Abstract
Background
Novelty-seeking behavior is related to the reward system in the brain and can predict the potential for addiction. Alcohol use is prevalent in HIV-1-infected patients and adversely affects anti-retroviral medication. The difference in vulnerability to alcohol addiction between HIV-1 infected and non-infected populations has not been fully investigated. This study was designed to determine whether HIV-1 proteins alter the effects of ethanol on novelty-seeking behavior using the HIV-1 transgenic (HIV-1Tg) rat as the study model and to examine the molecular mechanisms responsible for this behavior.
Methods
Both HIV-1Tg and F344 control rats were tested for baseline novelty-seeking behavior, then received either ethanol (1g/kg) at a concentration of 20% v/v or saline treatment for 13 days and then were re-tested for novelty seeking. Quantitative real-time PCR was conducted to examine the differences in expression of 65 genes implicated in novelty-seeking and alcohol addiction between strains and treatment groups.
Results
The HIV-1 proteins significantly enhanced baseline novelty-seeking behaviors in both the hole-board and open field tests. Chronic ethanol treatment significantly increased baseline novelty-seeking behavior in both strains, but the effects of ethanol appeared to be more robust and prominent in HIV-1Tg rats. Strain-specific patterns of altered gene expression were observed for dopaminergic, cholinergic, and glutamatergic signaling in the nucleus accumbens (NAc), suggesting the effects of HIV-1 proteins on the brain’s reward system. Chronic ethanol treatment was shown to greatly modulate the effects of HIV-1 proteins in these neurotransmitter systems.
Conclusions
Taken together, our findings indicate that HIV-1 proteins could modify novelty-seeking behavior at the gene expression level, and ethanol treatment may enhance this behavior in both strains but to a greater extent in HIV-1Tg rats.
Keywords: Addiction, alcohol, HIV-1 infection, novelty seeking, reward system, HIV-1Tg rat
Introduction
Alcohol abuse is a widespread problem that negatively impacts both the individual and society. Despite the millions of dollars dedicated to the treatment of drug addiction, current psychopharmacological therapies have not yet reached desired success rates (Kalivas and Volkow, 2011). Globally, nearly 370 million individuals are plagued with an alcohol use disorder (AUD), and some subsets of the population demonstrate even higher rates of alcoholism. For example, in the HIV-1 infected population, the prevalence of alcohol addiction has reached more than 50% (Burns, 2014). Such a high prevalence in this population is a serious and curious issue, as the viral infection and alcohol obviously interact in some way to amplify their detrimental effects (Silverstein and Kumar, 2014). Because of the extent of this issue, much research has focused on what personality traits make some individuals more vulnerable to alcohol addiction, and novelty seeking has emerged as a significant contributing factor (Cain et al., 2005, Cloninger, 1987, Koob, 2000).
Novelty seeking, one of the defining characteristics of a sensation-seeking personality, is often associated with susceptibility to substance abuse (Koob, 2000, Manzo et al., 2014, Wingo et al., 2015). Clinical human alcohol studies have established a link between AUD and the novelty-seeking trait (Koob, 2000). Individuals with high novelty seeking consistently display heavier drinking, higher rates of drug dependence, and higher relapse rates after treatment (Wingo et al., 2015). Rodent studies have confirmed these human findings suggesting a positive correlation between novelty-seeking behavior and alcohol addiction (Bienkowski et al., 2001, Manzo et al., 2014, Nowak et al., 2000, Parkitna et al., 2013, Bardo et al., 1996).
The molecular connections between novelty seeking and addiction have not been fully elucidated, although it is known that both are related to the mesocorticolimbic reward system in the brain (Bardo et al., 1996). It is known that dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) function as the main network by which the reward system becomes activated (Koob, 2000). Glutamatergic, serotonergic, cholinergic, and opioidergic transmission act as excitatory projections by activating the VTA–NAc circuit, whereas GABAergic neurons function as inhibitory projections to diminish signaling (Koob, 2000). However, the precise mechanisms by which responses to novelty and to addictive drugs produce such a phenotype remain to be further investigated.
To that end, differences in the vulnerability to alcohol addiction in HIV-1-infected and non-infected populations require further investigation at both the behavioral and molecular levels (Sarkar and Chang, 2013). There is a significant body of research established in the alcohol-dependent HIV-1-infected population, indicating the negative health consequences of excessive alcohol use (Silverstein and Kumar, 2014). Further, clinical studies in personality trait profiling in HIV patients showed that drug-dependent HIV-positive patients had higher scores in novelty seeking when compared with healthy subjects (Fassino et al., 2004). Given the high rate of alcohol dependence in the HIV-1-infected population, it is critical to determine whether HIV-1 proteins modify the novelty-seeking trait. If we know that these viral proteins affect vulnerability to alcohol dependence, successful treatments may be developed (Sarkar and Chang, 2013).
The goals of the present study were to 1) investigate the effects of HIV-1 proteins on baseline novelty-seeking behavior and 2) examine whether these viral proteins modify the effect of ethanol on molecular neurobiological substrates of the mechanism maintaining alcohol dependence and novelty-seeking behavior in the HIV-1 transgenic (HIV-1Tg) rat. In the current study, we utilized these rats in an attempt to mirror neuropathological conditions seen in HIV-1-infected individuals. These rats express 7 of the 9 HIV-1 proteins and display conditions similar to those observed in the HIV-1-infected population receiving highly active antiretroviral therapy (Moran et al., 2013, Sarkar and Chang, 2013). Regarding the first goal of this study, we hypothesized that HIV-1Tg rats would display higher scores in measures of novelty-seeking behavior than their genetic background F344 control rats under both baseline and ethanol treatment conditions. For our second goal, we hypothesized that there would be strain-specific differences in the expression of genes involved in novelty-seeking behavior and alcohol addiction, which are both modulated by the reward system in the brain.
Materials and methods
Animals
Male HIV-1Tg rats (N = 14) and F344 genetic background control rats (N = 23) (Harlan Industries, USA) at 7 to 8 weeks of age were used. Male rats were chosen in order to eliminate a potential confounding variable (sex), since previous reports have indicated that sex significantly influences novelty-seeking behavior (Hughes, 1968, Ray and Hansen, 2004, Tropp and Markus, 2001). Rats were housed two per cage in a temperature (20°C–22°C) and humidity (44%–55%) controlled environment on a 12-h light/dark cycle. Food and water were provided ad libitum. All behavioral experiments were conducted between 9:00 AM and 1:00 PM and were in accordance with the guidelines of the University of Virginia Animal Research Committee.
Alcohol and treatment
Rats from each strain were divided randomly into two groups: saline-treated control and ethanol (EtOH)-treated, designated as follows: F344_Saline (n = 10); F344_EtOH (n = 13); HIV-1Tg_Saline (n = 6); and HIV-1Tg EtOH (n = 8). Rats were tested for their baseline novelty-seeking behavior using the hole-board and open field tests, on days 1 and 2, respectively (defined as pre-treatment trials). To determine the effects of chronic ethanol treatment on novelty-seeking behavior, F344 and HIV-1Tg rats received a single intraperitoneal injection of either saline or ethanol at a dose of 1 g/kg/day for 13 days before starting post-treatment trials (Sarkar and Chang, 2013, Simms et al., 2008). Ethanol solution was prepared by diluting 200-proof (absolute) ethyl alcohol (Sigma-Aldrich, St. Louis, MO, USA) in 0.9% physiological saline to a final concentration of 20% vol/vol solution. On days 3–15, saline and ethanol treatments were conducted. On days 12 and 13, both hole-board and open field tests were conducted 10 min after injection. All rats received their last saline or ethanol injection on day 15 and were decapitated 10 min after the last injection. Because of congenital cataracts in HIV-1Tg rats (Vigorito et al., 2007), all behavioral experiments were conducted under dimmed red light to minimize visual differences between strains.
Behavioral tasks
Hole-board test
Novelty-seeking behavior was assessed by recording behavioral parameters during a 10-min session in the hole-board test (Boissier and Simon, 1962). The apparatus consisted of a grey PVC experimental box (78 × 78 cm) with 16 equally spaced holes (3.8 cm in diameter). Each rat was placed at the center of the box and allowed to move freely through the hole-board apparatus without reinforcements. The series of explored holes and novelty-seeking behavioral parameters (head dips, nose pokes, rearing events, and grooming events) were recorded by the Any-Maze video tracking system (Stoelting Co., Wood Dale, IL, USA).
Open field test
Further investigation of novelty-seeking behavior was conducted by recording locomotor activity during a 10-min session in the open field test (Welker, 1957). The apparatus consisted of a clear plastic box (50 × 50 × 50 cm). Each rat was placed at the center of the box and allowed to move freely through the apparatus. The Any-Maze video tracking system recorded total time mobile, as well as each time that the rat moved from the center of the box to the sides or from the sides to the center.
