Abstract
The complement system is an evolutionarily ancient component of immunity that revolves around the central component C3. With the recent description of intracellular C3 stores in many types of human cells, our view of the complement system has expanded. In this article, we hypothesize that a primitive version of C3 comprised the first element of the original complement system and initially functioned intracellularly and on the membrane of single-cell organisms. With increasing specialization and multicellularity, C3 evolved a secretory capacity that allowed it to play a protective role in the interstitial space. Upon development of a pumped circulatory system, C3 was synthesized in large amounts and secreted by the liver to protect the intravascular space. Recent discoveries of intracellular C3 activation, a C3-based recycling pathway and C3 being a driver and programmer of cell metabolism suggest that the complement system utilizes C3 to guard not only extracellular but also the intracellular environment. We predict that the major functions of C3 in all four locations (i.e., intracellular, membrane, interstitium and circulation) are similar: opsonization, membrane perturbation, triggering inflammation and metabolic reprogramming.
Keywords: C3, evolution, intracellular complement system, C3 recycling
The complement system
The complement system of higher vertebrates consists of nearly 60 fluid-phase and membrane proteins. The plasma components are part of proteolytic cascades and function to protect the host from microbes, remove debris and promote cell survival (1-3). The complement system’s essential role in innate immune protection is established. However, it’s now beginning to be understood that complement effector functions extend beyond the detection and destruction of bacterial and viral invaders. For example, increasing evidence points to a greater role than anticipated in adaptive immune responses (4-9), the “safe” handling of the host’s own garbage (10-12) and intracellular metabolic programming (13, 14).
At the heart of this immune system is its central and most abundant plasma component, C3. C3 cleavage by a protease (C3 convertase) is the convergence point of all three complement activation pathways (classical, alternative and lectin). C3 is composed of an alpha (α)- and a beta (β)-chain covalently linked by a disulfide bond (and also coupled by non-covalent forces). Proteolytic cleavage of the C3 α-chain by highly specific C3 convertases produces C3a (key inflammatory mediator) and C3b (major opsonin of the system). C3 can also be cleaved albeit generally less efficiently and specifically by proteases other than a C3 convertase, such as trypsin, plasmin (15, 16), thrombin (17), cathepsin L (14) and elastase (18, 19), resulting in C3b and C3a, as well as additional cleavage fragments.
C3 is a member of the evolutionarily old family of thioester containing proteins that includes α2-macroglobulin, C4 and CD109. It has been identified in organisms as early as sponges (porifera) (20). The internal thioester is protected by hydrophobic interactions in the native molecule and, upon cleavage of C3, a dramatic conformational change occurs that exposes the reactive thioester. This mechanism is a creative means for allowing a plasma protein to covalently attach to target surfaces by ester or amide linkages.
The complement system was originally identified as an effector arm of adaptive humoral (antibody-mediated) immunity (1, 2). Over 60 years later, it was realized that complement could be activated without a requirement for antibody recognition; i.e., the discovery of the alternative pathway (AP) identified an Ab independent function for complement in immunity (21-23). In addition, the lectin pathway was discovered about 20 years later as a third cascade in which lectins targeting pathogens and debris engaged this powerful effector system (24).
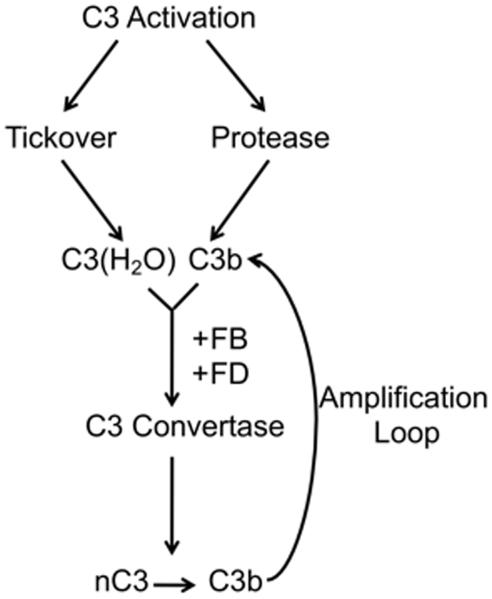
The thioester in the native C3 protein is subject to nucleophilic attack. In plasma, C3 ticks-over at a rate of 1-2% per hour to produce C3 with a hydrolyzed thioester bond known as C3(H2O) (22, 25). Although C3(H2O) can no longer covalently attach to a target surface, it is not inert. C3(H2O) serves as an initiator of the AP via its feedback loop (see Fig. 1). In this way, C3 serves as its own trigger to activate and amplify on foreign surfaces. The membrane expression of complement regulatory proteins (CD46, CD55, CD35) limits C3b deposition on healthy self as it is promptly inactivated relative to its ability to engage the AP’s feedback loop. The absence of such proteins on foreign cells allows for amplification by the AP [reviewed in (22, 23, 26)]. C3(H2O) can also be proteolytically modified to generate C3a (27) and possibly other C3 fragments that have biological activity.
