that nonresponding participants changed medications after 10 weeks. Although overall medication use appeared similar in the PT and no-PT groups, medication changes could have obscured PT effects. An additional possibility is the too-early or too-large reduction in frequency of PT. PT sessions occurred weekly in the acute study but only every four weeks in the extension. Indeed, compared to the no-PT groups, the PT groups unexpectedly improved significantly more at week 10 of the study (the end of the acute trial).
This is the first RCT to evaluate effects of PT on ADHD in children with ASD. The absence of a significant benefit on ADHD symptoms may result from our PT program not including behavioral strategies specifically designed to target ADHD symptoms such as self-management and dense schedules of reinforcement for staying on task. ADHD symptoms also may be less responsive to PT than other problem behaviors such as tantrums or aggression because they are less influenced by the social environment (e.g., parental discipline) and more by dispositional factors (e.g., preference for immediate reinforcement). Consistent with this view, although some studies show that PT can be efficacious for ADHD in children without ASD, particularly in preschoolers28 and in children without co-occurring conditions,29 the findings are not as clear and consistent as the findings on this intervention for other disruptive behaviors.28
Status of Acute Trial Responders at Week 34
Most responders (26 of 43, 60%) maintained their status through the end of the 24-week extension. Maintenance of responder status was especially high in ATX responders (19 of 28, 68%), suggesting that most (though not all) who benefit initially will continue to show benefits.
Open-Label ATX for Placebo Nonresponders
More than a third (37%) of placebo nonresponders achieved favorable ADHD outcomes in the open trial; 34% achieved favorable noncompliance outcomes. The 37% response rate compares with 60% in one small (N= 16) crossover RCT and 21% in a larger (N=97) parallel groups RCT in children with ASD.13 Response rates, dosing, and adverse events (AEs) in the extension were similar to those observed for ATX in the acute trial,17 indicating that ATX was effective for many participants even when placebo responders were removed from the sample.
A limitation is that the design required a complicated approach to analysis. One such complication was the use of different endpoints for different analyses (week 34 for examining maintenance of responder status, week 22 for comparing PT vs. no-PT, and week 20 for evaluating open-label ATX). These endpoints were selected based on a priori evidence from other studies that time to effect would be longer for PT than for ATX.14 Another complication was the sectioning of the sample into subgroups analyzed in different ways. We needed to do this because we intended the extension to answer several different questions rather than simply follow responders for 6 months to see if improvement was maintained. For example, in order to check for delayed effect of PT, it was necessary to retain as many participants as possible in the extension, including nonresponders at week 10. In order to maximize retention, the design had to allow for medication changes. Nevertheless, multiple medication changes may have confounded the comparison of PT versus no PT. Another limitation is that the use of two CGI outcomes could have led to confusion of the ADHD and noncompliance constructs when assigning separate CGI ratings. Other limitations include low power to detect differences among small subgroups in the extension, limited racial and ethnic diversity in the sample, and heavy reliance on rating scales as outcome measures.17
We followed children for eight months and examined many scenarios that clinicians routinely face in clinical practice. We found that most ATX responders maintain their response status over time. This confirms our conclusion from the acute trial that ATX is a first-line medication for ADHD symptoms in ASD. We also found that open-label ATX + PT surpassed open-label ATX-only. Because PT began prior to the open-label trial, this finding suggests that a viable treatment option is to start with PT-only and add ATX if the response is insufficient, consistent with findings on TD children with ADHD.30 However, we did not observe significant incremental improvement from beginning ATX and PT simultaneously in the acute trial. This finding is inconsistent with our original hypothesis.16 Although the finding could reflect inadequate statistical power or a scientific aberration of this study, it is the outcome we observed. A growing literature attests to the complementary benefits accruing from combined drug and behavioral treatments,10 so the challenge is to identify effective combinations for children with ASD and ADHD.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health to Ohio State University (5R01MH079080), University of Pittsburgh (5R01MH079082-05), and University of Rochester (5R01 MH083247), by Eli Lilly and Co., who provided atomoxetine and placebo, by the University of Rochester CTSA (UL1 RR024160) and the Ohio State University CTSA (UL1TR001070), from the National Center for Research Resources, and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Dr. Pan served as the statistical expert for this research.