Brain tissue collection
The animals were sacrificed by decapitation 10 min after their last saline or ethanol injection, and their brains were immediately removed. Although various brain regions were collected, only the tissues from the NAc are described in this report. Selection of the NAc for this study was based on research implicating its importance in the reward neurocircuitry of the brain (Flagel et al., 2010, Koob, 2000, Parkitna et al., 2013, Koob, 2006). The VTA–NAc network has been considered the main pathway by which an organism responds to rewarding stimuli, such as a novel environment or a drug of abuse (Koob, 2006). The NAc plays a central role in modulating the response to reward through projections in dopaminergic, cholinergic, GABAergic, serotonergic, opioidergic, and glutamatergic neurotransmission (Toda, 2012, Koob, 2006, Koob, 2000, Mahler et al., 2014, Wingo et al., 2015). By using a rat brain matrix (Paxinos and Watson, 1998), slices of approximately 1.0 mm were taken from each brain, and tissues from specific regions of interest were collected bilaterally from each slice with a 3.00-mm Harris Micro-Punch (GE Healthcare Life Sciences, Piscataway, NJ, USA). All punched tissues were stored at −80°C until use.
Quantitative RT-PCR (qRT-PCR) array
We used a custom-designed RT-PCR array with the goal of determining the mRNA expression levels of genes likely involved in the regulation of alcohol dependence and novelty-seeking behavior. Based on a PubMed literature search as well as pathway database information, including KEGG (Kyoto Encyclopedia of Genes and Genomes: http://www.genome.jp/kegg) and IPA (Ingenuity Pathway Analysis: http://www.ingenuity.com/), we identified 65 candidate genes. These genes can be grouped into the following systems: dopaminergic signaling (8 genes), opioid signaling (4 genes), serotonergic signaling (8 genes), GABAergic signaling (6 genes), cholinergic signaling (9 genes), and glutamatergic signaling (11 genes). In order to determine whether the viral protein-induced changes in the immune system impact the effect of ethanol in the brain, we also selected 19 genes implicated in the immune system.
The primers of each gene selected for the qRT-PCR assay were designed with Primer Express (v. 3.0) software (Applied Biosystems, Carlsbad, CA, USA) and spanned at least one intron to avoid amplifying genomic DNA. The primers had a melting temperature from 59°C to 61°C. Each pair of primers and their amplicon sequences were tested using the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov.proxy.its.virginia.edu/Blast.cgi) to ensure specificity of the designed primers for targeted genes. For a detailed list of primer sequences, please see Supplementary Table 1. Dissociation curves were employed to check the specificity of the primers before including them in the qRT-PCR array.
Total RNA was isolated from the NAc using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The purity and quantity of total RNA were measured at optical densities of 260 and 280 nm with NanoDrop 2000c (Thermo Scientific, Waltham, MA, USA). Then 2 μg of total RNA was reverse transcribed into first-strand cDNA using Superscript II reverse transcriptase. The cDNA mixture was incubated at 25°C for 10 min, 42°C for 1.5 h, and 70°C for 15 min. The PCR amplification was conducted as described previously (Cui et al., 2013, Nesil et al., 2015). Briefly, the product was amplified in a volume of 10μl containing 5.0 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems) and combined sense and antisense primers (2.5 μl; final concentration 20 nM) in a 384-well plate using the 7900HT Sequence Detection System (Applied Biosystems). The PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A cycle threshold was assigned at the beginning of the logarithmic phase of the amplification, and differences in the Ct values of the control and ethanol-treated groups were used to determine the relative expression of genes of interest. Melting curve analysis was applied to characterize the specificity of the amplifications.
Statistical analysis
Data are presented as the mean ± SEM, and significance is set at p < 0.05. All data were analyzed using SPSS (v.14.0) software (SPSS Inc. Chicago, Illinois, USA). The unpaired two-tailed Student’s t-test was performed to determine the significant differences between the two strains for hole-board and open field at the pre-treatment session. We also analyzed the post-treatment session data using two-way ANOVA to determine the interaction between strain and treatment groups. Post hoc comparisons were carried out by two-tailed paired and unpaired t-tests.
Expression of each gene of interest was first normalized to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and then analyzed using a comparative Ct method (Schmittgen and Livak, 2008). The relative expression of each gene was compared for different drug treatment groups of the same strain or different strains of the same drug treatment using the Student t-test. Significant alteration in mRNA was defined as a > 20% fold change with a p value < 0.05 (n = 4–6 per group).
Results
HIV-1 proteins enhanced novelty-seeking behaviors in pre-treatment trials
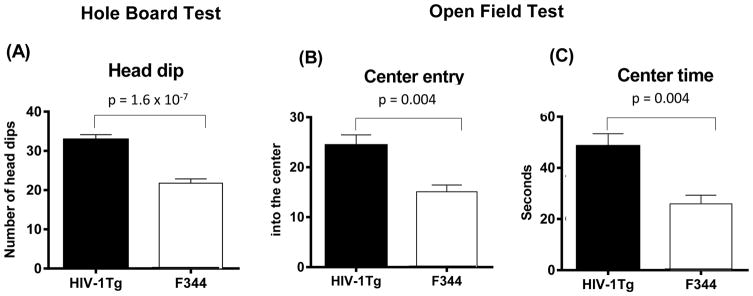
Figure 1 shows the strain differences in the measures of novelty-seeking behavior in the hole-board test (panel A) and the open field test (panels B, C) between HIV-1Tg and F344 rats. Statistical analysis of head dip scores for the pre-treatment session showed that HIV-1Tg rats displayed a higher frequency of head dipping in the hole-board test (t (37) = 6.44, p = 1.57 × 10−7 ) compared with F344 rats (Panel A). There was no significant difference in rearing, grooming, or time mobile scores between strains in the hole-board test (Table 1).
Figure 1.
HIV-1 protein effects on novelty-seeking behaviors in HIV-1Tg rats (N = 14) and F344 control rats (N = 23). (A) Number of head dips measured during the 10-min session in the hole-board test; (B) Number of center entries. (C) Center time measured during the 10-min session in the open field test. HIV-1Tg rats demonstrated significantly greater baseline novelty-seeking behaviors in each of these paradigms.
Table 1.
Novelty-seeking behavioral differences between HIV-1Tg and F344 rats as measured by hole-board and open field tests.
| Behavioral test | Behaviors | HIV-1Tg (Mean ± SEM) | F344 (Mean ± SEM) | P-value |
|---|---|---|---|---|
| Hole-Board Test | Head Dip | 32.84 ± 1.29 | 21.78 ± 1.11 | 1.57 × 10−7 |
| Rearing | 17.34 ± 1.10 | 14.43 ± 0.96 | 0.06 | |
| Grooming | 3.71± 0.41 | 4.47 ± 0.38 | 0.20 | |
| Time mobile | 329.70 ± 23.65 | 288.80 ± 17.89 | 0.17 | |
| Open Field Test | Center entry | 24.38 ± 2.09 | 15.09 ± 1.35 | 0.0004 |
| Center time | 48.48 ± 4.88 | 25.96 ± 3.37 | 0.0004 | |
| Time mobile | 300.50 ± 16.18 | 292.51 ± 16.88 | 0.74 |
Testing novelty-seeking behavior in the open field test yielded significant strain differences in the number of entries to the center (t (37) = 3.907; p = 0.0004) and time spent in the center of the arena (t (37) = 3.924; p = 0.0004). HIV-1Tg rats spent more time and made more entries into the center than F344 rats (Table 1).
Increased novelty-seeking behaviors in HIV-Tg rats in the post-treatment hole-board test
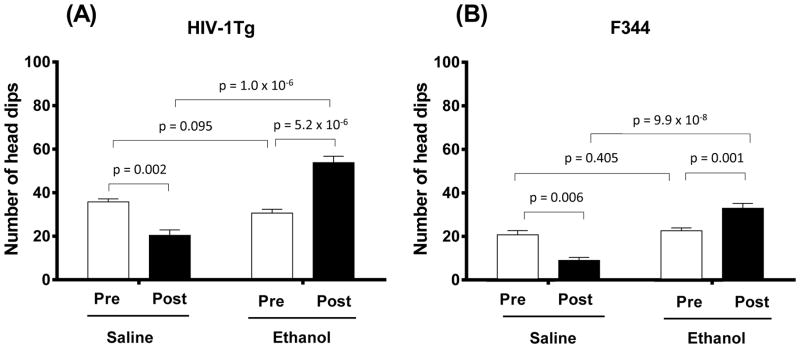
Figure 2 shows the effects of chronic ethanol treatment on the novelty-seeking behavior of HIV-1Tg and F344 rats in the hole-board test. Two-way ANOVA showed significant main effects of Treatment (F (1, 35) = 133.975; p = 1.6 × 10−13) and Strain (F (1, 35) = 43.292; p = 1.34 × 10−7) for the post-treatment session. Chronic ethanol treatment significantly increased the number of head dips in HIV-1Tg (p = 1.0 × 10−6; Fig. 2A) and F344 (p = 9.9 × 10−8; Fig. 2B) rats compared with the corresponding saline-control rats. Compared with pre-treatment session scores, both HIV-1Tg and F344 saline-treated groups displayed a significantly lower number of head dips during the post-treatment session (p = 0.0002 and p = 0.006, respectively). In contrast, chronic ethanol treatment significantly increased the number of head dips in both HIV-1Tg (p = 5.2 × 10−6) and F344 (p = 0.001) rats compared with their pre-treatment session scores (Fig. 2). Further, the percentage change from the pre-treatment head dip behavior was increased by 76% for the ethanol-treated HIV-1Tg group and by 44% for the ethanol-treated F344 group. We did not find significant differences between the pre- and post-treatment rearing, grooming, or time mobile scores in the ethanol-treated groups of either strain (Table 2). Two-way ANOVA of rearing, grooming, and time mobile scores for the post-treatment session revealed no significant main effect of either Treatment or Strain.