Figure 1. Alternative pathway activation and amplification.
nC3, native C3; AL, amplification loop; FB, Factor B; FD, Factor D.
Recently, studies have broadened the role of complement and have shifted our perspective on it functionalities. Instead of the older view of complement as operating almost exclusively outside of the cell, new evidence points to major roles in the cell interior. In this inner universe of interactions, C3 has several roles that we are only beginning to understand: a role for C3 and C3(H2O) stores, intracellular processing of C3b-coated pathogens, C3 as a controller of cellular reprogramming, and C3’s involvement in a complement-metabolism-inflammasome axis. Aside from many intracellular interactions within these mechanisms, C3 stores can be activated in a convertase-independent manner, suggesting that intracellularly C3 functions independent of other complement proteins. In this article we speculate that the complement system evolved from a primarily cell interior role to the modern day effector system of blood as well as retaining and broadening its intracellular connections. C3 is arguably the original complement system protein.
C3 evolution
The C3 protein likely arose, possibly in parallel, from common evolutionary origins via gene duplications of the structurally-related α2-macroglobulin protein (28, 29). The complement system was thought to be exclusive to vertebrates until the identification of a homologue of C3 in sea urchin coelomocytes (30). Subsequently, the absence of a C3 gene was documented in Drosophila, suggesting that the C3 gene arose somewhere in the deutrosome lineage (31). However, a recent report identified a gene for a C3-like protein in the sponge (Phylum porifera), indicating that the origins of the complement system are more ancient (20). Interestingly, even in the sponge, genes for Factor B and mannose binding lectin-associated serine proteases (MASPs) were identified indicating that a primitive AP and possibly a LP existed in these organisms (20). Importantly, to generate an AP convertase to cleave C3 requires only C3b, Factor B (FB) and Factor D (FD) (Fig. 1). Notably, the AP can proceed albeit relatively inefficiently in the absence of FD, suggesting that other non-specific proteases can substitute (32).
C-reactive protein (CRP) and β-amyloid activate the CP by binding to C1q. CRP is highly conserved through evolution and, prior to the emergence of Ab, CRP and related proteins including lectins, may likely have filled that role. These systems though lack memory, an enormous advantage of the adaptive immune system as well as the development of a second great opsonin, Ab. Of note, IgM and IgG then took advantage of the prior effector capabilities of the complement system. The AP and lectins predate in evolution immunoglobulins (Ig), which did not occur until the emergence of vertebrates (33). Immunoglobulins have been identified in bony fish but they are absent in the lamprey and hagfish [reviewed in (34)]. The development of Ig provided an important advantage since the recognition mechanism of the AP did not provide memory or specificity. The blood complement system evolved with the appearance of Ig to join its powerful effector system to the newly developing recognition systems of adaptive immunity. In line with this concept, the appearance of a CP of complement activation is not seen until the cartilaginous fishes (35, 36).
