Abstract
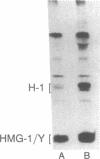
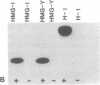
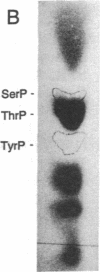
Mammalian high-mobility group I nonhistone protein (HMG-I) is a DNA-binding chromatin protein that has been demonstrated both in vitro and in vivo to be localized to the A + T-rich sequences of DNA. Recently an unusual binding domain peptide, "the A.T-hook" motif, that mediates specific interaction of HMG-I with the minor groove of DNA in vitro has been described. Inspection of the A.T-hook region of the binding domain showed that it matches the consensus sequence for phosphorylation by cdc2 kinase. Here we demonstrate that HMG-I is a substrate for phosphorylation by purified mammalian cdc2 kinase in vitro. The site of phosphorylation by this enzyme is a threonine residue at the amino-terminal end of the principal binding-domain region of the protein. Labeling of mitotically blocked mouse cells with [32P]phosphate demonstrates that this same threonine residue in HMG-I is also preferentially phosphorylated in vivo. Competition binding studies show that cdc2 phosphorylation of a synthetic binding-domain peptide significantly weakens its interaction with A + T-rich DNA in vitro, and a similar weakening of DNA binding has been observed for intact murine HMG-I protein phosphorylated by the kinase in vitro. These findings indicate that cdc2 phosphorylation may significantly alter the DNA-binding properties of the HMG-I proteins. Because many cdc2 substrates are DNA-binding proteins, these results further suggest that alteration of the DNA-binding affinity of a variety of proteins is an important general component of the mechanism by which cdc2 kinase regulates cell cycle progression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Chambers T. C., Langan T. A. Purification and characterization of growth-associated H1 histone kinase from Novikoff hepatoma cells. J Biol Chem. 1990 Oct 5;265(28):16940–16947. [PubMed] [Google Scholar]
- Churchill M. E., Suzuki M. 'SPKK' motifs prefer to bind to DNA at A/T-rich sites. EMBO J. 1989 Dec 20;8(13):4189–4195. doi: 10.1002/j.1460-2075.1989.tb08604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney J. E., Johnson K. R., Magnuson N. S., Sylvester S. R., Reeves R. High-mobility group protein HMG-I localizes to G/Q- and C-bands of human and mouse chromosomes. J Cell Biol. 1989 Nov;109(5):1975–1982. doi: 10.1083/jcb.109.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Elton T. S., Nissen M. S., Reeves R. Specific A . T DNA sequence binding of RP-HPLC purified HMG-I. Biochem Biophys Res Commun. 1987 Feb 27;143(1):260–265. doi: 10.1016/0006-291x(87)90659-0. [DOI] [PubMed] [Google Scholar]
- Elton T. S., Reeves R. Purification and postsynthetic modifications of Friend erythroleukemic cell high mobility group protein HMG-I. Anal Biochem. 1986 Aug 15;157(1):53–62. doi: 10.1016/0003-2697(86)90195-8. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Buratti E., Perissin L., Zorzet S., Balmain A., Portella G., Fusco A., Goodwin G. H. Analysis of the HMGI nuclear proteins in mouse neoplastic cells induced by different procedures. Exp Cell Res. 1989 Oct;184(2):538–545. doi: 10.1016/0014-4827(89)90352-2. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Pani B., D'Andrea P., Berlingieri M. T., Di Fiore P. P., Fusco A., Vecchio G., Philp R., Crane-Robinson C., Nicolas R. H. Elevated levels of a specific class of nuclear phosphoproteins in cells transformed with v-ras and v-mos oncogenes and by cotransfection with c-myc and polyoma middle T genes. EMBO J. 1987 Jul;6(7):1981–1987. doi: 10.1002/j.1460-2075.1987.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. R., Poccia D. L. Interaction of sperm histone variants and linker DNA during spermiogenesis in the sea urchin. Biochemistry. 1988 Jan 26;27(2):619–625. doi: 10.1021/bi00402a019. [DOI] [PubMed] [Google Scholar]