Figure 2.
Ethanol effects on head dip behavior as measured during the 10-min session in the hole-board test in HIV-1Tg (A) and F344 (B) rats. Each strain contained two experimental groups: saline treated and ethanol treated. Each experimental group was compared in pre-treatment baseline trials (“Pre”) with post-treatment trials (“Post”). Ethanol-treated HIV-1Tg rats (N = 8) showed significantly more head dips than saline-treated HIV-1Tg rats (N = 6). A similar pattern was seen in F344 control rats; ethanol-treated rats (N = 13) displayed more head dipping behavior than saline-treated rats (N = 10). Ethanol exposure significantly increased head dips in both HIV-1Tg and F344 rats compared with their baseline levels. Additionally, in both strains, saline-treated rats displayed relatively fewer head dips in the post-treatment trials compared with baseline levels.
Table 2.
Effect of ethanol on novelty-seeking behaviors in HIV-1Tg and F344 rats as measured by hole-board and open field tests.
| Behavioral Test | Behaviors | HIV-1Tg | F344 | ||||
|---|---|---|---|---|---|---|---|
| Pre-treatment (Mean ± SEM) | Post-treatment (Mean ± SEM) | P-value | Pre-treatment (Mean ± SEM) | Post-treatment (Mean ± SEM) | P-value | ||
| Hole-Board Test | Head Dip | 30.61 ± 1.74 | 53.77 ± 2.9 | 5.19 × 10−6 | 22.62 ± 1.30 | 32.69 ±2.38 | 0.0011 |
| Rearing | 4.42 ± 1.33 | 3.07 ± 0.95 | 0.09 | 7.06 ± 1.38 | 5.04 ±1.00 | 0.07 | |
| Grooming | 3.55 ± 0.53 | 4.13 ± 0.49 | 0.43 | 3.885 ± 0.52 | 3.61 ± 0.93 | 0.80 | |
| Time mobile | 301.5 ± 14.37 | 251.6 ± 21.65 | 0.07 | 286.7 ± 26.48 | 228.6 ±14.53 | 0.06 | |
| Open Field Test | Center entry | 23.78 ± 3.00 | 46.00 ± 2.88 | 1.64 x10−5 | 14.85 ± 1.78 | 24.62 ±2.56 | 0.004 |
| Center time | 47.59 ± 8.31 | 126.9 ± 10.09 | 6.71 × 10−5 | 26.75 ± 5.78 | 61.95 ±9.74 | 0.004 | |
| Time mobile | 282.8 ± 20.53 | 274.6 ± 16.52 | 0.76 | 288.8 ± 25.85 | 210.1 ±15.47 | 0.015 | |
Altered novelty-seeking behaviors in HIV-1Tg rats in the post-treatment open field test
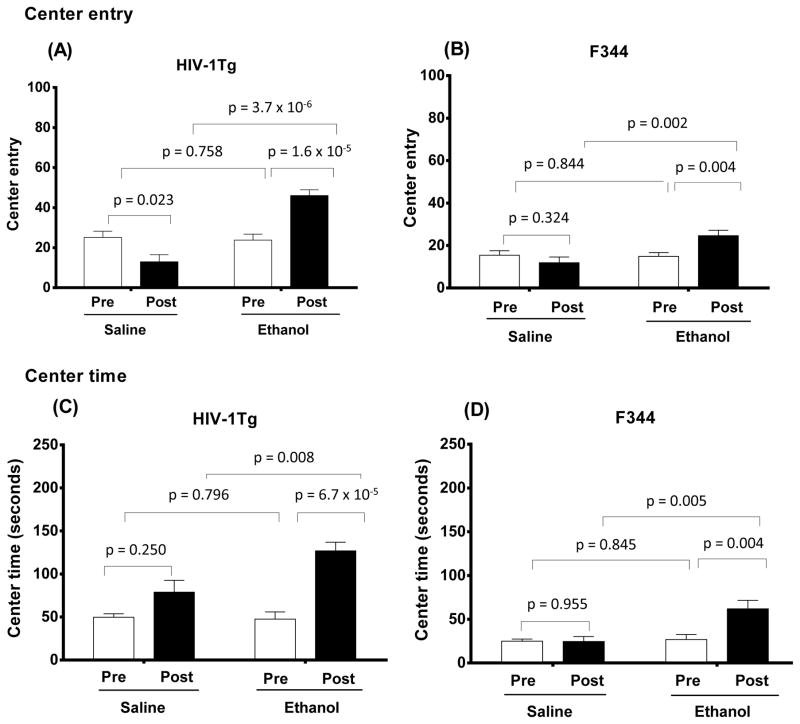
Analysis of number of entries into the center of the arena using two-way ANOVA revealed significant effects of Treatment (F (1, 35) = 60.758; p = 3.71 × 10−8) and Strain (F (1, 35) = 14.698; p = 0.001) for the post-treatment session. Chronic ethanol treatment induced higher center entry scores in both HIV-1Tg (Fig. 3A) and F344 (Fig. 3B) rats compared with pre-treatment (p = 1.6 × 10−5 and p = 0.002, respectively). The number of entries into the center was increased by 93% in the EtOH-treated HIV-1Tg rats and by 65% in the EtOH-treated F344 rats from their pre-treatment scores. In contrast, there was a significant reduction in center entry scores for post-treatment session scores in the HIV-1Tg saline-treated group, but not in the F344 saline-treated group compared with pre-treatment scores (p = 0.02; Fig. 3A).
Figure 3.
Ethanol effects on center entry (A, B) and center time (C, D) behaviors as measured by a 10-min session in the open field test in HIV-1Tg (A and C) and F344 (B and D) rats. Four experimental groups (saline-treated HIV-1Tg and F344 control rats and ethanol-treated HIV-1Tg and F344 rats) were tested. For each strain, saline- and ethanol-treated rats were tested in pre-treatment baseline trials (“Pre”) and in post-treatment trials (“Post”). Ethanol-treated HIV-1Tg rats (N = 8) showed significantly more center entries and spent more time in the center of the open field test than did saline-treated HIV-1Tg rats (N = 6). Similarly, ethanol-treated F344 rats (N = 13) displayed more center entries and spent more time in the center than saline-treated F344 rats (N = 10). Ethanol treatment also significantly increased both of these measures in HIV-1Tg and F344 rats compared with their baseline levels. In contrast, saline treatment decreased both center entries and center time in both rat strains, with one exception: saline-treated HIV-1Tg rats spent more time in the center of the arena in the post-treatment trials than at baseline (pre-treatment).
Analysis of the center time data during the post-treatment session indicated significant effects of Treatment (F (1, 35) = 12.115; p = 0.001) and Strain (F (1, 35) = 23.605; p = 0.00002). Ethanol-treated rats spent more time in the center of the arena than did saline-treated groups in both HIV-1Tg (p = 0.008; Fig. 3C) and F344 (p = 0.005; Fig. 3D) groups. Compared with values of the pre-treatment session, a significant increase in time spent in the center was detected in both HIV-1Tg (p = 6.7 × 10−5) and F344 (p = 0.004) rats at the end of the 12-day treatment with ethanol. Time spent in the center was increased by 167% in EtOH-treated HIV-1Tg rats and by 132% in EtOH-treated F344 rats from their pre-treatment scores. In contrast, time spent in the center did not significantly differ in the saline-treated strains between pre- and post-treatment sessions (Table 2). There were no significant effects of Treatment or Strain on time mobile scores during the post-treatment session.