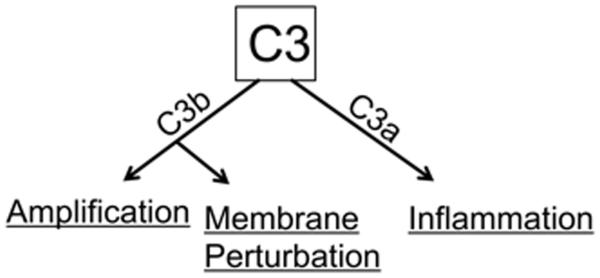
Selected aspects of the evolution of the complement system have been reviewed (37-39). In this article, we speculate on how the complement system accomplished at the protein level to protect an evolving host. We propose that this occurred in four major steps. The original complement system was an intracellular opsonic and cell-activating cascade of putatively even single-cell organisms (40). Next, the complement system was adapted to also function on plasma membranes and subsequently to be secreted into the surrounding milieu (the intercellular space). With the evolution of circulatory systems coupled with hepatocellular synthesis of most complement components, the modern day “guardian of the intravascular space” arose (Fig. 2). At all four sites, the functions are predicted to be the same– recognition of foreignness and altered self; opsonization and lysis of membranes; and induction of proinflammatory responses (Fig. 3).
Figure 2. Steps in the evolution of the complement system.

Four environments that C3 adapted to function in to defend the evolving host.
Figure 3. Functions of C3.
C3 activation produces C3b and C3a. C3b amplifies the cascade through the AP amplification loop, is an opsonin and leads to cell lysis. C3a is an inflammatory mediator.
The original complement system
Evidence indicates that the appearance of a C3-like protein occurred at least a billion years ago (39). Currently, the earliest evidence for this is found in porifera (sponges) (30). Sponges are arguably the sister group of all extant animals (41). Sponges are considered living fossils and are relatively simple, multicellular organisms lacking true tissues or organs. They circulate water through their body by choanocyte cells, which closely resemble single-celled choanoflagellates, the ancestors of sponges. We speculate that a C3-like protein was present prior to the emergence of sponges from single-cell choanoflagellates– the original complement system.
C3 would have functioned independently in a single-cell organism, providing a mechanism for constant surveillance of invading microbes. Recent work has demonstrated that C3b opsonized pathogens entering the cytoplasm are both marked for destruction and signal protective inflammasome type responses (42). From the view of a single-cell organism, this establishes the possibility of an ancient system whereby C3 provides immune protection. One can envision C3, possibly being stored in vesicles, providing an intracellular arsenal capable of quick release at the site of attack for defense against pathogenic invaders. The C3b and/or C3a generated could serve as a membrane injury and cytoplasmic alarm system in which recognition of non-self would trigger a stress response leading to cellular activation. This primitive system could operate without additional complement components. The C3 could be inherently unstable upon “release” or be susceptible to a variety of intracellular proteases (see below).
Thus, the requirements for this intracellular system are a pathogen recognition event, a protease to cleave C3 and an intracellular receptor to engage C3b and/or C3a to raise the alarm. We speculate that lectins, inside the cell or possibly present on the membrane and also perhaps carried in with the pathogen, would have triggered C3 activation. Of note, the lectin pathway can be activated in the absence of C2 (43), suggesting that in primitive organisms, the AP was triggered by lectins (44). Alternatively, a pathogen may have been sensed by a surveillance mechanism inside the cell similar to that of the modern day AP; that is, tickover of C3 to C3(H2O) or C3(H2O) released from a store could be an intracellular monitoring system for danger. This scenario would have allowed for recognition of non-self (foreign) antigens in the cytosol.
Upon sensing danger, cleavage of C3 could have occurred by proteases inside the cell. Multiple “non-specific” proteases can cleave C3. For example, cathepsin L (CTSL) has been shown to cleave C3 intracellularly in CD4+ T cells (14). Once danger was sensed and C3 was activated, a receptor is needed inside the cell to sound the alarm. There is evidence for a C3b receptor in the cytosol of numerous cell types. For example, C3b opsonized pathogens entering the cytoplasm induce an inflammasome response (42). This requires both C3b opsonization and a proper cytosolic location, indicating that there is a C3b receptor in the cytosol. Intracellular stores of CR3 and CR1 have been identified in distinct intracellular locations in human neutrophils (45, 46). We hypothesize that these or related receptors also function inside cells. These receptors may be representative of the original recognition molecules for C3b-opsonized pathogens.