The authors gratefully acknowledge the guidance and supervision of the data safety monitoring board, consisting of Edwin H. Cook, Jr., MD (University of Illinois at Chicago), Walter J. Meyer, MD (University of Texas-Galveston), Carson R. Reider, PhD (Ohio State University), and Wesley K. Thompson, PhD (University of California-San Diego).
Clinical trial registration information—Atomoxetine, Placebo and Parent Management Training in Autism (Strattera); http://clinicaltrials.gov/; NCT00844753.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented as a poster at the International Meeting for Autism Research, Salt Lake City, UT, May 13–16, 2015.
Disclosure: Dr. Aman has received research contracts, consulted with, served on advisory boards, or done investigator training for AMO Pharma Ltd., CogState, Inc., Confluence Pharmaceutica, CogState Clinical Trials, Ltd., Coronado Biosciences, Forest Research, Hoffman-La Roche, Lumos Pharma, MedAvante, Inc., ProPhase LLC, and Supernus Pharmaceuticals. Dr. Arnold has received research funding from Curemark, Forest, Eli Lilly and Co., Neuropharm, Novartis, Noven, Shire, Supernus, YoungLiving, NIH, and Autism Speaks; has consulted with or been on advisory boards for Arbor, Gowlings, Ironshore, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint; and has received travel support from Noven. Dr. Hollway has received financial support from Forest Research Institute, Supernus Pharmaceuticals, Sunovion, YoungLiving, and Autism Speaks. Dr. Tumuluru has received research funding from Roche, Curemark, Eli Lilly and Co., Autism Speaks, and NiA Pharmaceuticals. Dr. Hellings has served as an investigator for Autism Speaks-ATN, Forest, Shire, Sunovion, Supernus, YoungLiving Essential Oils, and Roche. Dr. Handen has received research funding from Roche, Curemark, Eli Lilly and Co., Autism Speaks, and NiA Pharmaceuticals. Drs. Smith, Silverman, Lecavalier, Hyman, Rice, Jr., Pan, and Mss. Buchan-Page and Brown report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Rosenberg RE, Kaufmann WE, Law JK, Law PA. Parent report of community psychiatric comorbid diagnoses in autism spectrum disorders. Autism Res Treatment. 2011;18:2011–20. doi: 10.1155/2011/405849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaat AJ, Lecavalier L. Disruptive behavior disorders in children and adolescents with autism spectrum disorders: A review of the prevalence, presentation, and treatment. Research in Autism Spectrum Disorders. 2013;7:1579–1594. [Google Scholar]
- 3.Lecavalier L, Leone S, Wiltz J. The impact of behavior problems on caregiver stress in young people with autism spectrum disorders. J Intell Disabil Res. 2006;50:172–183. doi: 10.1111/j.1365-2788.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 4.Sikora DM, Vora P, Coury DL, Rosenberg D. Attention-deficit/hyperactivity disorder symptoms, adaptive functioning, and quality of life in children with autism spectrum disorder. Pediatrics. 2012;130:S91–S97. doi: 10.1542/peds.2012-0900G. [DOI] [PubMed] [Google Scholar]
- 5.Research Units on Pediatric Psychopharmacology Autism Network A randomized, double-blind, placebo-controlled, crossover trial of methylphenidate in children with hyperactivity associated with pervasive developmental disorders. Arch General Psychiat. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- 6.Arnold LE. Contemporary Diagnosis and Management of Attention-Deficit/Hyperactivity Disorder. 3rd ed. Handbooks in Health Care Co.; Newtown, PA: 2004. [Google Scholar]
- 7.Arnold LE. Methylphenidate vs. Amphetamine: Comparative Review. J Attention Disorders. 2000;3:200–211. [Google Scholar]
- 8.Handen BL, Johnson C, Lubetsky M. Efficacy and safety of methylphenidate in children with autistic disorder. J Autism Dev Disord. 2000;35:245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- 9.Wong C, Odom SL, Hume K, et al. Evidence-Based Practices for Children, Youth, and Young Adults with Autism Spectrum Disorder. The University of North Carolina, Frank Porter Graham Child Development Institute, Autism Evidence-Based Practice Review Group; Chapel Hill: 2014. [Google Scholar]