HIV-1 proteins altered the expression of addiction-related genes in the NAc
Of the studied 65 genes, 21 were significantly altered by HIV-1 proteins (Table 3). HIV-1 proteins altered the expression of genes encoding the D1-like receptor subtype (Drd1), with 109% (p = 0.032) upregulation. HIV-1 proteins showed a dual effect on expression of serotonergic system-related genes. Htr1a, Htr7, and Slca4 showed significant upregulation with a range of 87%–276% (p = 0.0006–0.030), and 36% downregulation of Htr1b (p = 0.032). Of 6 genes involved in GABAergic signaling, only Gabra1 was significantly altered by HIV-1 proteins, with an upregulation of 92% (p = 0.013). HIV-1 proteins increased the expression of genes encoding subunits of nicotinic acetylcholine receptors (nAChRs) at a range of 44%–175% (p = 0.034–0.006) for Chrna2, Chrna3, Chrna4, Chrnb2, and Chrnb4. In the glutamatergic receptor signaling pathway, HIV-1 proteins significantly upregulated the expression of Gria1, Grm1, Grin1, Grin2c, and Grin2d by 40%–282% in the NAc (p < 0.04–0.0001). HIV-1 proteins displayed an opposing effect on immune system regulation at the mRNA level. The expressions of Il1r1, Il1r2, and Il1a were downregulated by 33%–60% (p = 0.021–0.004), and those of Il6r and Il12a were upregulated by 58% (p = 0.021) and 61% (p = 0.034), respectively.
Table 3.
A list of addiction-related genes whose expression levels differed significantly in the NAc between the F344 Saline and HIV-1Tg Saline (n= 6–8 per group).
| Gene Name | Gene Function | F344 _Saline (FS) | HIV-1Tg _Saline (HS) | Ratio (HS/FS) | P-Value |
|---|---|---|---|---|---|
| Drd1 | Dopamine receptor D1 | 4.72E-02 ± 6.43E-03 | 9.86E-02 ± 1.74E-02 | 2.09 | 0.032 |
| Htr1a | 5-Hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | 1.72E-03 ± 1.26E-04 | 6.46E-03 ± 9.05E-04 | 3.76 | 0.0006 |
| Htr1b | 5-Hydroxytryptamine (serotonin) receptor 1B, G protein-coupled | 2.99E-02 ± 2.99E-03 | 1.92E-02 ± 2.44E-03 | 0.64 | 0.032 |
| Htr7 | 5-Hydroxytryptamine (serotonin) receptor 7, adenylate cyclase-coupled | 2.29E-03 ± 1.71E-04 | 6.20E-03 ± 9.56E-04 | 2.71 | 0.0027 |
| Slc6a4 | 5-Hydroxytryptamine (serotonin) transporter | 8.75E-05 ± 1.16E-05 | 1.64E-04 ± 2.45E-05 | 1.87 | 0.030 |
| Gabra1 | Gamma-aminobutyric acid (GABA) A receptor, alpha 1 | 7.79E-02 ± 6.15E-03 | 1.50E-01 ± 2.57E-02 | 1.92 | 0.013 |
| Chrna2 | Cholinergic receptor, nicotinic, alpha 2 (neuronal) | 6.21E-05 ± 1.49E-05 | 1.71E-04 ± 2.92E-05 | 2.75 | 0.009 |
| Chrna3 | Cholinergic receptor, nicotinic, alpha 3 (neuronal) | 7.30E-04 ± 6.83E-05 | 1.17E-03 ± 8.45E-05 | 1.60 | 0.006 |
| Chrna4 | Cholinergic receptor, nicotinic, alpha 4 (neuronal) | 1.35E-03 ± 1.92E-04 | 2.34E-03 ± 3.35E-04 | 1.73 | 0.034 |
| Chrnb2 | Cholinergic receptor, nicotinic, beta 2 (neuronal) | 4.08E-02 ± 3.00E-03 | 5.85E-02 ± 7.31E-03 | 1.44 | 0.033 |
| Chrnb4 | Cholinergic receptor, nicotinic, beta 4 (neuronal) | 5.47E-04 ± 7.04E-05 | 8.29E-04 ± 7.55E-05 | 1.52 | 0.032 |
| Gria1 | Glutamate receptor, ionotropic, AMPA 1 | 1.40E-01 ± 9.19E-03 | 1.96E-01 ± 7.53E-03 | 1.40 | 0.006 |
| Grm1 | Glutamate receptor, metabotropic 1 | 1.56E-02 ± 1.14E-03 | 2.40E-02 ± 9.79E-04 | 1.54 | 0.001 |
| Grin1 | Glutamate receptor, ionotropic, N-methyl D-aspartate 1 | 1.08E-01 ± 1.00E-02 | 1.82E-01 ± 1.76E-02 | 1.69 | 0.007 |
| Grin2c | Glutamate receptor, ionotropic, N-methyl D-aspartate 2C | 1.58E-02 ± 1.55E-03 | 2.49E-02 ± 3.05E-03 | 1.57 | 0.045 |
| Grin2d | Glutamate receptor, ionotropic, N-methyl D-aspartate 2D | 5.95E-03 ± 4.94E-04 | 2.27E-02 ± 9.45E-04 | 3.82 | <0.0001 |
| Il1r1 | Interleukin 1 receptor, type I | 1.53E-03 ± 1.70E-04 | 6.06E-04 ± 1.69E-04 | 0.40 | 0.004 |
| Il1r2 | Interleukin 1 receptor, type II | 4.89E-04 ± 6.88E-05 | 2.37E-04 ± 4.23E-05 | 0.48 | 0.021 |
| Il1a | Interleukin-1 alpha | 2.24E-04 ± 1.37E-05 | 1.50E-04 ± 1.88E-05 | 0.67 | 0.019 |
| Il6r | Interleukin 6 receptor | 3.05E-03 ± 7.88E-05 | 4.83E-03 ± 5.55E-04 | 1.58 | 0.034 |
| Il12a | Interleukin 12A | 3.05E-03 ± 2.45E-04 | 4.91E-03 ± 6.37E-04 | 1.61 | 0.021 |
Chronic exposure to ethanol modifies addiction-related gene expression in the NAc of HIV-1Tg rats
To determine how chronic ethanol treatment impacts the expression of addiction-related genes in the NAc of HIV-1Tg rats, we examined the same set of genes. We found that chronic ethanol treatment significantly modified the expression of two, two, one, one, four, and four genes, respectively, in the dopaminergic, serotonergic, GABAergic, cholinergic, glutamatergic, and immune systems (Table 4).
Table 4.
A list of addiction-related genes whose expression levels differed significantly in the NAc between HIV-1Tg Saline and HIV-1Tg Ethanol (n=6–8 per group).
| Gene Name | Gene Function | HIV-1Tg_Ethanol (HE) | HIV-1Tg_Saline (HS) | Ratio (HE/HS) | P-Value |
|---|---|---|---|---|---|
| Drd1 | Dopamine receptor D1 | 7.46E-02 ± 2.87E-03 | 1.13E-01 ± 1.29E-02 | 0.66 | 0.042 |
| Drd2 | Dopamine receptor D2 | 2.94E-02 ± 5.54E-03 | 9.88E-02 ± 6.27E-03 | 0.30 | 0.0012 |
| Htr2a | 5-Hydroxytryptamine (serotonin) receptor 2A, G protein-coupled | 2.65E-02 ± 4.21E-03 | 1.15E-02 ± 1.73E-03 | 2.31 | 0.029 |
| Htr2c | 5-Hydroxytryptamine (serotonin) receptor 2C, G protein-coupled | 1.32E-05 ± 5.02E-07 | 7.67E-06 ± 4.62E-07 | 1.72 | 0.0006 |
| Gabra3 | Gamma-aminobutyric acid (GABA) A receptor, alpha 3 | 4.04E-02 ± 3.85E-03 | 2.13E-02 ± 4.69E-03 | 1.89 | 0.025 |
| Chrna3 | Cholinergic receptor, nicotinic, alpha 3 (neuronal) | 6.25E-04 ± 5.50E-05 | 1.24E-03 ± 6.45E-05 | 0.50 | 0.001 |
| Grm1 | Glutamate receptor, metabotropic 1 | 3.91E-03 ± 8.42E-04 | 8.27E-04 ± 6.89E-05 | 4.73 | 0.022 |
| Grin1 | Glutamate receptor, ionotropic, N-methyl D-aspartate 1 | 1.39E-01 ± 8.10E-03 | 1.82E-01 ± 1.76E-02 | 0.76 | 0.042 |
| Grin2c | Glutamate receptor, ionotropic, N-methyl D-aspartate 2C | 1.51E-02 ± 1.01E-03 | 2.49E-02 ± 3.05E-03 | 0.61 | 0.029 |
| Grin2d | Glutamate receptor, ionotropic, N-methyl D-aspartate 2D | 1.23E-02 ± 1.63E-04 | 2.27E-02 ± 9.45E-04 | 0.54 | 0.0004 |
| Il1r1 | Interleukin 1 receptor, type I | 1.86E-03 ± 2.68E-05 | 6.06E-04 ± 1.69E-04 | 3.06 | 0.001 |
| Il1r2 | Interleukin 1 receptor, type II | 1.76E-04 ± 5.35E-06 | 4.79E-04 ± 6.74E-05 | 0.37 | 0.011 |
| Il10ra | Interleukin 10 receptor, alpha | 1.04E-02 ± 9.33E-04 | 5.10E-03 ± 2.73E-04 | 2.05 | 0.005 |
| Il11 | Interleukin 11 | 2.02E-04 ± 9.42E-06 | 4.89E-04 ± 5.32E-05 | 0.41 | 0.006 |
Chronic exposure to ethanol significantly decreased the expression of Drd1 (44%; p = 0.042) and Drd2 (70%; p = 0.0012) in the dopaminergic system of HIV-1Tg rats. In the serotonergic system, ethanol treatment significantly upregulated Htr2a by 131% (p = 0.029) and Htr2c by 72% (p = 0.0006). Gabra3 was significantly upregulated by 89% (p = 0.025), and Chrna3 was downregulated by 50% (p = 0.001). Ethanol treatment increased expression of the gene encoding the metabolic glutamatergic receptor but decreased the expression of genes encoding the ionotropic glutamatergic receptors. The expression of Grm1 was significantly upregulated by 373% (p = 0.022), and the expressions of Grin1, Grin2c, and Grin2d were downregulated by 24%–46% (p = 0.042–0.0004). Ethanol treatment upregulated Il1r1 by 206% (p = 0.001) and Il10ra by 105% (p = 0.005), and downregulated Il1r2 by 63% (p = 0.011) and Il11 by 59% (p = 0006) in the NAc of HIV-1Tg rats.