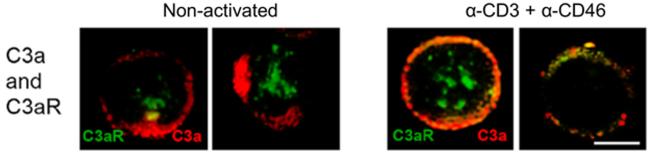
Intracellularly, C3a is also recognized. A C3aR homolog has been identified and functionally characterized in ascidians indicating that this is an evolutionarily old protein (47). Evidence for an intracellular functional C3aR derives from data demonstrating that C3aR/C3a interact and then translocate as a complex to the membrane following cell activation (14) (Fig. 4). Based on the ancient origin of the C3aR and its known intracellular functionality, we speculate that a C3aR-like protein operated to protect the host even in single-celled organisms.
Figure 4. C3a and C3aR are intracellular and translocate and colocalize upon CD4+ T cell activation.
Non-activated or anti-CD3 and anti-CD46 activated human peripheral blood CD4+ T cells were permeabilized and stained for C3a (red) and C3aR (green). Two representative examples of the migration of C3a and C3aR to the plasma membrane upon activation of human CD4+ T cells (right hand panel). Adapted from (14).
Another potentially ancient and key function of intracellular C3 is that of a metabolism modulator to support cell survival. This concept that C3 plays several somewhat distinct roles aligns with precepts of evolutionary biology purporting that nature conserves its most vital proteins by multi-tasking (48, 49). In addition to modulating the immune response (12, 14, 42), intracellular C3 supports cell survival and metabolism. In CD4+ T cells, the tonic intracellular cleavage of C3 is essential for cell survival (14). Also, autocrine C3b production and subsequent signaling through CD46 induces expression of the molecular machinery necessary for the metabolic reprogramming required to induce Th1 effector cells. Specifically, CD46 signaling drives expression of the amino acid transporter, LAT1, and the glucose transporter, GLUT1, and enhances glucose and amino acid uptake by T cells (13). Sensing of this nutrient influx by mTOR complex 1 (mTORC1) drives increased glycolysis and oxidative phosphorylation, supporting induction of Th1 effector cells (13). Although the idea that C3 plays a role, outside of host defense in cell survival and metabolism is relatively new, this may have been one of the original functions of C3-like proteins in single-cell organisms.
Cell membrane
As single cells evolved into multicellular organisms, the complement system needed to provide enhanced host defense against microbes. While C3 could have functioned relatively alone in a single-cell organism (both inside and on membrane), the development of multiple organ systems would have required a more complex approach to innate defense. The expansion of the complement system could have been designed to prevent microbes from entering the cellular environment in the first place. Evidence for this comes from work outlining an intracellular C3a/C3aR system in CD4+ T cells (14). Upon stimulation of CD4+ T cells via CD46 and CD3, C3a and C3aR translocate to the membrane where the complex induces a Th1 effector cell response (14). This membrane system required new activators, sensors (receptors) and regulators to protect the host. To meet these needs, additional intracellular complement components were required. As early as in the first multicellular animals, genes for Factor B and MASPs have been identified (20). This provided a primitive AP and potentially lectins that could direct C3 opsonization onto foreign membranes. Also, indirect evidence for a terminal pathway of complement first occurs in lancelets with the identification of a C6-like protein and lytic capacity of the humoral fluid (50). However, the earliest definitive presence of a terminal pathway is in the nurse shark (35). This points out that, in more primitive species, the complement systems main protective mechanism were opsonization and induction of inflammation. This required membrane receptors to recognize C3 fragments. Indeed, the appearance of membrane-bound complement receptors was an early evolutionary step in the complement system. An opsonic role for C3 has been demonstrated in tunicates (sea squirt), suggesting the presence of complement receptors in these primitive organisms (51). Genes for integrin-type receptors similar to CR3 and CR4 have been identified at least as early as in cnidarians (jelly fish, corals) (39).
A second important activity on the membrane is regulation. Each cell of a multicellular organism is, at least partially, dependent on the health of the other cells. This requires that the organism recognize and safely eliminate neighbors that are compromised. The AP of complement provides a constant surveillance mechanism to identify and remove altered and damaged self, in addition to patrolling for pathogens (1-3, 22). However, the lability of the thioester bond of C3 represents a dual-edged sword. Swift to attach to a foreign microbe or “tag” a damaged cell, it likewise robotically attacks such host cells. Thus, complement regulatory proteins evolved to regulate this system (reviewed in (2)). One ancient family is called the Regulators of Complement Activation (RCA, consists of a genetically-, structurally- and functionally-related set of regulators that interact with C3b and C4b (52). The family consists of complement receptor type one (CR1: CD35), complement receptor type two (CR2: CD21), membrane cofactor protein (MCP; CD46), decay accelerating factor (DAF: CD35), C4b binding protein (C4BP) and Factor H (and the family of related proteins).