- 10.Aman MG, Mcdougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Psychiatry. 2009;48:1143–1154. doi: 10.1097/CHI.0b013e3181bfd669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bearss K, Johnson C, Smith T, et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. JAMA. 2015;313:1524–1533. doi: 10.1001/jama.2015.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz S, Correll C. Efficacy and safety of atomoxetine in children and adolescents with attention deficit/hyperactivity disorder: Results from a comprehensive meta-analysis and metaregression. J Am Acad Child Psychiatry. 2014;53:174–187. doi: 10.1016/j.jaac.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Aman MG, Smith T, Arnold LE, et al. A Review of Atomoxetine Effects in Young People with Developmental Disabilities. Res Dev Disabil. 2014;35:1412–1424. doi: 10.1016/j.ridd.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans SW, Owens J, Bunford MN. Evidence-Based Psychosocial Treatments for Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J Clin Child Adolesc. 2014;43:527–551. doi: 10.1080/15374416.2013.850700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlong M, McGilloway S, Bywater T, et al. Behavioural and cognitive-behavioural group-based parenting programmes for early-onset conduct problems in children aged 3 to 12 years. The Cochrane database of systematic reviews. 2012;2:CD008225. doi: 10.1002/14651858.CD008225.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Silverman L, Hollway JA, Smith T, et al. A Multisite Trial of Atomoxetine and Parent Training in Children with Autism Spectrum Disorders: Rationale and Design Challenges. Res Autism Spectr Disord. 2014;8:899–907. doi: 10.1016/j.rasd.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handen BL, Aman MG, Arnold LE, et al. Atomoxetine, Parent Training, and Their Combination in Children With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:905–915. doi: 10.1016/j.jaac.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy W. ECDEU Assessment Manual of Psychopharmacology. National Institute of Mental Health US Dept. of Health, Education, and Welfare publication (ADM), Psychopharmacology Research Branch; Rockville, MD: 1976. pp. 76–338. [Google Scholar]
- 19.Barkley RA, Edelbrock C. Assessing situational variation in children's problem behaviors: the Home and School Situations Questionnaires. In: Prinz R, editor. Advances in Behavioral Assessment of Children and Families. JAI Press Inc; Greenwich, CT: 1987. pp. 157–176. [Google Scholar]
- 20.Chowdhury M, Aman MG, Scahill L, et al. The Home Situations Questionnaire–PDD Version: Factor structure and Psychometric Properties. J Intell Disabil Res. 2010;54:281–91. doi: 10.1111/j.1365-2788.2010.01259.x. [DOI] [PubMed] [Google Scholar]
- 21.Roid GH. Stanford-Binet Intelligence Scales. 5th ed. Riverside Publishing; Itasca, IL: 2003. [Google Scholar]
- 22.Mullen EJ. Mullen Scales of Early Learning. Pearson Assessments; Bloomington, MN: 1995. [Google Scholar]
- 23.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview Revised. Western Psychological Services; Torrance, CA: 2003. [Google Scholar]
- 24.Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Def. 1985;89:485–491. [PubMed] [Google Scholar]
- 25.Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. Am J Ment Def. 1985;489:492–502. [PubMed] [Google Scholar]
- 26.Aman MG, Singh NN, Turbott SH. Reliability of the Aberrant Behavior Checklist and the effect of variations in instructions. Am J Men Def. 1987;92:237–240. [PubMed] [Google Scholar]
- 27.Harfterkamp M, Buitelaar JK, Minderaa RB, et al. Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: an open-label extension study. J Child Adoles Psychop. 2013;23:194–199. doi: 10.1089/cap.2012.0012. [DOI] [PubMed] [Google Scholar]
- 28.Webster-Stratton C. Combining parent and child training for young children with ADHD. JCCAP. 2011;40:191–203. doi: 10.1080/15374416.2011.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PC, Niew WI, Yang HJ, et al. A meta-analysis of behavioral parent training for children with attention deficit hyperactivity disorder. RIDD. 2012;33(6):2040–2049. doi: 10.1016/j.ridd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Fabiano GA, Pelham WE, Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O'Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.