Dual effects of chronic ethanol treatment on addiction-related gene expression in the NAc of F344 rats
We then examined the molecular effects of chronic ethanol treatment in F344 control rats. Among the 65 genes examined, 24 were differentially expressed in ethanol-treated rats relative to their corresponding saline controls (Table 5). Chronic ethanol treatment significantly modified the expression of two, four, three, four, five, and six genes, respectively, in the dopaminergic, serotonergic, GABAergic, cholinergic, glutamatergic, and immune systems (Figures 4 and 5).
Table 5.
A list of addiction-related genes whose expression levels differed significantly in the NAc between F344 Saline and F344 Ethanol (n=6–8 per group).
| Gene Name | Gene Function | F344_Ethanol (FE) | F344_Saline (FS) | Ratio (FE/FS) | P-Value |
|---|---|---|---|---|---|
| Drd4 | Dopamine receptor D4 | 4.00E-05 ± 7.48E-06 | 1.60E-04 ± 1.21E-05 | 0.25 | 0.0003 |
| Drd5 | Dopamine receptor D5 | 5.04E-04 ± 5.91E-05 | 2.23E-04 ± 2.96E-05 | 2.26 | 0.013 |
| Htr1a | 5-Hydroxytryptamine (serotonin) receptor 1A, G protein-coupled | 6.71E-03 ± 1.56E-03 | 1.72E-03 ± 1.26E-04 | 3.91 | 0.004 |
| Htr3a | 5-Hydroxytryptamine (serotonin) receptor 3A, ionotropic | 1.08E-02 ± 5.63E-04 | 8.42E-03 ± 3.52E-04 | 1.28 | 0.007 |
| Htr7 | 5-Hydroxytryptamine (serotonin) receptor 7, adenylate cyclase-coupled | 4.20E-03 ± 2.26E-04 | 2.29E-03 ± 1.71E-04 | 1.83 | 0.0002 |
| Slc6a4 | 5-Hydroxytryptamine (serotonin) transporter | 1.53E-04 ± 1.87E-05 | 8.75E-05 ± 1.16E-05 | 1.75 | 0.027 |
| Gabra1 | Gamma-aminobutyric acid (GABA) A receptor, alpha 1 | 1.18E-01 ± 1.46E-02 | 7.79E-02 ± 6.15E-03 | 1.51 | 0.043 |
| Gabra2 | Gamma-aminobutyric acid (GABA) A receptor, alpha 2 | 1.08E-01 ± 7.38E-03 | 2.45E-01 ± 2.25E-02 | 0.44 | 0.004 |
| Gabra5 | Gamma-aminobutyric acid (GABA) A receptor, alpha 5 | 2.46E-02 ± 1.17E-03 | 1.71E-02 ± 1.19E-03 | 1.44 | 0.018 |
| Chrna2 | Cholinergic receptor, nicotinic, alpha 2 (neuronal) | 1.47E-04 ± 2.12E-05 | 4.95E-05 ± 9.99E-06 | 2.97 | 0.005 |
| Chrna4 | Cholinergic receptor, nicotinic, alpha 4 (neuronal) | 6.10E-03 ± 6.41E-04 | 1.65E-03 ± 8.97E-05 | 3.70 | 0.002 |
| Chrna5 | Cholinergic receptor, nicotinic, alpha 5 (neuronal) | 1.60E-03 ± 2.39E-04 | 6.22E-04 ± 4.67E-05 | 2.57 | 0.002 |
| Chrnb2 | Cholinergic receptor, nicotinic, beta 2 (neuronal) | 7.95E-02 ± 4.44E-03 | 4.33E-02 ± 2.60E-03 | 1.83 | 0.002 |
| Grm1 | Glutamate receptor, metabotropic 1 | 2.90E-02 ± 2.06E-03 | 1.56E-02 ± 1.14E-03 | 1.86 | 0.001 |
| Grm5 | Glutamate receptor, metabotropic 5 | 1.95E-01 ± 1.92E-02 | 1.27E-01 ± 1.39E-02 | 1.53 | 0.032 |
| Grin1 | Glutamate receptor, ionotropic, N-methyl D-aspartate 1 | 1.74E-01 ± 1.89E-02 | 9.17E-02 ± 2.45E-03 | 1.90 | 0.013 |
| Grin2b | Glutamate receptor, ionotropic, N-methyl D-aspartate 2B | 1.50E-01 ± 4.78E-03 | 2.45E-01 ± 5.52E-03 | 0.61 | 0.0002 |
| Grin2d | Glutamate receptor, ionotropic, N-methyl D-aspartate 2D | 1.03E-02 ± 1.13E-03 | 5.95E-03 ± 4.94E-04 | 1.74 | 0.0075 |
| Stat6 | Signal transducer and activator of transcription 6, interleukin-4 induced | 3.73E-03 ± 2.95E-04 | 2.53E-03 ± 1.22E-04 | 1.48 | 0.019 |
| Il1r1 | Interleukin 1 receptor, type I | 8.55E-04 ± 2.81E-04 | 1.77E-03 ± 1.16E-04 | 0.48 | 0.046 |
| Il1r2 | Interleukin 1 receptor, type II | 3.20E-04 ± 2.79E-05 | 4.89E-04 ± 6.88E-05 | 0.65 | 0.042 |
| Il1a | Interleukin-1 alpha | 1.49E-04 ± 2.39E-05 | 2.24E-04 ± 1.37E-05 | 0.66 | 0.039 |
| Il1b | Interleukin 1, beta | 1.38E-04 ± 1.78E-05 | 7.06E-05 ± 3.29E-06 | 1.95 | 0.020 |
| Il12a | Interleukin 12A | 3.93E-03 ± 2.64E-04 | 3.05E-03 ± 2.45E-04 | 1.29 | 0.046 |
Figure 4.
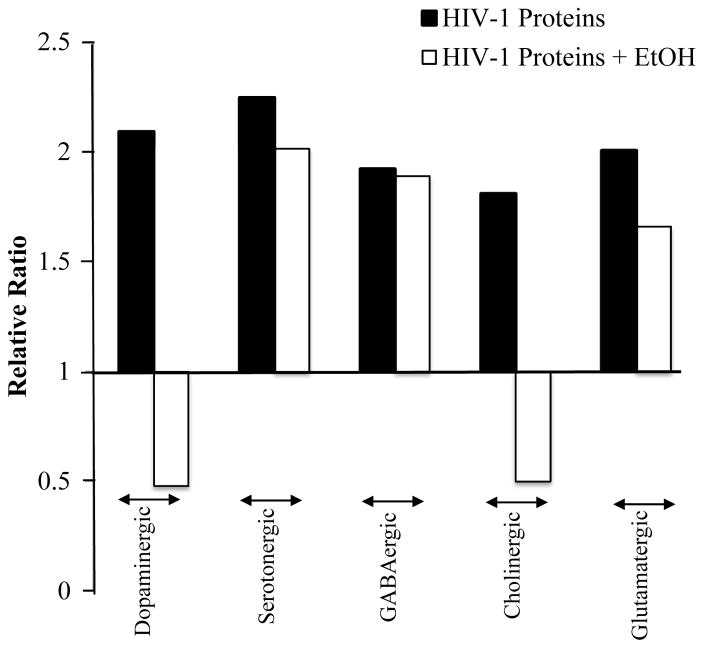
Interactive effects of HIV-1 proteins and ethanol on neurotransmitter gene expression in the NAc of HIV-1Tg and F344 rats at the signaling system level. These data were generated by quantitative RT-PCR and then analyzed using a comparative Ct method. The relative expressions of each gene were compared between saline-treated HIV-1Tg rats and saline-treated F344 rats (black) and between ethanol-treated and saline-treated HIV-1Tg rats (white). The mean value of each significantly altered gene ratio between saline-treated HIV-1Tg rats and saline-treated F344 rats in each gene family (dopaminergic, N = 1 gene; serotonergic, N = 4 genes; GABAergic, N = 1 gene; cholinergic, N = 5 genes; and glutamatergic, N = 5 genes) was calculated. The same procedure was followed for comparing ethanol-treated and saline-treated HIV-1Tg rats (dopaminergic, N = 2 genes; serotonergic, N = 2 genes; GABAergic, N = 1 gene; cholinergic, N = 1 gene; and glutamatergic, N = 4 genes). HIV-1 proteins significantly increased dopaminergic, serotonergic, GABAergic, cholinergic, and glutamatergic gene expression compared with F344 rats. Ethanol treatment reversed this trend in HIV-1Tg rats, downregulating all five neurotransmitter systems compared with saline-treated HIV-1Tg rats.