One of the intriguing features of the RCA members is that they consist largely or exclusively of complement control protein (CCP) modules. They are composed of ~60 amino acids of which 10-18 are highly conserved, as well as four invariant cysteines that define the CCP architecture (a loop within a loop structure) (53). The genomic organization and clustering of RCA genes are diverse among species that include mice, chicken, frog and teleost (the most common group of ray-finned bony fish [reviewed in (54)]. Both phylogenetic and genomic evidence indicates the emergence of the RCA by the time of cyclostomes (the most ancient class of vertebrates). A CD46-like molecule has been identified and characterized in bony fish (54). Genes containing the complement control protein (CCP) domains common to human complement regulatory elements have been identified as early as in ascidians (55).
Intercellular space
In order to protect multicellular organisms, complement evolved to secrete C3 into the surrounding fluid. This occurred prior to the evolution of closed circulatory systems and would have been an essential defense mechanism to prevent binding of microbes by organisms such as sponges that obtain nutrients by pulling water through their open body cavity. The role of interstitial C3 is analogous to that of the complement system in the circulation being designed to trigger a local inflammatory reaction. In mammals, C3 is still produced locally and present in the interstitial fluid and current knowledge indicates that local and systemic C3 each have unique functions. For example, the donor tissue production of C3 primes T cells that mediate transplant rejection and contributes to ischemia reperfusion injury (6). Similarly, it has been demonstrated that humoral responses to T cell dependent antigens are enhanced by local C3 production (7). This may reflect local complement defense mechanisms that were developed early in evolution and retained in more complex animals.
Multiple gene copies of C3 and FB have been identified in several cnidarian species (56). The C3 proteins in these species have distinct binding affinities to complement activating surfaces (57). This may have allowed for a more diverse recognition system that expanded the range of pathogens C3 identifies. Additionally, the titers of AP components are much higher in these species and active at a broad range of temperatures (58). This system is probably less necessary in higher vertebrates with the development of more specific recognition molecules (Ig).
Guardian of the intravascular space
Upon development in evolution of a pumped circulatory system carrying nutrients (a perfect culture system for microorganisms, especially bacteria), a means to react rapidly to identify and destroy invading bacteria was necessary. Opsonized organisms are required to prevent and resolve bacteremia. Without C3 or phagocytes, particularly neutrophils, humans (especially newborns) die of sepsis.
Full circle (A recycling pathway)
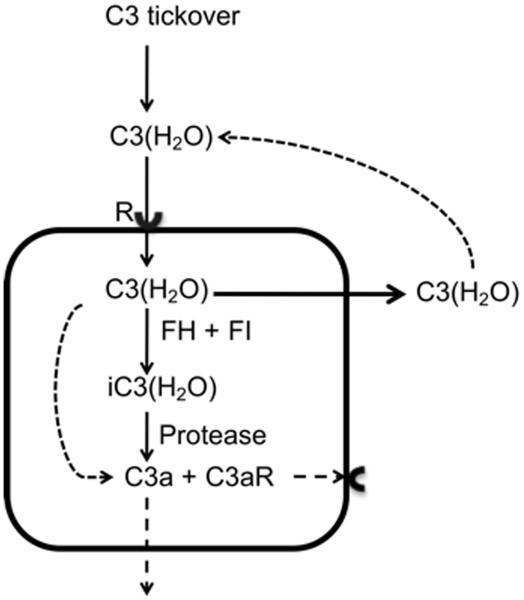
We have recently discovered that human cells recycle C3(H2O) from extracellular fluids (submitted for publication). We identified intracellular uptake and subsequent return of a significant portion of the loaded C3(H2O) to the extracellular space under steady state conditions. A portion of the C3(H2O) is metabolized producing C3a and potentially other biologically active fragments of C3 (Fig.5). With the evolutionary development of hepatocellular synthesis of C3, this uptake mechanism evolved to allow cells to continuously replenish intracellular C3 stores without the metabolic burden of synthesizing the C3. This also provides a continuous source of intracellular C3 in conditions where it is needed quickly (insufficient time for biosynthesis). Neutrophils represent an example of such a system; they contain stores of C3 (59) and properdin (60) that are rapidly secreted upon stimulation, facilitating directed AP amplification at sites of inflammation.
