Abstract
Emerging evidence has pointed to the importance of long non-coding RNAs (lncRNAs) in biological function and disease. However, lncRNAs remain largely unexploited in the vascular system. In this issue of ATVB, Zhao et al., identified a novel vascular smooth muscle cell-specific lncRNA, Myoslid. The investigators demonstrated that Myoslid, whose expression is transcriptionally controlled by SRF and myocardin, regulates smooth muscle cell proliferation and differentiation program, at least in part, by modulating the TGF-β pathway. This study has uncovered the involvement of non-coding RNAs in the already complicated gene regulatory networks in vascular biology, highlighting the potential of lncRNAs as novel therapeutic targets for cardiovascular disorders.
Cardiovascular disease remains the leading cause of mortality and morbidity worldwide. Over the past decades, intense studies on key molecules that control gene expression (transcription factors, epigenetic regulators) and signaling pathways (for example, the TGF-β, Notch, Hippo pathways) have led to a fundamental understanding of the regulatory mechanisms for cell proliferation, differentiation, migration and apoptosis in the cardiovascular system, resulting in the identification of potential therapeutic targets for cardiovascular diseases. Despite these advances, a deeper understanding of the intricate layers of gene regulation and molecular mechanisms by which the activity of the aforementioned pathways is regulated is necessary as a means towards precision medicine.
It is now recognized that the majority of our genome is actively transcribed to produce thousands of non-coding transcripts. In particular, long non-coding RNAs (lncRNAs), RNAs longer than 200 nucleotides in length, are widely expressed and participate in a variety of biological processes. In recent years, gene expression profiling, thanks to the increased depth of RNA-sequencing, has documented that thousands of lncRNAs are expressed in normal and diseased hearts 1. Several pioneering studies have linked the function of lncRNAs to the maintenance of cardiomyocyte fate (Braveheart) 2, 3; cardioprotection in response to stress (Myheart) 4, 5. Another study demonstrated that lincRNA-p21 controls cell proliferation and the pathological development of atherosclerosis 6. Mechanistically, lncRNAs are found in both the nucleus and cytoplasm, and have been suggested to regulate transcription and translation. However, the biological functions of most lncRNAs in the cardiovascular system remain largely unexplored.
Transcriptional control plays a vital role in smooth muscle gene expression and vascular function. Myocardin was initially identified as a “master” regulator of the smooth muscle gene program 7. Acting as a tissue-specific co-factor of Serum Response Factor (SRF), myocardin was shown to transactivate a battery of key smooth muscle genes 8, 9. In this study, Zhao et al., asked whether myocardin could regulate the expression of long non-coding RNA (lncRNA) genes in contractile vascular smooth muscle 10. As expected, upon profiling lncRNA expression in human coronary artery smooth muscle cells (HCASMCs) overexpressing myocardin, they identified 137 lncRNAs whose expression was significantly altered. Among them, Myoslid stood out as a novel, smooth muscle-specific lncRNA. They showed that expression of Myoslid is transcriptionally regulated by SRF and Myocardin. Myoslid appears to be a cytosolic lncRNA and its expression level is modest when compared to that of protein-coding genes. Overexpression and knockdown experiments indicated that Myoslid participates in the TGF-β signaling pathway to control VSMC differentiation 10.
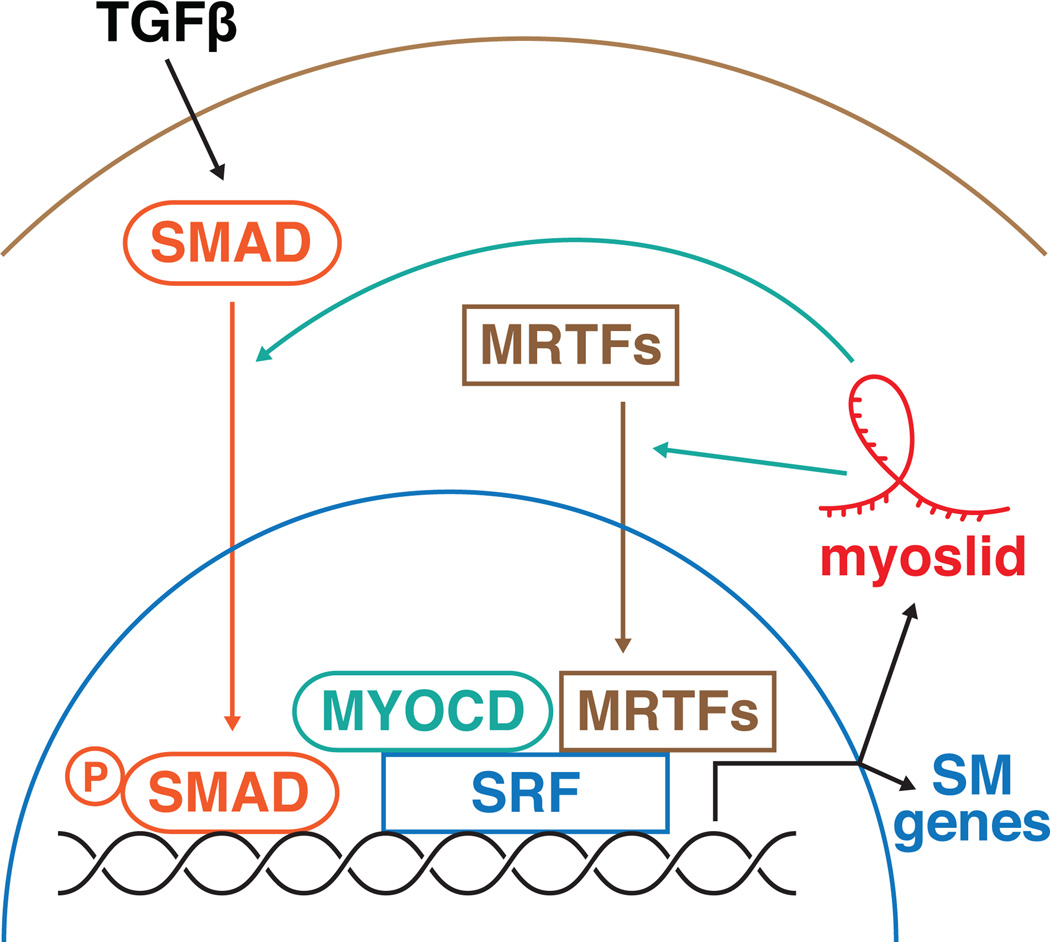