Figure 5.
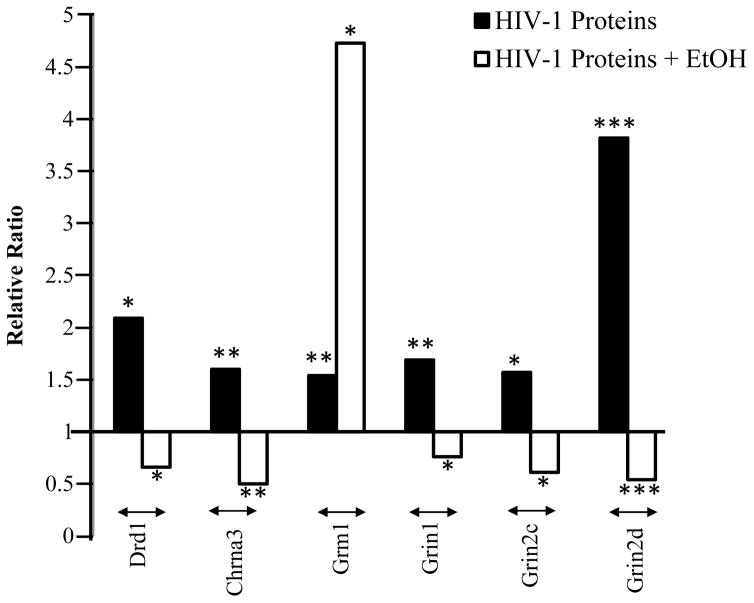
Interactive effects of HIV-1 proteins and ethanol on neurotransmitter gene expression in the NAc at the gene level. Quantitative RT-PCR was utilized to measure the expression of each gene, which was then analyzed using a comparative Ct method. The asterisks indicate the relative strength of p values: * marks a p value of < 0.05; ** indicates p < 0.01; and *** indicates p < 0.001. Six genes (DRD1, CHRNA3, GRM1, GRIN1, GRIN2C, and GRIN2D) were significantly altered in both comparisons: saline-treated HIV-1Tg versus saline-treated F344 rats (black) and ethanol-treated versus saline-treated HIV-1Tg rats (white). Except for one gene (GRM1), ethanol treatment downregulated all of these genes in the presence of HIV-1 proteins.
Chronic ethanol treatment differentially altered the expression of genes encoding dopaminergic receptor subtypes. In contrast to the effects of ethanol in HIV-1Tg rats, ethanol treatment increased the expression of D1-like receptor Drd5 by 126% in the NAc of F344 rats (p = 0.013). However, as observed in HIV-1Tg rats, ethanol treatment significantly decreased the expression of D2-like receptor Drd4 by 75% (p = 0.0003). In the serotonergic system, ethanol treatment significantly upregulated the expression of genes Htr1a, Htr3a, Htr7, and Slc6a4 by 28%–291% (p = 0.027–0.0002).
We observed gene-specific differential regulation of ethanol on the GABAergic signaling in the NAc of F344 rats. The expressions of Gabra1 and Gabra5 were significantly upregulated by ethanol by 44% (p= 0.043 and 51% (p = 0.018), and the expression of Gabra2 was downregulated by 56% (p = 0.004). The expressions of genes encoding nAChR subtypes – Chrna2, Chrna4, Chrna5, and Chrnb2 – were significantly upregulated by 83%–270% in F344 rats (p = 0.005–0.002). Chronic ethanol treatment increased the expression of genes encoding both metabotropic and ionotropic glutamatergic subtypes with the exception of one gene (Grin2b). The increased expression was found in metabotropic receptors Grm1 and Grm5 by 53% (p=0.032) and 86% (p = 0.001) and in ionotropic receptors Grin1 and Grin2d by 74% (p = 0.013) and 90% (p = 0.0075). Ethanol downregulated the expression of Grin2b by 39% (p = 0.0002).
In F344 rats, differential regulation by chronic ethanol exposure was observed in the expression of immune system-related genes. Ethanol treatment significantly increased the expressions of Stat6, Il1b, and Il12a by 29%–95% (p = 0.046–0.019) and decreased expressions of Il1r1, Il1r2, and Il1a by 34%–52% in F344 rats (p = 0.046–0.039).
Discussion
The present study examined the role of HIV-1 proteins in novelty-seeking behavior and how chronic ethanol treatment affects this behavior in the presence of viral proteins by using HIV-1Tg rats as an animal model. The HIV-1Tg rats showed markedly higher baseline novelty-seeking behavior than did F344 rats under both free-choice (hole-board) and inescapable (open field) testing conditions. Following chronic treatment, ethanol increased baseline novelty-seeking behavior in both strains, but the effects appeared to be more robust in HIV-1Tg rats. Strain-specific patterns of altered gene expression were observed for dopaminergic, cholinergic, and glutamatergic signaling in the NAc. Chronic ethanol treatment reversed the effects of HIV-1 proteins in the dopaminergic, cholinergic, and NMDA glutamatergic signaling; ethanol enhanced HIV-1 protein-induced upregulation of metabotropic glutamate receptor 5 gene (Grm5) in the NAc. Together, these findings indicate that HIV-1 proteins can modify novelty-seeking behavior by changing expression patterns of these genes. Further, chronic ethanol treatment enhanced this behavior in both strains, although to a greater extent in HIV-1Tg rats, possibly by interacting with genes that are affected by HIV-1 proteins.
Our study indicated that HIV-1 proteins alter the expression of genes that govern the brain’s reward circuits in the presence and absence of ethanol. Under baseline conditions, HIV-1 proteins produced significant upregulation of genes involved in dopaminergic, cholinergic, serotonergic, GABAergic, and glutamatergic signaling in the NAc. Chronic ethanol treatment reversed this viral protein-induced upregulation of these neurotransmitter systems. The most marked changes in gene expression were observed in dopaminergic and cholinergic signaling.
Effects of HIV-1 proteins alone and in combination with chronic ethanol treatment on novelty-seeking behavior
The most striking finding from this study is that HIV-1 proteins may have modulated the behavior of the animal host by increasing the response to novelty compared with that observed in healthy control subjects (F344 rats). In the pre-treatment trials, HIV-1Tg rats displayed more head dipping in the hole-board test, made more center entries, and spent more time in the center of the arena in the open field test than did F344 rats. HIV-1Tg rats displayed a high propensity to explore a new environment, regardless of whether test conditions reflected free-choice or inescapable novelty. Because the increase in behavioral response to novelty is highly associated with enhanced activity of the brain’s reward system, our results suggest that HIV-1 proteins could shape novelty-seeking behavior via changes in this system’s activity. These alterations may lead to a greater desire to use addictive substances, with greater hedonic impact of drugs of abuse in the HIV-1-infected population.
To determine the interactive effects of HIV-1 proteins and ethanol on novelty-seeking behavior, we extended our experiments by showing that chronic ethanol treatment caused significant enhancement in exploratory behavior of both strains compared with baseline levels. In drug-naïve rats, the first day of exposure to the environment is considered the only day that rodents perceive the environment to be highly novel (Lubow, 1976). As such, re-exposure to a novel environment typically leads to decreased novelty-seeking behavior in rodents. However, according to our results, chronic ethanol exposure may have induced a lack of habituation to novelty. It is possible that the memory impairment effects of ethanol (Vetreno et al., 2011) could have affected these results. However, given the low dosage of ethanol and the reliance of these tasks examining novelty-seeking behavior – and not learning or memory, it is likely that the post-treatment behavioral tests reflect the effects of ethanol on novelty-seeking behavior. The significant gene expression alterations observed in the brain’s reward system (Cloninger, 1987) of ethanol-treated rats further support this claim. It is interesting that there were no differences in time mobile scores and anxiety-related behaviors between treatment groups. One possible interpretation of this finding is that repeated ethanol exposure in rats could lead to development of tolerance to its anxiogenic effects (Escrig et al., 2012). Such tolerance may heighten ethanol’s rewarding effects (Koob, 2004) to a greater extent in HIV-1Tg rats. Therefore, our results suggest that the greater change in novelty-seeking behavior in HIV-1Tg rats is related to the complex interaction of ethanol and viral proteins on the activity of neurotransmission involved in the brain’s reward system.