Figure 5. C3(H2O) recycling pathway.
C3(H2O) is continuously internalized by cells and a majority returned to the extracellular milieu. Under steady state conditions, a fraction of the C3(H2O) is metabolized intracellularly. Loaded FH (61) and FI (unpublished observation) act on the C3(H2O), resulting in iC3(H2O). Multiple intracellular proteases could also cleave the C3(H2O) and iC3(H2O) releasing C3a and likely other active fragments. The C3a and C3aR could be engaged to signal intracellularly, on the membrane, or in the interstitial space. R; receptor.
We propose that this work extends recent studies showing that C3b opsonized pathogens entering the cytoplasm activate an immune response (12, 42). Although these studies were performed in the absence of a human complement source, we hypothesize that, in the presence of C3(H2O), loaded cells opsonize pathogens inside the cell resulting in, or enhancing, protective responses. Although C3(H2O) can not covalently opsonize pathogens, it could function as a weak opsonin by associating with microbes via non-covalent forces (i.e., hydrophobic interactions) or by attaching to properdin and as a focus for initiating the AP.
Importantly, these results point to new roles for C3(H2O). In the extracellular environment, C3(H2O) activates the AP, is regulated by cofactor activity, and is loaded into cells. Loaded C3(H2O) is also regulated by cofactor activity and susceptible to proteases to generate C3 fragments ((61) and our unpublished results), including ones containing C3a, that may function intracellularly (Fig. 6). Our group, in collaboration with the Kemper laboratory, has demonstrated that intracellular generation of C3a modulates a Th1 response in CD4+ T cells under specific activating conditions (14). Intracellularly, the C3a interacts with the C3a receptor (C3aR) and then translocates to the plasma membrane. We surmise that CD4+ T cells load and recycle C3(H2O) providing a source of C3a to function in this activation pathway. Finally, loaded C3(H2O) that is secreted (recycled) under steady state conditions may serve as a surveillance mechanism, and an initiator of the AP to guard both the intracellular and extracellular spaces.
Figure 6. Functions of C3(H2O) by location.
Intracellular C3(H2O) is a source of C3a that could then engage the C3aR, it is secreted and C3 fragments are generated by cofactor activity, some of which contain C3a. Extracellular C3(H2O) is regulated by cofactor activity, taken up into cells and initiates the AP.
Concluding remarks
The complement system is an ancient component of immunity that likely evolved from protection of a single celled organism to providing essential defense functions in the blood of vertebrates.
Acknowledgements
Support was provided by the National Institutes of Health (R01 GM0099111 and R01 AI041592 to J.P.A.), the National Institutes of Health Training in the Immunobiology of Rheumatic Disease (2T32 AR007279, to M.E.), the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (P30AR048335) and the National Institutes of Health Grant for the Washington University Institute of Clinical and Translational Sciences (3UL1 TR000448).