While much is known regarding the signaling and transcriptional control in the cardiovascular system there is comparatively less information on the function of lncRNAs and their placement in the vascular gene regulatory network. Myoslid is a new member of the vascular lncRNA pool that regulates cytoskeleton assembly and VSMC differentiation through its activation of contractile gene expression and concomitant suppression of VSMC proliferation and migration. Accordingly, maintenance of Myoslid expression in differentiated VSMCs appears to be essential as its downregulation correlates with the pathological de-differentiation of smooth muscle. Although Myoslid expression is not sufficient to induce differentiation it plays a critical function in this process by modulating the activities of MRTFs, and through feedback regulation of both the SRF/Myocd and TGF-β pathways to maintain the differentiated state. (Figure)
Figure.
The long non-coding RNA Myoslid regulate smooth muscle (SM) differentiation through modulation of the TGFβ and MRTF pathways in a feedback manner.
In their study, the investigators used both overexpression and siRNA-based knockdown to uncover the function of Myoslid in VSMC in vitro. It will be important to define the in vivo function of this lncRNA, using gain and loss-of function approaches in animal models. Ultimately, it will be essential to determine the role of human Myoslid in cardiovascular diseases. While this report focused on Myoslid the authors of this study identified over one hundred myocardin-induced lncRNAs. Given the central role of myocardin in smooth muscle differentiation it would be important to determine whether any of the other lncRNAs identified here also play a role in VSMCs. For example, do any of these lncRNAs modulate the TGF-β pathway? If not, then what smooth muscle pathways do these lncRNAs regulate? And, how many of these are smooth muscle-specific? Given recent reports demonstrating that some previously annotated lncRNAs encode micropeptides 11, 12, the possibility of Myoslid encoding a micropeptide also needs to be formally excluded. Additionally, the molecular mechanisms underlying Myoslid function remain to be fully understood. lncRNAs regulate gene expression through various mechanisms: recruitment of chromatin modifiers or transcriptional regulators in cis or in trans, or as molecular sponges by binding to and titrating endogenous mRNAs or miRNAs. Though the authors suggest that Myoslid lacks known miRNA seed sequences to function as a molecular sponge, its cytoplasmic enrichment strongly suggests this mode action to regulate MKL nucleocytoplasmic shuttling. It is equally attractive to test the hypothesis that Myoslid itself shuttles between nucleus and cytoplasm under pathophysiological conditions. Finally, whether Myoslid, and probably other myocardin-induced lncRNAs, is directly involved in human cardiovascular disease is an important link to determine in the future.
Acknowledgments
We thank members of the Naya and Wang laboratories for discussions. Research in our labs was supported by the American Heart Association, Muscular Dystrophy Association and the NIH (HL085635, HL116919, HL125925) (Wang); HL073304 (Naya).
References
- 1.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 2.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–583. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kataoka M, Huang ZP, Wang DZ. Build a braveheart: the missing linc (RNA) Circ Res. 2013;112:1532–1534. doi: 10.1161/CIRCRESAHA.113.301519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien HC, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HS, Quertermous T, Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Wang DZ. An epigenetic "LINK(RNA)" to pathological cardiac hypertrophy. Cell metabolism. 2014;20:555–557. doi: 10.1016/j.cmet.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 9.Miano JM. Myocardin in biology and disease. J Biomed Res. 2015;29:3–19. doi: 10.7555/JBR.29.20140151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Zhang W, Lin M, Wu W, Jiang P, Tou E, Xue M, Richards A, Jourd'heuil D, Asif A, Zheng D, Singer HA, Miano JM, Long X. MYOSLID Is a Novel Serum Response Factor-Dependent Long Noncoding RNA That Amplifies the Vascular Smooth Muscle Differentiation Program. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel-Duby R, Olson EN. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, Reese AL, McAnally JR, Chen X, Kavalali ET, Cannon SC, Houser SR, Bassel-Duby R, Olson EN. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]