It is important to note the selection of the dose of ethanol utilized in this study. Each ethanol-treated rat received a single, daily intraperitoneal injection of 20% (vol./vol.) ethanol/saline solution at a dose of 1 g/kg. The reasoning for choosing this dosage was two-fold. First, several studies have demonstrated that rats exposed to 20% v/v ethanol solutions have escalated drinking behaviors (Chang et al., 2010, Mill et al., 2013, Li et al., 2013). Such behaviors have been shown to be analogous to the transition to compulsive alcohol use among humans, a hallmark of alcohol addiction (Cloninger, 1987) Secondly, studies in alcohol addiction in F344 rats have shown that daily minimum consumption of 1 g/kg of 20% ethanol solution induced alcohol dependence (Mill et al., 2013). Duration of at least 10 days of intraperitoneal injections of ethanol has been demonstrated to be sufficient to produce withdrawal symptoms among rats, an indication of alcohol dependence (Morales et al., 2011). Further, the delivery of 1 g/kg of ethanol by oral or intraperitoneal injections impacted the regulation of brain mRNA levels in rats (Li et al., 2013, Chang et al., 2010). After conducting this study, our assumption on dose selection was shown to be accurate, as we demonstrated significant changes in gene expression between strains.
Both the post-treatment behavioral tests and collection of tissues were conducted 10 minutes after injections. Studies have demonstrated excellent correlation between ethanol levels in the brain and in systemic blood, at times as early as 10 min after intraperitoneal injection of ethanol (Cunningham CL, 1997, Gentry et al., 1983). Peak brain ethanol levels have been shown to occur between 10 and 25 min after injection (Gentry et al., 1983, Mattucci-Schiavone and Ferko, 1984). Considering this information and that the behavioral tests are 10 min in duration, it was decided that beginning the tasks 10 min after injections would produce the most meaningful results, as the rats would be performing during peak BECs.
HIV-1 protein-induced changes in the expression of genes that govern the function of the brain’s reward circuits in the presence and absence of ethanol
We selected NAc for our gene expression analyses because of its key role in the reward system of the brain, as well as in drug addiction (du Hoffmann and Nicola, 2014, Koob, 2000, Mohan et al., 2011). We analyzed the expression of genes implicated in alcohol addiction and novelty-seeking behavior between strains (Cloninger, 1987, Sarkar et al., 2013). Our results demonstrated that HIV-1Tg rats showed significant upregulation of genes involved in dopaminergic, cholinergic, and glutamatergic signaling in the NAc. These results are consistent with previous findings showing that HIV-1 proteins alter excitatory neurotransmitter systems, mainly by changing the expression of NMDA receptors and by impairing dopaminergic and cholinergic function (Moran et al., 2013, Neri et al., 2007, Nesil et al., 2015, Potter et al., 2013). These alterations lead to dysfunction in neurotransmission between synapses and, ultimately, to neuronal cell death in the brains’ mesolimbic system (Avdoshina et al., 2013). Our results support the idea that observed increases in the expression of neurotransmitter receptor subunits could reflect an attempt to compensate tonic extracellular dopamine in the NAc following glutamate-mediated excitotoxicity (Koob and Volkow, 2010). Previous findings have shown that glutamatergic, dopaminergic, and cholinergic neurotransmitter systems are centrally involved in reward processing, including novelty-seeking behavioral output (Koob and Volkow, 2010, Krebs et al., 2011, Potter et al., 2013). Thus, it is reasonable to assume that molecular and cellular adaptations in response to HIV-1 proteins in the brain could lead to a high behavioral response to novelty in HIV-1Tg rats.
It is worth mentioning that the observed alterations in the expression of neurotransmitter receptors and novelty-seeking behavioural output may be linked to neuroinflammation caused by HIV-1 viral proteins in the NAc. Our results showed that HIV-1 proteins increased expression of genes encoding the Il-6 receptor subunits and decreased expression of genes encoding Il-1 receptor subunits. These results indicate divergent effects of HIV-1 proteins in the immune signalling pathways in the NAc. Given that cytokines are major players in the modulation of both excitatory and inhibitory synapses in the brain, a possible disturbance between IL-1 and IL-6 signalling may increase the severity of immune responses to HIV infection, which induces synaptic dysfunction and abnormal response to novelty in HIV-1Tg rats.
We also demonstrated that HIV-1 protein-induced upregulation of neurotransmitter receptors could be modulated by ethanol in the NAc. Chronic ethanol treatment downregulated Drd1 expression in HIV-1Tg rats compared with their saline-treated counterparts. However, this finding is in contrast to ethanol’s well-established ability to enhance extracellular dopamine concentrations in the NAc by increasing dopaminergic neuronal activity in the VTA and by increasing the immune signaling pathways, particularly that of IL-1 (Feduccia et al., 2012, Tabakoff and Hoffman, 2013, Vengeliene et al., 2008). Regarding the interactive effects of ethanol and IL-1 on dopaminergic signaling in the NAc, our results showed that chronic ethanol treatment significantly upregulated expression of the Il1r1 receptor subunit, which is a key regulator in development of alcohol dependence. One possible interpretation of the observed effects of ethanol on Drd1 expression is that ethanol could increase extracellular dopamine concentrations above the baseline through activation of IL-1 signaling, resulting in a compensatory mechanism. This mechanism may occur to correct the disturbance of dopamine levels in the NAc of HIV-1Tg rats. Regarding the molecular mechanisms that implicate the critical role of Drd1 gene function in the NAc in regulating the degree of alcohol consumption (Li, 2013), our results point out that Drd1 may be a molecular target where HIV-1 proteins and ethanol functionally interact. As such, this role could help to explain the widespread co-morbidity between impulsive personality and alcohol consumption in HIV-1-infected patients (Durvasula and Miller, 2014). Further research examining dopaminergic targets at the protein level, such as monitoring dopamine release in HIV-1Tg rats, may supplement our findings. Although the activity of the NAc is mainly regulated by dopaminergic and glutamatergic afferents projecting from the frontal cortex, amygdala, and hippocampus, it can also be modified by cholinergic interneurons within the NAc (Besheer et al., 2010, Dichter et al., 2012, Koob and Volkow, 2010). In the current study, ethanol exposure decreased expression of Chrna3, Grin1, Grin2c, and Grin2d but increased expression of the metabotropic glutamate receptor subunit 5 (mGrm5) in HIV-1Tg rats relative to their saline-treated counterparts. With respect to downregulation of Chrna3 in the NAc, our results are in accord with previous studies showing that chronic ethanol treatment decreases cholinergic activity in the NAc, which has shown to be correlated with addiction-like behaviors (Clarke and Adermark, 2015, Jupp and Dalley, 2014, Tuesta et al., 2011). Further, recent studies have shown that mGluR5 activity in the NAc is required for the expression of ethanol’s reinforcing effects and novelty-seeking behavior (Cozzoli et al., 2009, Gass and Olive, 2008). Parkitna and colleagues demonstrated that mGluR5 signaling in the dopaminergic neurons controls novelty-seeking behavior and escalation of alcohol intake in mice (Parkitna et al., 2013). Thus, the observed ethanol-induced alterations in the dopaminergic and cholinergic systems in HIV-1Tg rats could help to explain the robust enhancement of their response to novelty, which is controlled mainly by metabotropic glutamate receptor 5 rather than NMDA receptors.
In sum, our study demonstrated that HIV-1 proteins may alter the function of central reward in the NAc by modulating the expression of neurotransmitter receptors implicated in both novelty-seeking behavior and alcohol addiction (Figure 6). These differential expression patterns could explain the increased novelty-seeking behavior observed in the HIV-1Tg rats compared with F344 rats. These results suggest a link between HIV-1 infection and vulnerability to alcohol addiction. Specifically, we demonstrated that adaptations in response to HIV-1 proteins in the brain neurotransmitter systems may lead to enhanced vulnerability to the rewarding effects of alcohol in HIV-1-infected persons.
Figure 6.