Footnotes
Conflict of interest statement: The authors have declared that no conflict of interest exists.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. The New England journal of medicine. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. Second of two parts. The New England journal of medicine. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 4.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 6.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nature reviews Immunology. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 7.Carroll MC. The complement system in regulation of adaptive immunity. Nature immunology. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 8.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nature immunology. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Molecular immunology. 2014;58:98–107. doi: 10.1016/j.molimm.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Molecular immunology. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Baudino L, et al. C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1503–1508. doi: 10.1073/pnas.1316877111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolev M, et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity. 2015;42:1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor FB, Jr., Ward PA. Generation of chemotactic activity in rabbit serum by plasminogen-streptokinase mixtures. The Journal of experimental medicine. 1967;126:149–158. doi: 10.1084/jem.126.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seya T, Nagasawa S, Matsukura M, Hasegawa H, Atkinson JP. Generation of C3d,g and C3d by urokinase-treated plasma in association with fibrinolysis. Complement. 1985;2:165–174. doi: 10.1159/000467857. [DOI] [PubMed] [Google Scholar]
- 17.Amara U, et al. Molecular intercommunication between the complement and coagulation systems. Journal of immunology. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlo JR, Spitznagel JK, Studer EJ, Conrad DH, Ruddy S. Cleavage of membrane bound C3bi, an intermediate of the third component of complement, to C3c and C3d-like fragments by crude leucocyte lysosomal lysates and purified leucocyte elastase. Immunology. 1981;44:381–391. [PMC free article] [PubMed] [Google Scholar]
- 19.Claesson R, Kanasi E, Johansson A, Kalfas S. A new cleavage site for elastase within the complement component 3. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2010;118:765–768. doi: 10.1111/j.1600-0463.2010.02655.x. [DOI] [PubMed] [Google Scholar]
- 20.Poole AZ, Kitchen SA, Weis VM. The Role of Complement in Cnidarian-Dinoflagellate Symbiosis and Immune Challenge in the Sea Anemone Aiptasia pallida. Frontiers in microbiology. 2016;7:519. doi: 10.3389/fmicb.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann PJ. The amplification loop of the complement pathways. Advances in immunology. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
- 23.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. Journal of immunology. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 24.Ohta M, Okada M, Yamashina I, Kawasaki T. The mechanism of carbohydrate-mediated complement activation by the serum mannan-binding protein. The Journal of biological chemistry. 1990;265:1980–1984. [PubMed] [Google Scholar]
- 25.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. The Journal of experimental medicine. 1981;154:856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson JP, Farries T. Separation of self from non-self in the complement system. Immunology today. 1987;8:212–215. doi: 10.1016/0167-5699(87)90167-8. [DOI] [PubMed] [Google Scholar]
- 27.Pangburn MK, Muller-Eberhard HJ. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. The Journal of experimental medicine. 1980;152:1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sottrup-Jensen L, et al. Common evolutionary origin of alpha 2-macroglobulin and complement components C3 and C4. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:9–13. doi: 10.1073/pnas.82.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen BJ, et al. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 30.Al-Sharif WZ, Sunyer JO, Lambris JD, Smith LC. Sea urchin coelomocytes specifically express a homologue of the complement component C3. Journal of immunology. 1998;160:2983–2997. [PubMed] [Google Scholar]
- 31.Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Ma M, Ippolito GC, Schroeder HW, Jr., Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14577–14582. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litman GW, et al. Phylogenetic diversification of immunoglobulin genes and the antibody repertoire. Molecular biology and evolution. 1993;10:60–72. doi: 10.1093/oxfordjournals.molbev.a040000. [DOI] [PubMed] [Google Scholar]
- 34.Laird DJ, De Tomaso AW, Cooper MD, Weissman IL. 50 million years of chordate evolution: seeking the origins of adaptive immunity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6924–6926. doi: 10.1073/pnas.97.13.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen JA, Festa E, Smith DS, Cayer M. The complement system of the nurse shark: hemolytic and comparative characteristics. Science. 1981;214:566–569. doi: 10.1126/science.7291995. [DOI] [PubMed] [Google Scholar]