- Green G. R., Poccia D. L. Phosphorylation of sea urchin sperm H1 and H2B histones precedes chromatin decondensation and H1 exchange during pronuclear formation. Dev Biol. 1985 Mar;108(1):235–245. doi: 10.1016/0012-1606(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S., Packman L. C., Thomas J. O. Phosphorylation at clustered -Ser-Pro-X-Lys/Arg- motifs in sperm-specific histones H1 and H2B. EMBO J. 1990 Mar;9(3):805–813. doi: 10.1002/j.1460-2075.1990.tb08177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzmanowski A., Cole R. D. Flanking sequences of Xenopus 5 S RNA genes determine differential inhibition of transcription by H1 histone in vitro. Mitotic phosphorylation of H1 decreases its inhibitory power. J Biol Chem. 1990 Jun 25;265(18):10726–10732. [PubMed] [Google Scholar]
- Johnson K. R., Disney J. E., Wyatt C. R., Reeves R. Expression of mRNAs encoding mammalian chromosomal proteins HMG-I and HMG-Y during cellular proliferation. Exp Cell Res. 1990 Mar;187(1):69–76. doi: 10.1016/0014-4827(90)90118-t. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Elton T. S., Barr P. J., Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J Biol Chem. 1988 Dec 5;263(34):18338–18342. [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989 May;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe J. C., Lee M. G., Nurse P., Picard A., Doree M. Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature. 1988 Sep 15;335(6187):251–254. doi: 10.1038/335251a0. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Characterization of highly phosphorylated subcomponents of rat thymus H1 histone. J Biol Chem. 1982 Dec 25;257(24):14835–14846. [PubMed] [Google Scholar]
- Langan T. A., Gautier J., Lohka M., Hollingsworth R., Moreno S., Nurse P., Maller J., Sclafani R. A. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol. 1989 Sep;9(9):3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Fredriksen M., Laland S. G. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983 Feb 21;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Laland S. G. On the phosphorylation of low molecular mass HMG (high mobility group) proteins in Ehrlich ascites cells. FEBS Lett. 1985 Jan 28;180(2):275–279. doi: 10.1016/0014-5793(85)81085-1. [DOI] [PubMed] [Google Scholar]
- Lund T., Skålhegg B. S., Holtlund J., Blomhoff H. K., Laland S. G. Fractionation and identification of metaphase-specific phosphorylated forms of high-mobility-group proteins. Eur J Biochem. 1987 Jul 1;166(1):21–26. doi: 10.1111/j.1432-1033.1987.tb13477.x. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Palvimo J., Linnala-Kankkunen A. Identification of sites on chromosomal protein HMG-I phosphorylated by casein kinase II. FEBS Lett. 1989 Oct 23;257(1):101–104. doi: 10.1016/0014-5793(89)81796-x. [DOI] [PubMed] [Google Scholar]
- Piggott J. R., Rai R., Carter B. L. A bifunctional gene product involved in two phases of the yeast cell cycle. Nature. 1982 Jul 22;298(5872):391–393. doi: 10.1038/298391a0. [DOI] [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Nissen M. S. The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem. 1990 May 25;265(15):8573–8582. [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Freedlender E. F. Sites of in vivo phosphorylation of histone H5. Biochemistry. 1978 May 16;17(10):1884–1890. doi: 10.1021/bi00603a013. [DOI] [PubMed] [Google Scholar]
- Suzuki M. SPKK, a new nucleic acid-binding unit of protein found in histone. EMBO J. 1989 Mar;8(3):797–804. doi: 10.1002/j.1460-2075.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Vartiainen E., Palvimo J., Mahonen A., Linnala-Kankkunen A., Mäenpä P. H. Selective decrease in low-Mr HMG proteins HMG I and HMG Y during differentiation of mouse teratocarcinoma cells. FEBS Lett. 1988 Feb 8;228(1):45–48. doi: 10.1016/0014-5793(88)80581-7. [DOI] [PubMed] [Google Scholar]
- Zeilig C. E., Langan T. A. Studies on the mechanism of activation of mitotic histone H1 kinase. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1372–1379. doi: 10.1016/0006-291x(80)91625-3. [DOI] [PubMed] [Google Scholar]