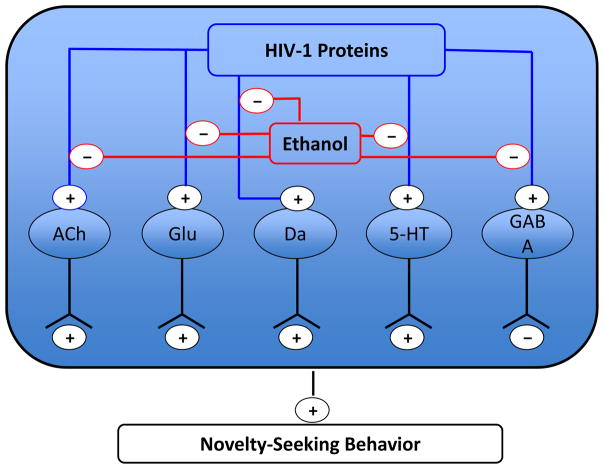
Summary of the effects of HIV-1 proteins and chronic ethanol treatment on neurotransmitters in the NAc. (+) indicates an excitatory projection, and (-) indicates an inhibitory projection. We found that HIV-1 proteins enhance the signaling activity of the following neurotransmitters: acetylcholine (ACh), glutamate (Glu), dopamine (Da), serotonin (5-HT), and gamma-aminobutyric acid (GABA). Ethanol treatment interacted with HIV-1 proteins to produce decreased signaling activity of these five neurotransmitters. HIV-1 proteins alone and in the presence of ethanol led to greater novelty-seeking behavior.
Also, our study indirectly suggests that HIV-1 infection leads to an increased vulnerability to drug addiction compared with the general population. Future studies investigating the mechanisms by which HIV-1 proteins create a signaling system in the brain that is more vulnerable to addiction would extend the clinical applications of this field’s body of relevant knowledge. Finally, our research findings may offer implications for clinical applications by using novelty-seeking behavioral tests among HIV-1-infected patients during alcohol cessation therapies. This approach would be useful in monitoring patients’ clinical status, assessing their risk for relapse, and evaluating pharmacopsychotherapies.
Supplementary Material
Acknowledgments
This study was supported, in part, by US National Institutes of Health grants DA-012844 to MDL and DA-026356 to SLC and MDL.
Footnotes
Financial disclosures
All authors of this study declare no conflict of interest regarding the works reported.
References
- Avdoshina V, Bachis A, Mocchetti I. Synaptic dysfunction in human immunodeficiency virus type-1-positive subjects: inflammation or impaired neuronal plasticity? J Intern Med. 2013;273:454–465. doi: 10.1111/joim.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biological psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36:525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Boissier JR, Simon P. The exploration reaction in the mouse. Preliminary note. Therapie. 1962;17:1225–1232. [PubMed] [Google Scholar]
- Burns L. World Drug Report 2013 United Nations Office on Drug and Crime. Drug and alcohol review. 2014;33:216–216. [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R, Adermark L. Dopaminergic Regulation of Striatal Interneurons in Reward and Addiction: Focus on Alcohol. Neural Plast. 2015;2015:814567. doi: 10.1155/2015/814567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WY, Zhao S, Polanowska-Grabowska R, Wang J, Wei J, Dash B, Chang SL, Saucerman JJ, Gu J, Li MD. Identification and characterization of poly(I:C)-induced molecular responses attenuated by nicotine in mouse macrophages. Mol Pharmacol. 2013;83:61–72. doi: 10.1124/mol.112.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, OD, Howard CE. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learning & Behavior. 1997;25:31. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula R, Miller TR. Substance abuse treatment in persons with HIV/AIDS: challenges in managing triple diagnosis. Behav Med. 2014;40:43–52. doi: 10.1080/08964289.2013.866540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrig MA, Pardo M, Aragon CM, Correa M. Anxiogenic and stress-inducing effects of peripherally administered acetaldehyde in mice: similarities with the disulfiram-ethanol reaction. Pharmacol Biochem Behav. 2012;100:404–412. doi: 10.1016/j.pbb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Fassino S, Leombruni P, Amianto F, Abbate-Daga G. Personality profile of HIV outpatients: preliminary results and remarks on clinical management. Psychotherapy and psychosomatics. 2004;73:361–365. doi: 10.1159/000080389. [DOI] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:83. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry RT, Rappaport MS, Dole VP. Serial determination of plasma ethanol concentrations in mice. Physiol Behav. 1983;31:529–532. doi: 10.1016/0031-9384(83)90077-x. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Behaviour of male and female rats with free choice of two environments differing in novelty. Anim Behav. 1968;16:92–96. doi: 10.1016/0003-3472(68)90116-4. [DOI] [PubMed] [Google Scholar]
- Jupp B, Dalley JW. Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. British journal of pharmacology. 2014;171:4729–4766. doi: 10.1111/bph.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RM, Heipertz D, Schuetze H, Duzel E. Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI. Neuroimage. 2011;58:647–655. doi: 10.1016/j.neuroimage.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Li J, Li J, Liu X, Qin S, Guan Y, Liu Y, Cheng Y, Chen X, Li W, Wang S, Xiong M, Kuzhikandathil EV, Ye JH, Zhang C. MicroRNA expression profile and functional analysis reveal that miR-382 is a critical novel gene of alcohol addiction. EMBO Mol Med. 2013;5:1402–1414. doi: 10.1002/emmm.201201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubow RE, Rifkin B, Alex M. The context effect: The relationship between stimulus preexposure and environmental preexposure determines subsequent learning. Journal of Experimental Psychology Animal Behavior Processes. 1976;2:38–47. [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature neuroscience. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo L, Gomez MJ, Callejas-Aguilera JE, Donaire R, Sabariego M, Fernandez-Teruel A, Canete A, Blazquez G, Papini MR, Torres C. Relationship between ethanol preference and sensation/novelty seeking. Physiology & behavior. 2014;133:53–60. doi: 10.1016/j.physbeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Mattucci-Schiavone L, Ferko AP. Sampling of orbital sinus blood closely reflects brain ethanol content in rats. Physiol Behav. 1984;33:895–898. doi: 10.1016/0031-9384(84)90224-5. [DOI] [PubMed] [Google Scholar]
- Mill DJ, Bito-Onon JJ, Simms JA, Li R, Bartlett SE. Fischer rats consume 20% ethanol in a long-term intermittent-access two-bottle-choice paradigm. PLoS One. 2013;8:e79824. doi: 10.1371/journal.pone.0079824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Pendyam S, Kalivas PW, Nair SS. Molecular diffusion model of neurotransmitter homeostasis around synapses supporting gradients. Neural Comput. 2011;23:984–1014. doi: 10.1162/NECO_a_00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–1624. doi: 10.1111/j.1530-0277.2011.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Experimental neurology. 2013;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri E, Musante V, Pittaluga A. Effects of the HIV-1 viral protein TAT on central neurotransmission: role of group I metabotropic glutamate receptors. International review of neurobiology. 2007;82:339–356. doi: 10.1016/S0074-7742(07)82018-6. [DOI] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Molecular brain. 2015;8:43. doi: 10.1186/s13041-015-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, Ingraham CM, McKinzie DL, McBride WJ, Lumeng L, Li TK, Murphy JM. An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav. 2000;66:113–121. doi: 10.1016/s0091-3057(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Parkitna JR, Sikora M, Golda S, Golembiowska K, Bystrowska B, Engblom D, Bilbao A, Przewlocki R. Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biological psychiatry. 2013;73:263–270. doi: 10.1016/j.biopsych.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; New York: 1998. [Google Scholar]
- Potter MC, Figuera-Losada M, Rojas C, Slusher BS. Targeting the glutamatergic system for the treatment of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 2013;8:594–607. doi: 10.1007/s11481-013-9442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Hansen S. Temperament in the rat: sex differences and hormonal influences on harm avoidance and novelty seeking. Behav Neurosci. 2004;118:488–497. doi: 10.1037/0735-7044.118.3.488. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Chang SL. Ethanol concentration-dependent alterations in gene expression during acute binge drinking in the HIV-1 transgenic rat. Alcohol Clin Exp Res. 2013;37:1082–1090. doi: 10.1111/acer.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Mao X, Liu C, Chang SL. Age- and ethanol concentration-dependent effects of acute binge drinking in the HIV-1 transgenic rat. Alcohol Clin Exp Res. 2013;37(Suppl 1):E70–78. doi: 10.1111/j.1530-0277.2012.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Silverstein PS, Kumar A. HIV-1 and alcohol: interactions in the central nervous system. Alcohol Clin Exp Res. 2014;38:604–610. doi: 10.1111/acer.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S. The role of the striatum in addiction. Brain Nerve. 2012;64:911–917. [PubMed] [Google Scholar]
- Tropp J, Markus EJ. Sex differences in the dynamics of cue utilization and exploratory behavior. Behav Brain Res. 2001;119:143–154. doi: 10.1016/s0166-4328(00)00345-4. [DOI] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–995. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. British journal of pharmacology. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Welker WI. “FREE” VERSUS “FORCED” EXPLORATION OF A NOVEL SITUATION BY RATS. Psychological Reports. 1957;3 [Google Scholar]
- Wingo T, Nesil T, Choi JS, Li MD. Novelty Seeking and Drug Addiction in Humans and Animals: From Behavior to Molecules. J Neuroimmune Pharmacol. 2015 doi: 10.1007/s11481-015-9636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.