- 36.Nonaka M, Smith SL. Complement system of bony and cartilaginous fish. Fish & shellfish immunology. 2000;10:215–228. doi: 10.1006/fsim.1999.0252. [DOI] [PubMed] [Google Scholar]
- 37.Farries TC, Atkinson JP. Evolution of the complement system. Immunology today. 1991;12:295–300. doi: 10.1016/0167-5699(91)90002-B. [DOI] [PubMed] [Google Scholar]
- 38.Dodds AW, Law SK. The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and alpha 2-macroglobulin. Immunological reviews. 1998;166:15–26. doi: 10.1111/j.1600-065x.1998.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka M, Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58:701–713. doi: 10.1007/s00251-006-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Friec G, Kemper C. Complement: coming full circle. Archivum immunologiae et therapiae experimentalis. 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 41.Pisani D, et al. Genomic data do not support comb jellies as the sister group to all other animals. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15402–15407. doi: 10.1073/pnas.1518127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selander B, et al. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. The Journal of clinical investigation. 2006;116:1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson JP, Frank MM. Bypassing complement: evolutionary lessons and future implications. The Journal of clinical investigation. 2006;116:1215–1218. doi: 10.1172/JCI28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Shea JJ, Brown EJ, Seligmann BE, Metcalf JA, Frank MM, Gallin JI. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. Journal of immunology. 1985;134:2580–2587. [PubMed] [Google Scholar]
- 46.Sengelov H, Kjeldsen L, Kroeze W, Berger M, Borregaard N. Secretory vesicles are the intracellular reservoir of complement receptor 1 in human neutrophils. Journal of immunology. 1994;153:804–810. [PubMed] [Google Scholar]
- 47.Melillo D, et al. First identification of a chemotactic receptor in an invertebrate species: structural and functional characterization of Ciona intestinalis C3a receptor. Journal of immunology. 2006;177:4132–4140. doi: 10.4049/jimmunol.177.6.4132. [DOI] [PubMed] [Google Scholar]
- 48.Warnefors M, Hartmann B, Thomsen S, Alonso CR. Combinatorial Gene Regulatory Functions Underlie Ultraconserved Elements in Drosophila. Molecular biology and evolution. 2016 doi: 10.1093/molbev/msw101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ScienceDaily. Oxford University Press; 2016. Nature conserves its most vital DNA by multitasking, researchers show. Molecular biology and evolution. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Wang C, Wang Y, Wei R, Jiang G, Ju H. Presence and characterization of complement-like activity in the amphioxus Branchiostoma belcheri tsingtauense. Zoological science. 2003;20:1207–1214. doi: 10.2108/zsj.20.1207. [DOI] [PubMed] [Google Scholar]
- 51.Nonaka M, et al. Opsonic complement component C3 in the solitary ascidian, Halocynthia roretzi. Journal of immunology. 1999;162:387–391. [PubMed] [Google Scholar]
- 52.Hourcade D, et al. Analysis of the human regulators of complement activation (RCA) gene cluster with yeast artificial chromosomes (YACs) Genomics. 1992;12:289–300. doi: 10.1016/0888-7543(92)90376-4. [DOI] [PubMed] [Google Scholar]
- 53.Norman DG, Barlow PN, Baron M, Day AJ, Sim RB, Campbell ID. Three-dimensional structure of a complement control protein module in solution. Journal of molecular biology. 1991;219:717–725. doi: 10.1016/0022-2836(91)90666-t. [DOI] [PubMed] [Google Scholar]
- 54.Tsujikura M, Nagasawa T, Ichiki S, Nakamura R, Somamoto T, Nakao M. A CD46-like molecule functional in teleost fish represents an ancestral form of membrane-bound regulators of complement activation. Journal of immunology. 2015;194:262–272. doi: 10.4049/jimmunol.1303179. [DOI] [PubMed] [Google Scholar]
- 55.Azumi K, et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: "waiting for Godot". Immunogenetics. 2003;55:570–581. doi: 10.1007/s00251-003-0606-5. [DOI] [PubMed] [Google Scholar]
- 56.Sunyer JO, Tort L, Lambris JD. Structural C3 diversity in fish: characterization of five forms of C3 in the diploid fish Sparus aurata. Journal of immunology. 1997;158:2813–2821. [PubMed] [Google Scholar]
- 57.Sunyer JO, Tort L, Lambris JD. Diversity of the third form of complement, C3, in fish: functional characterization of five forms of C3 in the diploid fish Sparus aurata. The Biochemical journal. 1997;326:877–881. doi: 10.1042/bj3260877. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunyer JO, Lambris JD. Evolution and diversity of the complement system of poikilothermic vertebrates. Immunological reviews. 1998;166:39–57. doi: 10.1111/j.1600-065x.1998.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 59.Botto M, Lissandrini D, Sorio C, Walport MJ. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. Journal of immunology. 1992;149:1348–1355. [PubMed] [Google Scholar]
- 60.Wirthmueller U, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. Journal of immunology. 1997;158:4444–4451. [PubMed] [Google Scholar]
- 61.Martin M, et al. Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell death and differentiation. 2016;23:903–911. doi: 10.1038/cdd.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]